Chapter 10 Electrodiagnostic Medicine II
Clinical Evaluation and Findings
Clinical Assessment: History and Physical Examination
Physical Examination
In many cases, these signs can, when combined with the history, point to the correct condition. Other tests can further clarify the clinical picture. These include Phalen’s test for carpal tunnel syndrome. Palpation over the elbow to reproduce symptoms in someone with suspected ulnar neuropathy can be helpful. Straight leg raise testing for persons with suspected lumbosacral radiculopathy is useful when clearly positive. For persons with less clear conditions, palpation over the site of discomfort can help define trigger points or tendinopathies. Shoulder impingement signs can identify bursitis and bicipital tendonitis to help the electrodiagnostician in assessment for suspected C5 or C6 radiculopathy. Finklestein’s test for de Quervain’s tenosynovitis is useful for persons presenting with wrist pain. Lateral epicondylitis can mimic C6 radiculopathy. Lateral epicondylitis is characterized by reproduction of pain with palpation over the extensor forearm muscles and with resisted wrist extension. These examinations can help put the clinical scenario in the proper context.
Cannon et al.23 examined the ability of physical examination and delineation of upper limb musculoskeletal disorders (myofascial pain, shoulder impingement, lateral epicondylitis, de Quervain’s tenosynovitis) to predict the outcomes of electrodiagnostic testing (normal study, cervical radiculopathy, or another electrodiagnostically confirmed diagnosis). They found that the total prevalence of musculoskeletal disorders was 42%. The prevalence in those with a normal study was 69%, compared with 29% in those with cervical radiculopathy (p <0.0001) and 45% in those with another diagnosis (p = 0.02). While the prevalence of certain musculoskeletal disorders made having a normal electrodiagnostic evaluation significantly more likely, the high prevalence among both patients with normal studies and those with radiculopathy and other disorders limited the usefulness of this information in precisely predicting study outcome. Consequently the presence of upper limb musculoskeletal disorders should not preclude electrodiagnostic testing when it is otherwise indicated.
These investigators found similar results for the lower limb.22 In a sample of 170 patients who were referred for suspected lumbosacral radiculopathy, they found the total prevalence of musculoskeletal disorders (myofascial pain, trochanteric bursitis/iliotibial band syndrome, or plantar fasciitis) in the sample was 32%. The prevalence in those with a normal study was 55%, compared with 21% in those with lumbosacral radiculopathy (p <0.0001). As in the case for suspected cervical radiculopathy, the high prevalence among both patients with normal studies and those with radiculopathy and other disorders limits the usefulness of this information in predicting study outcome. Because it is common for patients to have two or more problems, the presence of a musculoskeletal disorder should not preclude electrodiagnostic testing when radiculopathy is suspected.22
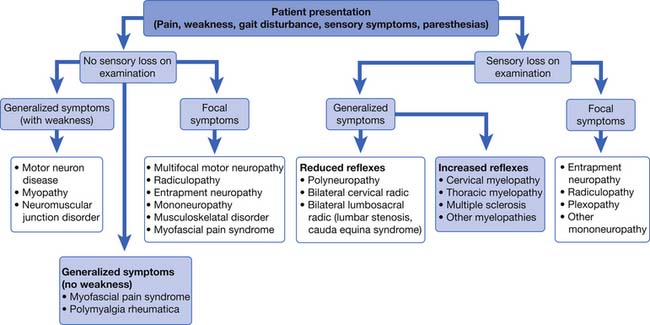
An algorithmic approach to using physical examination and symptom information to tailor the electrodiagnostic evaluation is shown in Figure 10-1. In this approach, the patient’s physical examination signs of sensory loss and weakness create a conceptual framework for approaching these sometimes daunting problems. For the purposes of this discussion, generalized findings are defined as being present in two or more limbs. While helpful, there are many exceptions to this taxonomy. The electrodiagnostician must refocus the diagnostic effort as data are acquired. Sensory loss is a key finding on examination for someone presenting with generalized symptoms, and provides a branch point in the algorithmic approach to tailoring the electrodiagnostic study (see Figure 10-1). Myopathies, neuromuscular junction disorders, motor neuron disease, and multifocal motor neuropathy are all characterized by preservation of the sensory system. In contrast, polyneuropathies, bilateral radiculopathies, myelopathies, and central nervous system disorders frequently result in reduced sensation. In persons with no sensory loss or weakness on examination, the electrodiagnostician should maintain a heightened suspicion for myofascial pain syndrome or fibromyalgia, polymyalgia rheumatica, or multiple other musculoskeletal disorders.11
Purpose of Electrodiagnostic Testing
Electrodiagnostic testing excludes conditions in the differential diagnosis and alters the referring diagnostic impression 42% of the time.71 Electrodiagnostic testing can to some extent suggest severity or extent of the disorder beyond the clinical symptoms. Involvement of other limbs can be delineated, or the involvement of multiple roots can be demonstrated, such as in the case of lumbosacral spinal stenosis. Finally, there is utility in solidifying a diagnosis. For example, an unequivocal radiculopathy on electromyography (EMG) provides greater diagnostic certainty and identifies avenues of management.
Types of Electrodiagnostic Tests
Motor and Sensory Nerve Conduction Studies
NCSs have been found to be safe. In a well-designed study involving patients with dual-chamber pacemakers and implanted cardiac defibrillators, NCSs including median motor testing with Erb’s point stimulation on the left side of the body resulted in no change in the function of these devices, nor did any stimulation show up on any of the electrocardiogram tracings.123 These investigators concluded that conventional NCSs have no effects on these implanted devices, and their findings should diminish the anxiety of electrodiagnosticians when working with such patients.123 These findings further support the safety of performing such testing in persons with implanted cardiac devices and dispel some of the concern expressed in previous recommendations.6
Sensory nerve conductions should always be incorporated when assessing a patient.7 In the upper limb, multiple sensory nerves are easily accessible and allow assessment for both entrapments and polyneuropathies. In the lower limbs, perhaps the most readily accessible is the sural nerve. Indeed, in the recent position paper on polyneuropathies, this nerve was felt to be an excellent nerve for screening a patient for distal symmetric polyneuropathy.58
Motor NCSs should be performed in almost all situations.7 Examiners should assess the morphology of the waveform, its latency, amplitude, and conduction velocity. Conduction should be performed across any suspected sites of entrapment or injury. Enough nerves should be studied to be able to determine whether a generalized condition is present.
The elderly frequently have symptoms that prompt electrodiagnostic testing, and for this reason understanding the implications of nerve conduction testing in this age-group is vital to understand and interpret normal from abnormal.60 Falco et al.60 examined the upper limb nerves using standardized testing in a group of healthy elderly persons. They found that upper limb conductions compared favorably with those reported for younger groups. Age had some influence on sensory NCS latencies and amplitudes These researchers concluded that age has smaller effects on NCSs than was previously thought.60 These investigators meticulously controlled temperature, and this might have contributed to the NCS findings, because the limbs of the elderly are often somewhat colder than younger adults. They stressed the need for temperature control. It is also possible that their stringent selection criteria for “healthy” selected out a special group of the elderly who were in markedly better health than their peers.
In a similar companion study, these investigators61 studied lower limb NCSs in healthy elderly persons. In a similarly well-designed study they found that nerve conduction velocities and distal latencies did not change with age from 60 to 89 years of age. Distal amplitudes did change significantly with a reduction in the elderly groups. What was particularly striking was that the sural sensory response was obtained in 98% of the elderly and the superficial peroneal sensory (medial dorsal cutaneous nerve) in 90%. This means that an unobtainable sural response should be considered abnormal and not simply attributed to age-related changes.61 The same reservations, however, as stated above for the upper limb studies pertain here as well.
Nerve conduction parameters are often obtained serially over time to monitor a disease process. The issue of stability and reproducibility of NCS testing is an important one. In a large multicenter trial, the reproducibility of NCSs if temperature and distances are well controlled is excellent, demonstrating excellent intraclass correlation coefficients ranging from about 0.7 to 0.9.12 The major sources of variability were the patients, with the sites of testing showing remarkable stability of NCS parameters. This means that NCSs done with meticulous technique and control for temperature can be used to judge changes in nerve function caused by disease processes. For large-scale clinical trials the authors point out the ability to reduce sample sizes because of the accuracy and stability of NCSs.12
H-Reflexes
The H-reflex is an electrophysiologically recorded Achilles muscle stretch reflex. It is typically generated by recording over the gastrocnemius and soleus muscles, and stimulating the tibial nerve in the popliteal fossa. Care must be taken to perform this test correctly. It is a submaximally elicited reflex response. The stimulus duration is 1 ms, and the stimulus should be slowly increased by 3- to 5-mA increments. The patient should be relaxed, and the stimulus frequency should be less than once per second. The H-reflex is consistent in latency and morphology, and occurs when the motor response over the gastrocsoleus is submaximal. As the stimulus current is gradually increased, the H-reflex will reaches its maximum amplitude, and then extinguish as the motor response becomes maximal. Many researchers have evaluated its sensitivity and specificity with respect to lumbosacral radiculopathies, and generally found a range of sensitivities from 32% to 88%.95,98,102,119,140 The specificity, however, has been reported at 91% for H-reflexes in lumbosacral S1 radiculopathy.102 The H-reflex can also help separate S1 radiculopathy from L5 radiculopathy, the latter being more likely to have a normal reflex.102
F-Waves
F-waves are late responses involving the motor axons and axonal pool at the spinal cord level. They can be elicited in most upper and lower limb muscles, and are typically tested by stimulating the median, ulnar, peroneal, or tibial nerves when recording from muscles they innervate. In contrast to H-reflexes, they are elicited by maximal stimulation of the nerve. They vary in morphology and latency, although when recording multiple F-waves, they should fall roughly within the same latency period. When responses that look like F-waves are seen across the screen at widely varying latencies, the examiner should turn up the machine sound and determine whether background motor unit firing resulting from poor relaxation is responsible for these responses. F-waves demonstrate low sensitivities for radiculopathy, and so have a limited role in such evaluations.95,122,129 However, they are quite useful for assessing persons for whom polyneuropathy is suspected (see below).
Needle or Pin Electromyography
One of the most important means of evaluating for neuromuscular diseases is EMG using a monopolar pin or coaxial needle (in this chapter, “needle” is used to mean either pin or needle). We will deal primarily with needle EMG and not surface EMG. Surface EMG has no clinical utility over standard needle EMG.104 EMG testing, a vital part of electrodiagnostic evaluations, provides highly useful information and, although somewhat uncomfortable, poses minimal risks to the patient. The American Association of Neuromuscular and Electrodiagnostic Medicine (AANEM) supports the Occupational Health and Safety Administration rule that mandates the use of gloves and universal precautions. Disposable needles are now readily available and are sufficiently inexpensive that they should be used. It is recommended that surface electrodes be cleaned with a 1:10 dilution of household bleach or 70% isopropyl alcohol solution between patients. Disposable surface electrodes are inexpensive, widely available, and easy to use.6 Needlestick injuries are reported by the majority of electrodiagnosticians to have occurred at least once.103 The most common preventable reason for injury was a perceived lack of time.103 Using a device to hold the plastic sleeve that the needle is packaged in, then replacing the needle using a one-handed technique, is recommended to help prevent needlesticks.
In most cases, it makes no difference whether the skin is prepared before needle insertion.6 Some examiners use alcohol as an antiseptic, and certainly if the skin is not clean then alcohol represents a useful skin preparation. There are no clear contraindications with respect to lymphedema as long as caution is used to sterilize the skin and not traverse an infected space or an area of clearly taut skin that could weep serous fluid after the insertion. Care should also be used to avoid cellulitis.8 The needle should not penetrate infected skin or open ulcerations or wounds. Needle EMG is not listed by the American Heart Association as a procedure requiring prophylactic antibiotic treatment to prevent endocarditis.4,6 Hemorrhagic complications are quite rare and are not usually symptomatic when they occur.67
In one case series, minor paraspinal muscle hematomas were noted on MRI performed shortly after needle EMG examination in 4 of 17 patients who were not receiving anticoagulants. These hematomas were small and of no clinical significance.24 In this report, the authors state that in their review of the literature, there has never been a case report of paraspinal hematoma compressing the spinal roots or the epidural space.24 Although clinicians should always weigh carefully the risk-to-benefit ratio of testing, a person with appropriate levels of anticoagulation for venous thromboembolism can be safely studied with little risk of neurologic complications. Appropriate pressure should be applied to the area after testing to prevent intramuscular hematoma. The paraspinal muscles are such an important region to study, not only for persons with suspected radiculopathy but in neuromuscular evaluations as well, that electrodiagnosticians should examine them whenever reasonably possible.
Needle EMG is a somewhat painful procedure and can be distressing to some patients. Patient education can help reduce anxiety. Recently a prospective, double-blind, randomized, placebo-controlled crossover study showed that 400 mg of ibuprofen taken 2 hours before EMG in a group of healthy volunteers reduced significantly the perceived pain immediately after the EMG. It did not, however, reduce the memory of pain a day after study.57 Another means of decreasing pain is to use a cold topical spray agent to the skin over the muscle being tested and to pinch the skin slightly before inserting the needle.
As noted previously, the electrodes for standard EMG generally fall into two categories. Monopolar needles are those in which the needle is coated with Teflon except for the tip, and the differences in potential from the tip of the electrode to a nearby surface electrode are recorded. Concentric needles are those with a fine wire running through the center of an insulated shaft that is electrically referenced to an outer metal shaft. Concentric needles are most useful for quantitative motor unit analysis, and are now commercially available in disposable form. Background interference is minimized with concentric needles, and the ground electrode can remain in one place for a given limb.
Insertional activity is examined by moving the needle through the muscle briefly and observing the amount and duration of electrical noise produced. This sound is mechanically evoked muscle depolarizations caused by the advancement of the needle. Insertional activity and spontaneous activity are usually examined using three or four insertions in each of four muscle quadrants. Smaller muscles, such as the abductor pollicis brevis, can be adequately assessed with fewer needle movements. After brief small movement of the needle, insertional activity usually persists for no more than a few hundred milliseconds.85 Insertional activity can be decreased in the case of atrophied muscle or placement of the needle into fatty tissue. Increased insertional activity is activity that lasts for more than about 300 ms after the needle stops advancing. It is sometimes difficult to sort this out from spontaneous activity, and such determination is a qualitative assessment. Diffuse abnormal insertional activity with prolonged trains of positive sharp waves (PSWs) in essentially every muscle, yet without any symptoms or disability, has been described as “EMG disease.”138 This is a rare condition that is infrequently encountered in clinical practice.
Spontaneous activity consists of electrical discharges occurring after the needle movement has stopped. Daube and Rubin36 highlight the usefulness and importance of auditory pattern recognition in the delineation of spontaneous activity of different types. A variety of potentials can be seen when the needle is not moving. If the needle is in an end-plate region, then end-plate noise can be heard. This noise is generated from miniature end-plate potentials, which sound like noise generated by a seashell held close to the ear. End-plate spikes are fully propagated action potentials from the end-plate region. These are typically biphasic potentials with an initially negative (upward) deflection. They can be mistaken for fibrillation potentials, but they fire irregularly, a distinction easily made visually on the display screen as well as by listening carefully. They tend to have a sputtering sound and are associated with excessive pain. The needle should be moved to a new location after it enters an end-plate region, to reduce pain and enter muscle more conducive to assessment for fibrillation potentials.
Fibrillation potentials represent spontaneous discharges of single muscle fibers, and are the most important EMG finding in clinical EMG. They are usually of short duration, and they can be biphasic (PSWs) or triphasic (fibrillation potentials).35,53,85 They represent individual muscle fiber depolarizations. One hypothesis is that PSWs arise from suprathreshold single muscle fiber discharges induced by and originating in close proximity to a perielectrode crushed membrane that then propagate away from the electrode.56 A smaller population of PSWs conforms to that of a blocked fibrillation potential.56 Such muscle membrane irritability can be caused by the mechanical stimulation of the needle, but persists after needle movement has stopped. Fibrillations result from motor axonal loss that is not balanced by reinnervation. They are seen in any condition causing denervation, including nerve disease, inflammatory myopathies, and direct muscle trauma.112 It is an interesting phenomenon that in persons with complete spinal cord injury, muscles innervated by roots below the level of the lesion demonstrate fibrillation potentials.86,131 Such prevalence of these findings in the legs of spinal cord–injured individuals with complete myelopathies can make electrodiagnostic testing for such patients indeterminate. Similar fibrillations and positive waves can be seen in stroke patients as well, and such findings should be interpreted with caution in the hemiplegic limb.66,77
Fibrillation potentials as well as PSWs are graded using a 0 to 4 grading scheme.68 This grading scale is described as follows:
The finding of a fibrillation or PSW in only one area of the muscle that is not easily reproducible is probably of uncertain significance and can represent an end-plate spike. The density of fibrillation potentials does not necessarily correlate with the degree of nerve damage and loss of axons. The compound muscle action potential (CMAP) gives a better estimate of the proportion of axons remaining. The innervation ratio is the average size of the motor unit expressed as a ratio between the total number of extrafusal muscle fibers and the number of innervating motor axons. For small extraocular muscles, this ratio is 1 to 3. For large muscles such as the gastrocnemius, this ratio increases to 1934 muscle fibers per motor axon. This means that the loss of relatively few motor axons results in many fibrillating muscle fibers.85
In a sample of persons with lumbosacral radiculopathy, the interrater reliability of identifying spontaneous activity on needle EMG was disquietingly low.81 For individual muscles, agreement on the presence of spontaneous activity was 64% for the paraspinal muscles, yet up to 92% in the vastus medialis (Spearman ρ = 0.655, p <0.01).81 Training level played a strong role in reliability, with residents scoring much lower on concordance than attending physicians. This study points out the need to closely supervise residents and fellows when doing needle EMG. The paraspinal muscles in particular posed identification difficulties and underscore the need to examine firing rate to ensure that discharges are firing regularly before calling them fibrillations or PSW.55
Motor unit morphology involves placing the needle near a group of muscle fibers such that the rise time of the motor unit action potential (MUAP) is sharp (less than 300 μs). At this location, a proper quantification of the MUAP can be made. Polyphasic potentials are those with greater than four phases.85 Serrated potentials have the same clinical relevance, but the turns do not cross the baseline. Polyphasic potentials are associated with reinnervation of denervated motor units when the duration of these MUAPs is increased. This is seen in chronic motor axonal loss from an entrapment, a radiculopathy, or other axonal polyneuropathy. If the polyphasic potentials are short in duration, they can be associated with myopathies or neuromuscular junction disorders. If profound axonal loss has occurred and the remaining axons are reinnervating many muscle fibers, polyphasic satellite potentials can be seen that are of short duration and appear myopathic. Large motor units, recruited with low force and greater than 6 mV in amplitude, are seen in chronic or old axonal loss conditions where there has been substantial reinnervation and reorganization of the motor unit. Remember that motor unit morphology assessment is generally a qualitative statement and less clear than the presence or absence of fibrillations, particularly for less skilled electrodiagnosticians. Such motor unit changes, however, when clear and profound can be very helpful, particularly in the proper clinical context. Quantitative EMG is necessary to precisely characterize motor unit morphology, but is rarely necessary in routine clinical EMG testing.
Recruitment is another important parameter to assess. For this evaluation, it is most important to examine low to moderate levels of muscle contractions. Full-force contractions fill the screen with overlapping motor unit potentials, rendering assessment of individual motor units impossible. At low force levels, there should be one or two firing units at about 10 Hz. As greater force is generated, more units will be recruited, and the firing rates for those already recruited increase. In normal situations, the ratio of highest firing rate to the number of units seen on a 100-ms display screen is less than five. This means that if the firing rate is 20 Hz, about four distinct motor units should be seen.85 When this firing ratio is increased to more than 10, it indicates a dropout of motor units and is termed reduced recruitment.
Other spontaneous discharges can be seen beyond fibrillations and positive waves that are more continuous and unique. One such discharge is the complex repetitive discharge (CRD). This machine-like discharge is of constant firing frequency and demonstrates consistent morphology with abrupt starts and stops. In Table 10-1, the firing characteristics are shown, along with some of the conditions in which they can be found. They are seen in conditions with chronic denervation, as well as in inflammatory myopathies. They are thought to be generated by a pacemaker muscle fiber with ephaptic conduction to other muscle fibers.
| Characteristic | Details |
|---|---|
| Appearance | May take any form, but this form is constant from one potential complex to the next |
| Rhythm | Regular |
| Frequency | 10-100 Hz |
| Amplitude | 50-1000 μV |
| Stability | Abrupt onset and cessation |
| Observed in | Myopathies: Polymyositis, limb girdle dystrophy, myxedema, Schwartz-Jampel syndrome. Neuropathies: poliomyelitis, spinal muscular atrophy, amyotrophic lateral sclerosis, hereditary neuropathies, chronic neuropathies, carpal tunnel syndrome. “Normal”: iliopsoas, biceps brachii |
Modified from Dumitru D: Needle electromyography. In Dumitru D, editor: Electrodiagnostic medicine, Philadelphia, 1995, Hanley & Belfus.
Myokymic potentials are grouped potentials that fire at regular rates. They have the characteristic sound of marching soldiers and are often seen in radiation plexopathies (Table 10-2). Myotonic discharges are fast firing with waxing and waning sounds somewhat like a dive bomber. The characteristics and conditions in which they are found are shown in Table 10-3.
Table 10-2 Characteristics of Myokymic Discharges
| Characteristic | Details |
|---|---|
| Appearance | Normal motor unit action potentials |
| Rhythm | Regular |
| Frequency | 0.1-10 Hz |
| Burst frequency | 20-250 Hz |
| Stability | Persistent firing or occasional abrupt cessation |
| Observed in | Facial: Multiple sclerosis, brainstem neoplasm, polyradiculopathy, Bell palsy, normal Extremity: radiation plexopathy, chronic nerve compression (carpal tunnel syndrome, radiculopathy), rattlesnake venom |
Modified from Dumitru D: Needle electromyography. In Dumitru D, editor: Electrodiagnostic medicine, Philadelphia, 1995, Hanley & Belfus.
Table 10-3 Characteristics of Myotonic Discharges
| Characteristic | Details |
|---|---|
| Appearance | Brief spikes, positive waveform |
| Rhythm | Wax and wane |
| Frequency | 20-100 Hz |
| Amplitude | Variable (20-300 μV) |
| Stability | Firing rate alterations |
| Observed in | Myopathies: myotonic dystrophy, myotonia congenita, paramyotonia, polymyositis, acid maltase deficiency, hyperkalemic periodic paralysis Other: chronic radiculopathy, chronic peripheral neuropathy |
Modified from Dumitru D: Needle electromyography. In Dumitru D, ed.itor: Electrodiagnostic medicine, Philadelphia, 1995, Hanley & Belfus.
Single-fiber EMG is a specialized technique with particular usefulness in the evaluation of persons with suspected neuromuscular disorders, myasthenia gravis, and Lambert-Eaton myasthenic syndrome (LEMS). A specialized electrode is used to examine two muscle fibers at a time and derive “jitter” values. It is a sensitive test for these disorders but can also be abnormal in persons with amyotrophic lateral sclerosis (ALS) and polyneuropathy.
Extent of Electrodiagnostic Testing
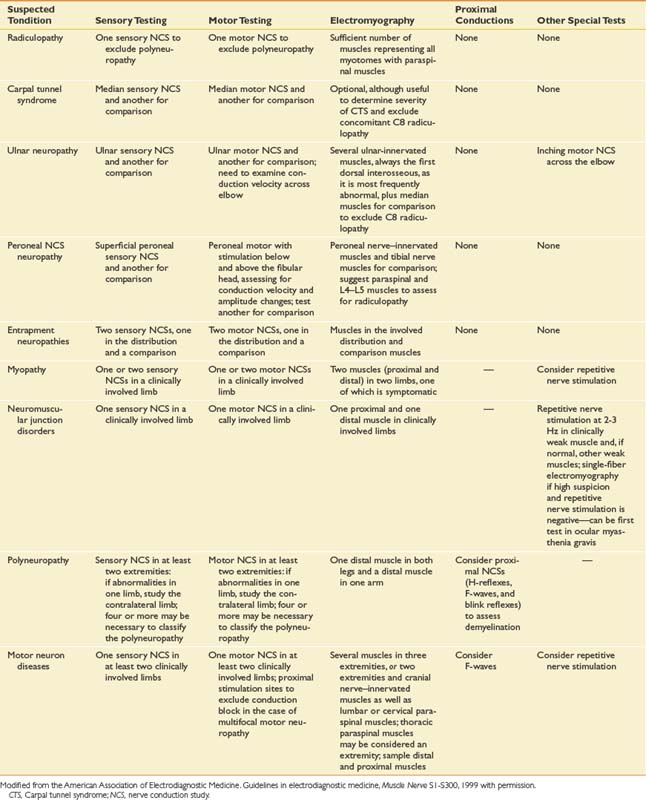
Guidelines from the AANEM, published in 1999,5 provide valuable guidance regarding the extent of appropriate electrodiagnostic testing. The guidelines and recommendations are summarized in Table 10-4.5 Electrodiagnostic testing is uncomfortable for patients, and for this reason it is important to examine only the minimum number of nerves and muscles needed to make the diagnosis. Guidelines in electrodiagnostic medicine provide parameters for testing and give clinicians and insurers critical information regarding how many tests should be conducted in most cases (e.g., what is enough and what is excessive). The AANEM guidelines, synthesized from the scientific literature as well as expert clinical opinions, provide an important framework for establishing consistency in judging what constitutes sufficient testing. From time to time, cases occur for which these guidelines are inadequate, and additional testing is needed; however, for most cases these guidelines are entirely applicable. These guidelines were developed to improve electrodiagnostic patient care, as well as to combat unscrupulous providers of electrodiagnostic services in the United States who perform excessive studies far beyond what is necessary to make a diagnosis, simply for the purpose of increasing the electrodiagnostic fee.
In a policy statement, the AANEM addressed the necessary testing for the identification of distal symmetric polyneuropathy (DSP).58 In this critical review and policy synthesis, the importance of screening for DSP was highlighted. To do this with accuracy, these authors recommended that a sural sensory and peroneal motor study be conducted in the symptomatic limb. These tests are sensitive for DSP and are easily performed. It was recommended that if these two studies are normal, there is typically no need for additional testing for DSP. It is worth noting that acute inflammatory demyelinating polyneuropathy (AIDP) and chronic inflammatory demyelinating polyneuropathy (CIDP) might only demonstrate conduction abnormalities with the proximal plexus and roots involved such that the distal conductions are normal (especially early in the course).
Limitations of Electrodiagnosis
Unfortunately, very few electrodiagnostic findings are clearly specific for any single diagnostic entity. Repetitive nerve stimulation at 2 or 3 Hz can reveal decrements in neuromuscular junction disorders, but also in motor neuron disease, myopathies, peripheral neuropathies, and myotonic disorders.80 Fibrillations and PSWs are seen in polyneuropathies, motor neuron disease, inflammatory myopathies, radiculopathies, and entrapment neuropathies. Marked facilitation of the CMAP to more than 400% of the baseline amplitude after a brief contraction in persons with LEMS is one finding that is unique to this uncommon disease.74,80,120,135
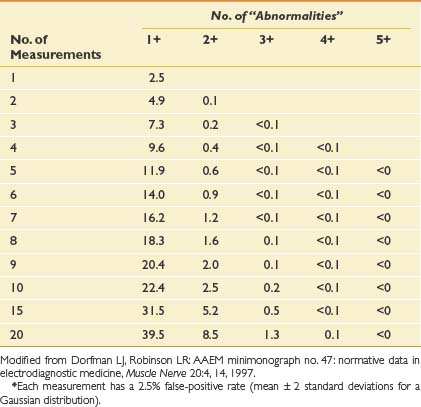
One important issue related to electrodiagnostic medicine is the possibility of false-positive results. In Table 10-5, the probabilities of finding false-positive results on the basis of chance alone are shown according to the number of independent measures.49 It is important to realize that if five measurements are performed, there is a 12% chance of having one false-positive result. If nine measurements are made, there is a 20% chance of a spuriously false-positive result. If there are two abnormal results when six or more tests are conducted, however, the likelihood that they are false-positive tests is quite low, less than about 1%. This underscores the need for electrodiagnosticians to critically examine their findings and not overdiagnose a disorder based on one subtle abnormality. If one electrodiagnostic parameter is markedly abnormal, far beyond the upper limit of normal, this can be compelling, particularly when that abnormality is consistent with the clinical impression. The study should be repeated to make certain of its validity. Two abnormalities indicating the same diagnosis, however, are far more likely to represent true findings and a clear underlying disorder.
Table 10-5 Probability (%) of Finding False-Positive “Abnormal” Results, on the Basis of Chance, According to the Number of Independent Measurements Made∗
These issues are another reason why it is critically important that the electrodiagnostician have sufficient training to be able to interpret the findings appropriately in the clinical context of the patient.
Nerve Conduction and Needle Electromyelographic Studies: Proper Technique for Temperature, Age, and Height
The well-known effects of temperature on nerve conduction velocity, distal latency, amplitude, and neuromuscular transmission should be prevented by maintaining the upper limbs at 35° C and the lower limbs at 32° C.37 Normative reference values provide the electrodiagnostician with information that allows a determination as to whether a particular nerve conduction parameter is significantly different from that in a person without symptoms or known neurologic disorders. Besides temperature, age and height have been demonstrated to influence electrodiagnostic findings, and these factors must be taken into consideration. Buschbacher16–20 has published extensive normative information derived from large samples, which account for height and age.
Standards of Practice for Electrodiagnostic Medicine
The AANEM has addressed the need to set standards for the evaluation and interpretation of studies in response to the burgeoning and disquieting practice of decoupling nerve conductions and needle electromyography (EMG). It is the organization’s contention that electrodiagnostic studies should be performed by physicians properly trained in electrodiagnostic medicine, that interpretation of NCS data alone, absent face to face patient interaction and control over the process provides substandard care, and that performance of NCS without needle EMG has the potential of compromising patient care.9 Considerable concern exists that there has been a dramatic increase in NCSs without EMG testing.9 EMG testing is critical for the assessment of radiculopathies and plexopathies as well as to complement NCSs and give additional information about entrapments and nerve injuries. Interpreting NCS findings without the benefit of a focused history and examination is inappropriate as well.9
Communication of the consultation results to the referring physician and other health care providers is essential. The AANEM recommends that the electrodiagnostic report contain (1) a description of the problem and the reason for referral; (2) a focused history and examination; (3) tabular NCS and EMG information with normative reference values; and (4) temperature and limbs studied.8 It is recommended that the study results are defined as normal or abnormal, and a probable physiologic diagnosis is presented. Any limitations to the study should be included.8 An optional section after this summary can be a synthesis of the clinical and electrophysiologic information into clinical recommendations or a stronger set of conclusions. Such a section should be separate from the electrophysiologic testing. The EMG and NCS data and the conclusions they support should stand alone.
The standards for ethical and professional conduct were summarized in an AANEM publication.99 This was the synthesis of principles derived from the American Academy of Neurology and the American Medical Association. The electrodiagnostic consultant must be competent professionally and must place the safety, privacy, and patient’s best interests first. The consultant should perform evaluations that represent the prevailing standards of electrodiagnostic practice. Practices such as receiving a fee for making a referral or giving a fee in exchange for a patient referral (e.g., fee-splitting) are not appropriate.99 Expert witnesses should not provide services under a contingency fee arrangement; rather they should receive compensation for actual services provided. Other issues that are clearly important are compliance with state and federal regulations as well as Health Insurance Portability and Accountability Act privacy laws.99
Pediatric Electrodiagnosis
In terms of equipment required for pediatric electrodiagnosis, a pediatric bipolar stimulating probe is available and is useful for small children.134 Monopolar needles are generally used because their diameter is smaller and insertional resistance is less, which is due to their Teflon coating. It is critically important to maintain a skin surface temperature of 36° to 37° C to avoid spurious results.134 There are strong advocates for and against the use of sedation and analgesia during electrodiagnostic examination.134 Performance of all conscious sedation and anesthesia should be done by specialists in pediatric anesthesia. When general anesthetic is required, children with possible neuromuscular diseases should not be given halogenated inhalation agents such as halothane because of the risks for malignant hyperthermia.128 Nerve conduction testing should include both sensory and motor studies. The needle examination, although difficult, should always be part of the diagnostic evaluation. Despite the challenging nature of the needle examination, a complete needle EMG is necessary to reach appropriate conclusions. A single muscle exploration is insufficient for diagnosis in a child.134
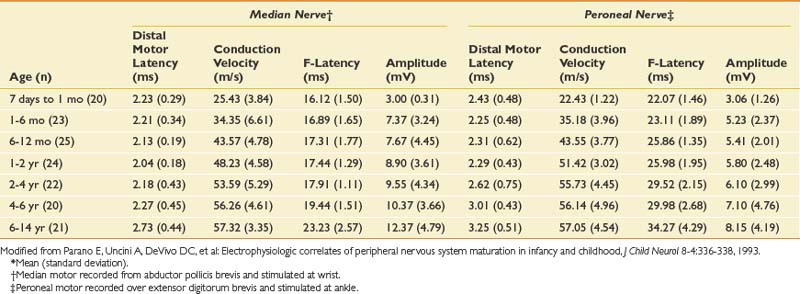
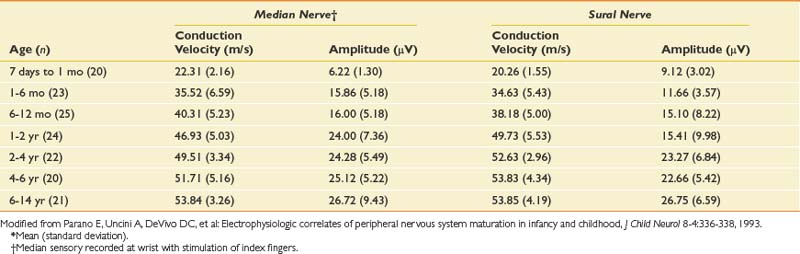
The conduction velocities in newborns are about half of those found in adults. There is a rapid increase in values during the first year of life. Median values for conduction velocities in children and adults are equalized by 5 years of age.134 Tables 10-6 and 10-7 show values for infants and newborns.109 Motor conduction velocities in full-term infants should be no less than 20 m/s.134 Clinicians who examine newborns, infants, and children should have this information readily available in their laboratories.
Late responses evaluate an infant’s proximal peripheral nervous system. They offer several advantages including reduced problems with temperature control, longer conduction distance, reduced measurement error, and a single stimulation site. In contrast to adults, the H-reflex can be elicited from any muscle during infancy, but most responses are gradually and variably suppressed by 1 year of age.82,134 The tibial nerve, however, retains its electrophysiologic H-reflex through life.
The needle EMG examination in infants is challenging and should begin with the muscles of highest diagnostic yield such as those in a weak limb. Needle manipulation and repositioning are often necessary because of the greater relative amount of adipose tissue and reduced muscle activation. Muscle relaxation to examine for spontaneous or normal insertional activities is best obtained by placing the muscle at its shortest length from origin to insertion.134 The infant’s foot and hand intrinsic muscles can best be used to study insertional and spontaneous activity because they are usually not very active.134 These distal muscles, however, exhibit high levels of end-plate noise because of the relatively larger end-plate regions, which can be confused with fibrillation potentials.134 Extensor muscles are not activated as often by the neonate and therefore are good choices as well for evaluation of spontaneous and insertional activity.134
Assessment of motor unit morphology and recruitment is difficult. Motor unit potentials in the pediatric population differ from those in the adult. Motor unit potentials in the newborn are usually biphasic or triphasic, their amplitudes range from 100 to 700 μV, and they are shorter in duration at 5 to 9 ms.82,134 Recruitment of motor units is often difficult to interpret because there is typically no voluntary control. When looking for motor unit morphology and recruitment, it is advisable to examine flexor muscles, because there is usually strong flexor tone and muscle activation. It is important to remember that this is a difficult examination, and overinterpretation is common for the inexperienced practitioner.
Repetitive nerve stimulation is used to evaluate presynaptic and postsynaptic neuromuscular disorders.26,31 Immobilization of the arm or leg and maintenance of warm body and limb temperatures are critical for optimizing sensitivity for this testing. Use of 20-Hz stimulation to assess for postactivation decrement repair or to look for postexercise facilitation is necessary. This is painful, and an anesthetized, immobile infant is optimal to derive a meaningful study. Stimulated single-fiber EMG can be performed and is an ideal, sensitive means of testing for neuromuscular junction disorders.26,31
The hypotonic infant poses diagnostic challenges, with hypotonia being the most common reason infants are referred for electrodiagnostic examination. Within this category, the most common cause for generalized hypotonia is a central nervous system disorder.134 In fact, only 10% to 20% of infants and children with hypotonia have a neuromuscular etiology.134 Perhaps the most common neuromuscular diagnosis in hypotonic infants is spinal muscular atrophy.
Several peripheral nerve entities occur in the newborn. Partial facial paralysis or asymmetric facies might occur, and motor NCSs and needle EMG of the facial nerve and its corresponding innervated muscles are useful to clarify the mononeuropathy. Side-to-side comparisons of the CMAPs can give an estimate of the degree of axonal loss and some prognostic information. Brachial plexopathies at birth are rare, occurring in 1.5 per 1000 live births.46 These brachial plexus palsies are difficult to predict and are unrelated to identifiable obstetric characteristics such as gestational age, oxytocin augmentation, delivery mode (forceps, spontaneous, breech, or cesarean), or birth weight.46 However, the duration of labor and presence of shoulder dystocia were significantly related to the occurrence of brachial plexus palsy.46 Erb palsy involves the upper plexus and C5 or C6 roots. A Klumpke or lower plexus palsy primarily demonstrates C8 or T1, and lower trunk involvement. The needle EMG can help confirm the site of pathology and location of primary involvement.134 In one series, supracostoclavicular space narrowing caused by anatomic variations such as cervical ribs or fibrous bands was identified as a predisposing factor that rendered the brachial plexus more prone to injury.10
A particularly interesting case report discussed the intrauterine onset of a peroneal neuropathy with fibrillations found within 20 hours of birth in the affected muscles, underscoring the onset before delivery and arguing against obstetric trauma as the cause.78 In this case report, the authors discuss other cases in which onset predated the delivery, as evidenced by fibrillations in clinically affected muscles shortly after birth. These findings have profound implications for medicolegal issues surrounding brachial plexus palsies and other mononeuropathies in newborns. For situations in which a neurologic deficit is found at the time of delivery, an electrodiagnostic study shortly afterward (within 24 hours) can help elucidate whether such a deficit developed intrauterine or during delivery. Intrauterine onset of a focal neuropathy or plexopathy will demonstrate fibrillation potentials in the weak muscles if tested by EMG shortly after delivery.78
Mononeuropathies and Entrapment Neuropathies
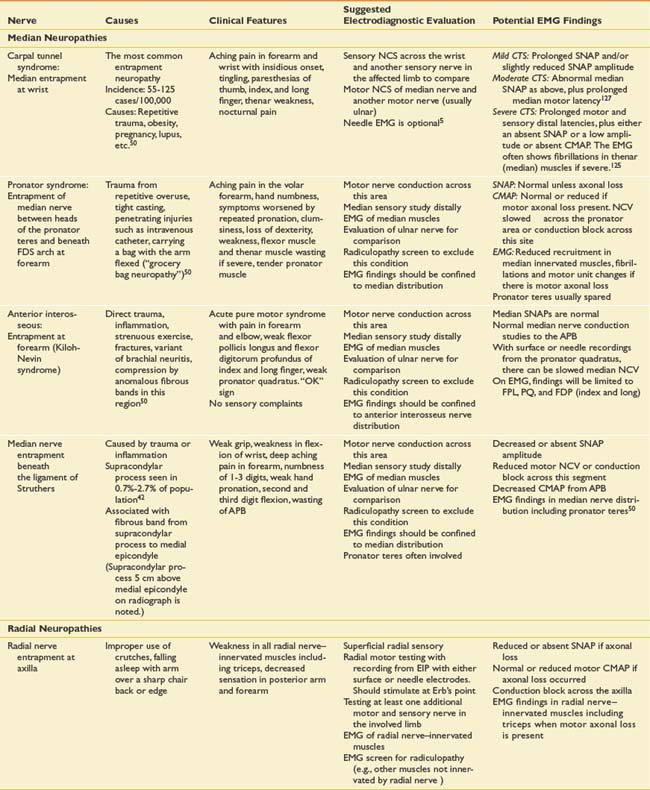
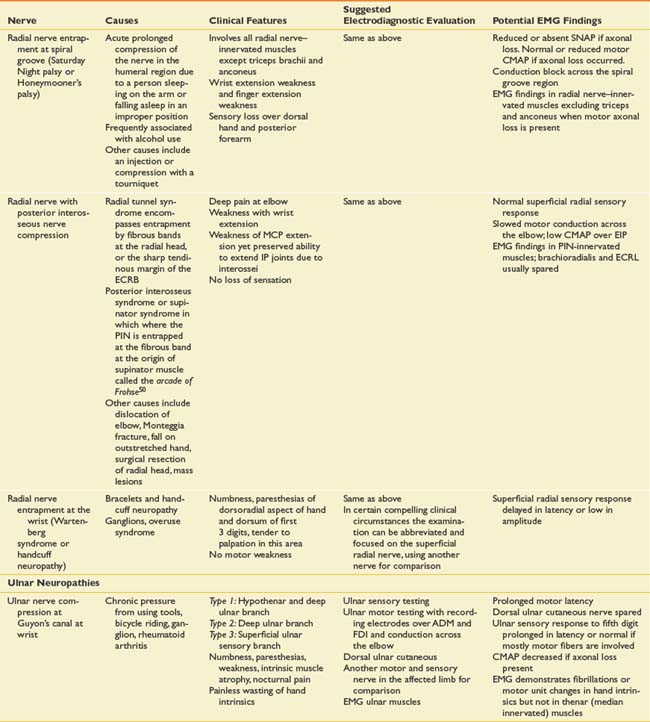
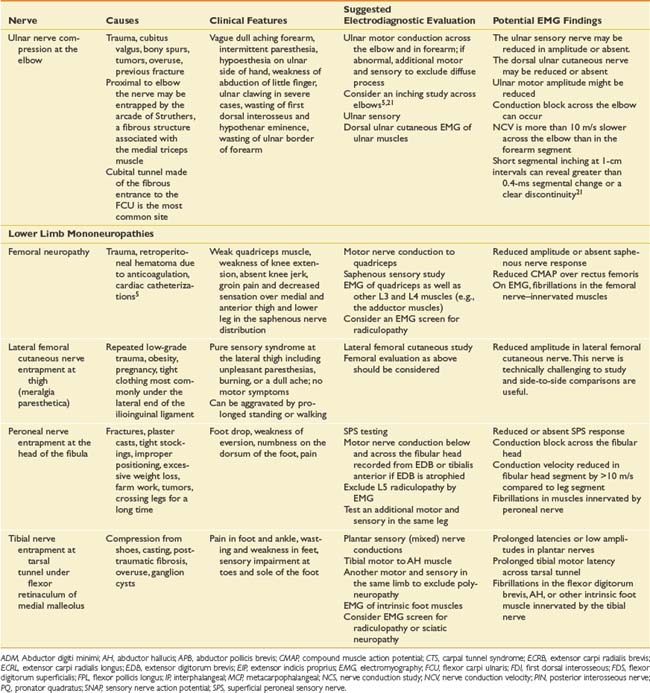
Suspected mononeuropathies are the most common reasons for electrodiagnostic laboratory referral. Top among these conditions is median neuropathy at the wrist or carpal tunnel syndrome. Other entrapments include ulnar neuropathy at the elbow, common peroneal neuropathy at the fibular head, and tibial nerve entrapment at the ankle (tarsal tunnel syndrome). In addition to these entrapments, focal nerve injuries can occur with penetrating trauma and fractures. The common entrapments and nerve injuries, their anatomic causes, and their electrodiagnostic correlates are given in Table 10-8.
The approach to assessing for mononeuropathies is to fully test the nerve in question and another nerve in the affected limb for comparison. Stimulation both below and above the suspected site of injury is imperative to assess for conduction block or conduction slowing. EMG of selected muscles innervated by the particular nerve is required. If these muscles are abnormal, the study should be expanded to ensure that these EMG findings are confined to a single peripheral nerve distribution and that they are not due to a radiculopathy, plexopathy, or polyneuropathy. The electrodiagnostician should have a low threshold for testing the same nerves in the opposite limb. Sorting out a focal process versus a generalized process such as polyneuropathy is imperative.
Brachial Plexopathies
Brachial plexopathies are important causes of shoulder and neck region pain.28,29,139 The EMG examination is critical and should be extensive and detailed, particularly for proximal muscles and muscles in which there is clinical weakness.110 Sensory nerve action potentials (SNAPs) are a key part of the evaluation. SNAPs are readily available for C6 (radial to the thumb), C7 (median to digit 2), and C8 (ulnar to digit 5) root levels in the hand, and L5 (superficial peroneal sensory) and S1 (sural sensory) in the leg. Other SNAPs, such as the lateral antebrachial cutaneous (C5, C6) or the medial antebrachial cutaneous (C8, T1), can provide additional information to the study. SNAPs are generally low in amplitude or absent with postganglionic plexopathies, and help differentiate between radiculopathies and plexopathies. The exception to this rule is in the case of traumatic plexopathy when a root is avulsed. This severe injury with little chance of recovery should be recognized. In this case, the patient’s weakness is profound and the CMAPs are low or absent, but the SNAPs are preserved. A myelogram or MRI often reveals a traumatic meningocele associated with such root avulsions.
The amplitudes of CMAPs should be elicited when possible in the weak muscles and compared with the uninvolved side. After Wallerian degeneration has occurred, this gives a rough estimate of the amount of axonal loss. It is thought that CMAPs less than 10% to 20% of the uninvolved side portend poor prognosis; however, the literature supporting this statement is weak and in large measure derived from Bell’s palsy experience.32,38
Radiculopathies
By far the most useful test for confirming the presence of a radiculopathy is needle EMG. Needle EMG testing with a sufficient number of muscles and at least one motor and one sensory NCS should be performed in the involved limb.7 The NCSs are necessary to exclude polyneuropathy. An EMG study is considered diagnostic for radiculopathy if EMG abnormalities are found in two or more muscles innervated by the same nerve root and different peripheral nerves, yet muscles innervated by adjacent nerve roots are normal.140 This assumes that other generalized conditions such as polyneuropathy are not present. The need for EMG, particularly in relationship to imaging of the spine, has been highlighted.117 Needle EMG is particularly helpful given that the false-positive rates for MRI of the lumbar spine are high, with 27% of normal subjects having a disk protrusion.76 For the cervical spine, the false-positive rate for MRI is lower, with 19% of subjects demonstrating an abnormality, but only 10% showing a herniated or bulging disk.13 Radiculopathies can occur without structural findings on MRI, and likewise without EMG findings. The EMG evaluates only motor axonal loss or motor axon conduction block (reduced recruitment is seen), and for these reasons a radiculopathy affecting the sensory root will not yield abnormalities by EMG. If the rate of denervation is balanced by reinnervation in the muscle, then spontaneous activity is less likely to be seen with EMG. The sensitivity for EMG ranges from 49% to 92%.73,83,90,133,136
The specificity of needle EMG was recently addressed in a group of asymptomatic persons in comparison with a radiculopathy group. When considering the typical distribution of findings to identify a radiculopathy (two abnormal muscles not innervated by the same peripheral nerve), and using six muscle screens and considering only spontaneous activity as abnormal, the needle EMG had 100% specificity. There were no false-positive tests. This is valuable information that supports the usefulness of needle EMG as a confirmatory test that complements spinal imaging.132
Cervical and lumbar paraspinal muscles are important to examine with EMG when assessing for radiculopathy. If positive, they localize the site of pathology to the spine or root level. When assessing paraspinal muscles, only fibrillations, PSWs, CRDs, or myotonic discharges have clinical relevance. Recruitment findings and motor unit morphology for these muscles have not been well established. There are no normative data regarding motor unit morphology in either the cervical or lumbar paraspinal muscles to which precise recruitment comparisons can be made. Likewise, it requires quantitative EMG to accurately assess motor unit size, duration, and polyphasicity—time-consuming tasks. Examiners should not overcall radiculopathies based on reduced “recruitment” or “increased polyphasicity” of the paraspinal muscles. Paraspinal muscles show either spontaneous activity or other abnormal discharges (CRDs), and therefore they localize the lesion to the root, but not to a specific level. There is considerable overlap in paraspinal muscles, with single roots innervating muscle fibers above and below their anatomic levels. For this reason, the level of radiculopathy cannot be delineated by paraspinal EMG alone, but rather is based on the root level that best explains the distribution of limb muscles with EMG abnormalities.
The lumbar paraspinal muscle examination can be easily performed and should be studied in persons with suspected radiculopathies. Haig et al.68–70 derived a means of quantifying the degree of findings in the paraspinals by means of an index derived by adding the fibrillations found in a standard set of muscle insertions. Such quantitative assessment distinguishes patients with radiculopathies and spinal stenosis with greater precision. In this mini–paraspinal mapping screen, the lumbar paraspinal muscles are identified, and four separate areas in the lumbar paraspinal region are studied.68 Fibrillations scored greater than 3 on the Mini-PM scale are abnormal. More recently this technique was validated in a group of asymptomatic older adults.27 The stricter criterion of abnormal spontaneous activity on needle examination, and paraspinal mapping scores greater than 6 being considered abnormal, serve to lower the risk of false-positive EMG testing. These findings in a well-conducted and blinded study suggest that EMG is less likely to be abnormal (false positive) in asymptomatic adults than MRI when quantified by paraspinal muscles mapping.27
Dumitru et al.55 examined the lumbosacral paraspinal muscles and intrinsic foot muscles with monopolar EMG. These investigators recorded potentials and found that there were irregularly firing potentials with similar waveform characteristics as fibrillations and PSWs. By excluding irregularly firing potentials (atypical end-plate spikes) and considering only regularly firing potentials with appropriate morphology consistent with fibrillations, they found that only 4% of these subjects had false-positive paraspinal EMG findings. This well-designed quantitative study underscores the need to assess both firing rate and rhythm, as well as discharge morphology, when evaluating for fibrillations and positive waves in the lumbar paraspinal muscles. Fibrillations fire regularly, and this should be confirmed by listening carefully and seeing consistent intervals between fibrillations on the display screen. Electrodiagnosticians should take care not to overcall paraspinal muscle EMG findings by mistaking irregularly firing end-plate spikes for regularly firing fibrillations.
How Many and Which Muscles to Study
A prospective multicenter study evaluating patients referred to participating electrodiagnostic laboratories with suspected cervical radiculopathy was conducted to address the issue regarding how many muscles are sufficient to confidently identify an electrodiagnostically confirmable cervical radiculopathy.40 There were 101 patients with electrodiagnostically confirmed cervical radiculopathies representing all cervical root levels. When paraspinal muscles were one of the screening muscles, five-muscle screens identified 90% to 98% of radiculopathies, six-muscle screens identified 94% to 99%, and seven-muscle screens identified 96% to 100% (Table 10-9). When paraspinal muscles were not part of the screen, eight distal limb muscles recognized 92% to 95% of radiculopathies. Six-muscle screens including paraspinal muscles yielded consistently high identification rates, and studying additional muscles led to marginal increases in identification. Individual screens useful to the electromyographer are listed in Table 10-9. These findings were consistent with those derived from a large retrospective study.96
Table 10-9 Six-Muscle Screen Identifications of Patients With Cervical Radiculopathies
| Muscle Screen | Neuropathic∗ (%) | Spontaneous Activity† (%) |
|---|---|---|
| Six Muscles Without Paraspinals | ||
| Deltoid, APB, FCU, triceps, PT, FCR | 93 | 66 |
| Biceps, triceps, FCU, EDC, FCR, FDI | 87 | 55 |
| Deltoid, triceps, EDC, FDI, FCR, PT | 89 | 64 |
| Biceps, triceps, EDC, PT, APB, FCU | 94 | 64 |
| Six Muscles with Paraspinals | ||
| Deltoid, triceps, PT, APB, EDC, PSM | 99 | 83 |
| Biceps, triceps, EDC, FDI, FCU, PSM | 96 | 75 |
| Deltoid, EDC, FDI, PSM, FCU, triceps | 94 | 77 |
| Biceps, FCR, APB, PT, PSM, triceps | 98 | 79 |
APB, Abductor pollicis brevis; EDC, extensor digitorum communis; FCR, flexor carpi radialis; FCU, flexor carpi ulnaris; FDI, first dorsal interosseus; PSM, cervical paraspinal muscles; PT, pronator teres.
∗ The “neuropathic” column indicates the identification rates when looking for all types of subtle neuropathic findings.
† The spontaneous activity column indicates identification rates when only fibrillations or positive sharp waves are considered.
Modified from Dillingham TR, Lauder TD, Andary M, et al: Identification of cervical radiculopathies: optimizing the electromyographic screen, Am J Phys Med Rehabil 80(2):84-91, 2001.
A similar prospective multicenter study was conducted to assess the optimal screening electrodiagnostic assessment for persons with suspected lumbosacral radiculopathy.39,44 In this study, there were 102 patients with electrodiagnostically confirmed lumbosacral radiculopathies representing all lumbosacral root levels. When paraspinal muscles were one of the screening muscles, four-muscle screens identified 88% to 97%, five-muscle screens identified 94% to 98%, and six-muscle screens identified 98% to 100% (Table 10-10). When paraspinal muscles were not part of the screen, identification rates were lower for all screens, and eight distal muscles were necessary to identify 90%. Other retrospective studies are consistent with these findings.33,96,97
Table 10-10 Six-Muscle Screen Identifications of Patients With Lumbosacral Radiculopathies
| Muscle Screen | Neuropathic∗ (%) | Spontaneous Activity† (%) |
|---|---|---|
| Six Muscles Without Paraspinals | ||
| ATIB, PTIB, MGAS, RFEM, SHBF, LGAS | 89 | 78 |
| VMED, TFL, LGAS, PTIB, ADD, MGAS | 83 | 70 |
| VLAT, SHBF, LGAS, ADD, TFL, PTIB | 79 | 62 |
| ADD, TFL, MGAS, PTIB, ATIB, LGAS | 88 | 79 |
| Six Muscles with Paraspinals | ||
| ATIB, PTIB, MGAS, PSM, VMED, TFL | 99 | 93 |
| VMED, LGAS, PTIB, PSM, SHBF, MGAS | 99 | 87 |
| VLAT, TFL, LGAS, PSM, ATIB, SHBF | 98 | 87 |
| ADD, MGAS, PTIB, PSM, VLAT, SHBF | 99 | 89 |
| VMED, ATIB, PTIB, PSM, SHBF, MGAS | 100 | 92 |
| VMED, TFL, LGAS, PSM, ATIB, PTIB | 99 | 91 |
| ADD, MGAS, PTIB, PSM, ATIB, SHBF | 100 | 93 |
ADD, Adductor longus; ATIB, tibialis anterior; LGAS, lateral gastrocnemius; MGAS, medial gastrocnemius; PSM, lumbar paraspinal muscles; PTIB, tibialis posterior; RFEM, rectus femoris; SHBF, short head of biceps femoris; TFL, tensor fascia lata; VLAT, vastus lateralis; VMED, vastus medialis.
∗ The “neuropathic” column indicates the identification rates when looking for all types of subtle neuropathic findings.
† The spontaneous activity column indicates identification rates when only fibrillations or positive sharp waves are considered.
Modified from Dillingham TR, Lauder TD, Andary M, et al: Identification of cervical radiculopathies: optimizing the electromyographic screen, Am J Phys Med Rehabil 80(2):84-91, 2001.
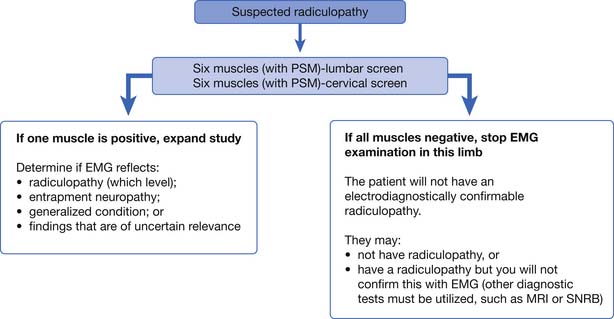
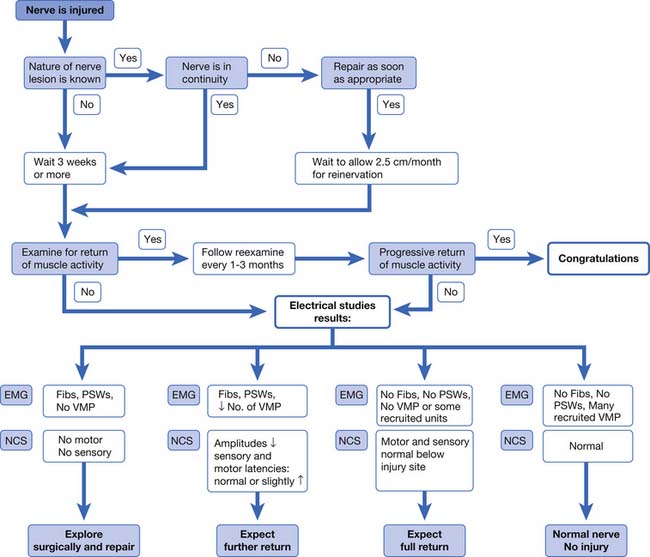
It is an important caveat that if one of the six muscles studied in the screen is positive, there is the possibility of confirming electrodiagnostically that a radiculopathy is present. In this case, the examiner must study additional muscles to determine the radiculopathy level and to exclude a mononeuropathy. If the findings are found in only a single muscle, they remain inconclusive and of uncertain clinical relevance. If none of the six muscles are abnormal, the examiner can be confident of not missing the opportunity to confirm electrodiagnostically that a radiculopathy is present. The patient might still have a radiculopathy (purely sensory or very mild case), but other tests such as MRI will be necessary to confirm this clinical suspicion. This logic is illustrated in Figure 10-2.
In the past, a well-defined temporal course of events was thought to occur with radiculopathies, despite the absence of studies supporting such a relationship. It was a commonly held notion that in acute lumbosacral radiculopathies, the paraspinal muscles denervated first, followed by distal muscles, and that reinnervation started with paraspinal muscles and then with distal muscles. This paradigm was addressed with a series of investigations.42–44115 For both lumbosacral and cervical radiculopathies, symptom duration had no significant relationship to the probability of finding spontaneous activity in paraspinal or limb muscles. This simplistic explanation, although widely quoted in the older literature, does not explain the complex pathophysiology of radiculopathies. Electrodiagnosticians should not invoke this relationship to explain the absence or presence of fibrillations in a particular muscle.
Traumatic Nerve Injuries
Trauma to the peripheral nervous system often accompanies other traumatic injuries. Electrodiagnosis is a valuable means of identifying the location of a peripheral nerve lesion and can, to some extent, assess the magnitude of the nerve injury.54 Electrodiagnostic testing has important implications but also clear limitations regarding precise nerve injury classification.15 Recovery can be expected to be days to weeks in the case of an injury resulting only in neurapraxia. Months may be required for recovery in peripheral nerve injuries showing substantial axonal loss. NCSs distal to the site of the lesion after Wallerian degeneration has taken place can help delineate the degree of axonal loss. The side-to-side amplitudes can be compared, yielding a semiquantitative means of determining the degree of axonal loss. What must be appreciated is the considerable variability in side-to-side motor and sensory amplitudes. Intraoperative nerve action potential assessment across individual fascicles can further define whether a fascicle has axons in continuity.79,87–89
A major use of electrodiagnosis is to identify electrophysiologic evidence of spontaneous regeneration that precedes clinical findings of recovery.100 In the case of a traumatically injured limb, the electrodiagnostician can usually determine the general location of the lesion and delineate whether axonal loss has occurred. If remaining motor units are firing, the electrodiagnostic examination can determine with certainty that there is not complete neurotmesis (section of the nerve).
After complete nerve section, failure at the neuromuscular junction occurs in 2 to 5 days in animals.65,105,106 In partial nerve injuries, regeneration occurs through axonal sprouting from undamaged axons in proximity to denervated muscle fibers. Axonal sprouting can reinnervate muscle fibers within days. Axonal regeneration occurring at the site of injury, on the other hand, requires months to reinnervate the denervated muscle fibers.106
Chaudhry and Cornblath25 studied wallerian degeneration in humans. They found that motor-evoked responses were absent in 9 days, sensory responses were absent by 11 days, and denervation potentials were seen 10 to 14 days after injury. These results indicate that the motor and sensory responses reliably assess the status of the nerve after 11 days. It takes 3 weeks for fibrillations to fully develop and be consistently recorded by needle EMG.
Kraft92 studied fibrillation potential amplitudes and their relationship to time after injury. The mean fibrillation amplitude in the first 2 months postinjury was 612 μV. This important study showed that 1 year after traumatic nerve injury, no population of fibrillation potentials was greater than 100 μV in amplitude. These findings can help determine whether fibrillations are the result of recent or remote denervation.
An informative study regarding fibrillation potential generation resulting from direct muscle injury was reported by Partanen and Danner.112 They studied 43 patients after muscle biopsy. At 6 to 7 days after biopsy, about half the subjects showed fibrillations on needle EMG testing. At 16 days, all subjects revealed fibrillation potentials in the biopsied muscles, and these fibrillations persisted for up to 8 months. These findings suggest that direct muscle trauma can result in denervation potentials and can occur in the absence of any nerve injury.106,112 Such information is important when evaluating traumatically injured limbs. Areas of muscle damage or surgical scars should be avoided because fibrillation potentials in these areas are uninterpretable.
Electrodiagnostic Assessment of Nerve Injuries
The pertinent issues in electrodiagnosis of peripheral nerve injuries are localization of the injury, pathophysiology of the lesion, severity of the dysfunction, and progress of reinnervation.40 Frykman et al.64 presented an algorithmic approach to the assessment and management of traumatic peripheral nerve injuries based on the clinical examination and electrodiagnostic findings (Figure 10-3). In those patients for whom little or no recovery is evident at 3 weeks by clinical examination, electrodiagnostic studies are performed. If no voluntary motor units and no motor or sensory responses are elicited, surgical exploration can be considered. If fibrillations are present, yet some voluntary units are noted along with reduced but present sensory or motor responses, then a partial nerve injury can be diagnosed, and further recovery can be reasonably expected. In a mild or neurapraxic injury with no fibrillations and normal sensory and motor amplitudes, a full recovery can be expected.64 Although somewhat simplistic, this approach provides a conceptual framework for consolidating electrodiagnostic and clinical information into a treatment plan. The algorithm by Frykman has been updated and slightly modified regarding the presence of voluntary MUAPs.64 Some newer surgical approaches to peripheral nerve injury are based on intraoperative NCSs. Information derived from peripheral nerve imaging techniques (MRI and ultrasound imaging) might well play an important role. However, the Frykman approach is a reasonable conceptual framework for managing nerve injuries.
Generalized Disorders
Electrodiagnostic testing is an extension of the physical examination, and as such should be placed within a conceptual framework of physical findings and patient symptoms. Figure 10-1 provides a conceptual framework for utilizing the clinical scenario and examination findings to address the highest probability disorders. For the purposes of this discussion, generalized disorders are those affecting more than one limb. The guidelines in Table 10-4 provide a framework for structuring the examination to evaluate for these disorders.
Polyneuropathy
Evaluation for polyneuropathy involves identifying peripheral nervous system involvement. The electrodiagnostician should also strive to categorize the type of neuropathy based on its electrodiagnostic features. Donofrio and Albers47 compiled a comprehensive list of polyneuropathies classified by their characteristic electrodiagnostic findings. Determining whether sensory, motor, or both types of nerves are involved, and whether the primary type of pathology is demyelination or axonal loss, allows the electrodiagnostician to narrow the list of potential etiologies for the polyneuropathy (Box 10-1).
Box 10-1 Classification of Polyneuropathies by Their Electrodiagnostic Characteristics
Modified from Donofrio P, Albers J. AAEM minimonograph no. 34: polyneuropathy: classification by nerve conduction studies and electromyography, Muscle Nerve; 13:889-903, 1990.
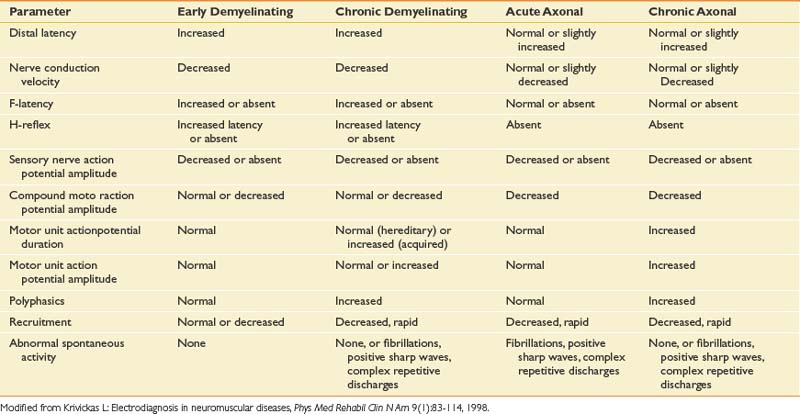
The electrodiagnostic changes seen with demyelinating and axonal neuropathies are shown in Table 10-11. Persons with polyneuropathies involving acute or recent motor axonal loss show fibrillations and PSWs in predominantly distal muscles. The distribution of EMG findings in polyneuropathies is such that distal lower limb muscles often demonstrate the greatest involvement. In persons with a more chronic process with either old axonal loss or slow axonal loss that is balanced with reinnervation and reorganization of the motor unit, large-amplitude, long-duration MUAPs can be seen.84,93
F-waves measure longer neurologic pathways and are important in diagnosing polyneuropathies. They are helpful in identifying diabetic polyneuropathy, acute inflammatory demyelinating polyneuropathy (AIDP), and chronic inflammatory demyelinating polyradiculoneuropathy (CIDP).63,108 F-waves and H-reflexes are important tests for assessing proximal segments of the peripheral nervous system and can be helpful for neuropathy evaluations.
AIDP (or Guillain-Barré syndrome) is the most common, acute, rapidly progressive polyneuropathy in clinical practice and must be recognized when present.3 Patients typically present with sensory symptoms and weakness, with disease progression over 2 to 4 weeks. Sensory nerve conduction abnormalities, reduced amplitude or prolonged distal latency, particularly in the median nerve, are observed but can take 4 to 6 weeks to peak.50,51 Motor NCSs reveal prolonged distal latencies, temporal dispersion and conduction block, or slowed velocities in 80 to 90% of these patients. Reduced CMAPs in ulnar nerve– and median nerve–innervated muscles that are 10% to 20% of normal values signify a poor prognosis and can provide early information to guide clinical management.107 In severe cases, EMG findings of fibrillations and PSWs can be seen if motor axonal loss has occurred. Facial muscle weakness and bulbar muscle involvement are commonly found in patients with AIDP.137 Nerve conduction findings of slowed conduction velocity, temporal dispersion, prolonged distal latencies, or prolonged F-waves should be found in two or more nerves to support a diagnosis of AIDP.2,137 In some cases, late responses (F-waves and H-reflexes) are the only findings noted on electrodiagnostic testing early on in persons with AIDP (see Chapter 11).
CIDP represents a demyelinating polyneuropathy of chronic nature with fluctuating weakness and stepwise progression. Weakness and sensory symptoms similar to those of AIDP are found, but the chronicity is longer. In CIDP, sensory responses are usually absent in both upper and lower limbs, and the nerve conduction velocities are reduced.107 EMG findings depend on the rate of disease progression and can reveal reduced recruitment, fibrillation potentials, polyphasic potentials, and reorganized MUAPs with large amplitudes and increased durations. Sensory nerve conduction testing was recently shown to be a highly specific, yet less sensitive marker for differentiating CIDP from axonal polyneuropathy.14
Multifocal motor neuropathy is a disorder that is sometimes confused with motor neuron disease. Patients present with asymmetric weakness in a single body region, frequently the hand, but without sensory symptoms. Progression is slow and spans many years.25,111,114,130 Sensory nerves are usually normal on electrodiagnostic testing. Motor nerve testing reveals conduction block in multiple nerves at sites not prone to focal entrapments: midforearm, midleg, arm, and brachial plexus. Distal motor latencies and amplitudes are usually normal. Proximal motor nerve stimulations at Erb’s point are important to exclude conduction block over proximal nerve segments (see Chapter 11).
Diabetes is on the rise in the United States, with an increasing incidence and prevalence.72 Diabetes often confounds the accurate diagnosis of radiculopathy and spinal stenosis.1,30 Inaccurate recognition of sensory polyneuropathy, diabetic amyotrophy, or mononeuropathy can lead to unnecessary surgical interventions. The pattern of involvement can take the form of symmetric polyneuropathies, or focal and multifocal patterns of mononeuropathies. The proximal lower limb can be involved, with pain and weakness in patients with diabetic amyotrophy. The sensory nerve involvement, particularly in the legs, often results in complaints of numbness, tingling, burning, aching, and pain. Up to 80% of patients with diabetes having clinical polyneuropathies will demonstrate an abnormality of the SNAP.113,118 Sensory findings usually precede motor findings. Motor nerve conduction is 15% to 30% below normal. EMG can show fibrillations and motor unit changes along with reduced recruitment.50,51
Accurate assessment for polyneuropathy requires heightened suspicion coupled with sufficient testing. Unfortunately, nonphysicians perform 17% of studies in the United States.45 In a sample of 6381 diabetic patients undergoing electrodiagnostic testing in 1998, identification rates were highest for physiatrists, osteopathic physicians, and neurologists (12.5%, 12.2%, and 11.9%, respectively).41 Podiatrists and physical therapists identified 2.4% and 2.1%, respectively, as having polyneuropathy—rates about one sixth that of physiatrists and neurologists, despite controlling for case mix differences. Nonphysician providers who did not recognize polyneuropathy in this group of patients with diabetes performed almost exclusively EMG testing (>90%) at the expense of NCSs. These findings underscore the need for high-quality consultations of sufficient scope by well-trained physicians to accurately diagnose patients with complex disorders.41
Alcoholic polyneuropathy is a common peripheral nerve disease accounting for almost 30% of all cases of generalized polyneuropathy.124 This clinical entity usually occurs in the setting of longstanding alcoholism and nutritional deficiency. Substantial weight loss often occurs before or concurrent with the development of polyneuropathy. Presenting symptoms typically include pain, dysesthesias, and weakness in the feet and legs. Alcoholic polyneuropathy is an axonal loss disorder affecting both sensory and motor nerves (see Box 10-1).47 Low SNAP amplitudes are seen in the legs, and EMG reveals fibrillations and positive waves in distal muscles.
Hereditary sensory and motor neuropathies (HSMNs) are a diverse group of polyneuropathies that are rapidly becoming better characterized through genetic studies, and are reviewed in Chapter 47.50,51 From an electrophysiologic standpoint, they are distinctly different from acquired polyneuropathies because of the relative lack of temporal dispersion and conduction block. HSMN type 1 is the hypertrophic variety with onion bulb formation and axonal atrophy.84 Symptoms begin in the first two decades, and markedly reduced nerve conduction velocities are seen but without substantial temporal dispersion. HSMN type 2 presents in adult life or later. Patients have severe distal muscle atrophy and weakness. NCSs show mild slowing, but EMG reveals large, reorganized MUAPs with fibrillations and PSWs. Dejerine-Sottas (HSMN type 3) is a severe type of neuropathy appearing in infancy with delayed motor development, and reveals the slowest nerve conduction velocities—less than 10 m/s and often as low as 2 to 3 m/s.89,93
The AANEM, in conjunction with the American Academy of Neurology and the American Academy of Physical Medicine and Rehabilitation, determined that a formal case definition for polyneuropathy was needed.58 The results of their recommendations were recently published. They described that no single reference standard was most appropriate for distal symmetric polyneuropathy. The most accurate diagnosis comprised a combination of clinical signs, symptoms, and electrodiagnostic findings. Electrodiagnostic findings should be included as part of the case definition because of their higher level of specificity. Electrodiagnostic studies are sensitive and specific validated measures for the presence of polyneuropathy.
This panel recommended a simplified NCS protocol for screening for the presence of distal symmetric polyneuropathy.58 Sural sensory and peroneal motor nerve conductions performed in one lower limb, taken together, were thought by this group to be the most sensitive tests for detecting a distal symmetric polyneuropathy. If both studies are normal, there is no evidence of typical distal symmetric polyneuropathy, and in such a situation no further NCSs are necessary. If one of these tests is abnormal, however, then additional NCSs are recommended. This would include at least the ulnar sensory, median sensory, and ulnar motor nerves in one upper limb. A contralateral sural sensory and one tibial motor NCS can also be performed at the discretion of the examiner. These experts recommended caution when interpreting median and ulnar studies, because of the possibility of an abnormality caused by compression at the wrist or elbow.58 They further recommended that if a response is absent for any of the nerves studied, NCSs of the contralateral nerve should be performed. If a peroneal motor response is absent, an ipsilateral tibial motor nerve conduction should also be performed. These tests (sural sensory and peroneal motor) do not exclude all acquired (e.g., AIDP) or hereditary polyneuropathies; they only exclude distal symmetric polyneuropathies. The electrodiagnostician should structure evaluation for these other suspected conditions with examination of weak muscles and involved nerve distributions.
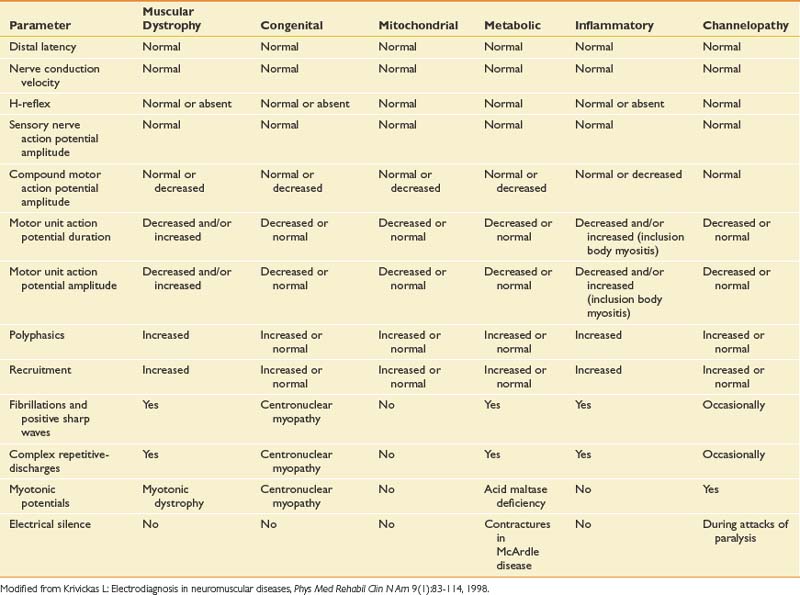
Myopathies
Myopathic disorders include acquired inflammatory types such as polymyositis and dermatomyositis, as well as congenital myopathies, metabolic myopathies, muscular dystrophies, and mitochondrial myopathies. A detailed discussion is found in Chapter 48, and the interested reader is directed to other specialized references for a complete description of the rarer forms of myopathy. Unfortunately, the electrodiagnostic examination is less sensitive or specific for detecting myopathies than for any other group of neuromuscular diseases.93 Myopathies are one of the most challenging disorders to identify and classify by electrodiagnosis because no findings are completely specific for myopathy. There is often patchy involvement of proximal muscles, and the EMG findings can vary depending on the severity and duration of the myopathy. Characteristic findings on EMG can indicate myopathy, yet the precise etiology of the myopathy requires other testing, such as muscle biopsy and genetic testing. The electromyographic findings for myopathies are shown in Table 10-12. NCSs are usually normal except when a muscle is atrophic, and then the CMAP can be reduced. Sensory NCS are normal unless a concomitant polyneuropathy exists.
Needle EMG is the most helpful part of the examination. In the acute inflammatory myopathies polymyositis and dermatomyositis, the characteristic findings are fibrillations and PSWs, CRDs, and early or increased recruitment of short-duration, polyphasic, low-amplitude MUAPs.116 Early recruitment or increased recruitment refers to the case in which more motor units are recruited than would be expected to generate a low muscular force. This is because in myopathies, more diseased muscle fibers are necessary to generate force than in the normal situation. This can also result from the motor units having fewer remaining muscle fibers. At low force levels, many units are present on the display screen. Proximal limb girdle and paraspinal muscles are frequently involved. In progressive muscular dystrophies such as Duchenne and Becker, fibrillations and PSWs are widespread, with occasional CRDs and myotonic discharges.93 Inclusion body myositis accounts for 30% of all inflammatory myopathies and requires muscle biopsy to diagnose the condition. The findings are similar to those seen in polymyositis, with fibrillations and PSWs more widespread and prominent.93
Neuromuscular Junction Disorders
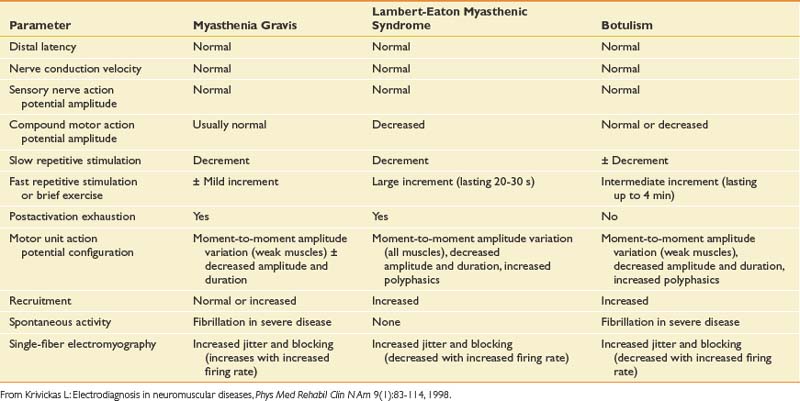
Disorders of the neuromuscular junction can be classified as either presynaptic or postsynaptic. The presynaptic disorders are LEMS and botulism. Myasthenia gravis is a postsynaptic disorder.74,80,120 These rare causes of generalized weakness demonstrate characteristic electrodiagnostic features (Table 10-13), making them readily identifiable to the electrodiagnostician with a heightened suspicion for these entities.
Myasthenia gravis is an autoimmune disorder caused by antibodies directed at the acetylcholine receptors of skeletal muscle. Electrodiagnostic techniques most useful for identifying this disorder are repetitive nerve stimulation and single-fiber EMG.74 Elevated acetylcholine receptor antibodies have been demonstrated in patients with generalized and ocular myasthenia gravis in 81% and 51% of cases, respectively. Elevated acetylcholine receptor antibody levels can be seen in those with penicillamine-induced myasthenia, in some elderly patients with autoimmune diseases, and in first-degree relatives of patients with myasthenia gravis. Decrementing responses in a distal hand muscle on repetitive nerve stimulation at 2 or 3 Hz are seen in 68% of persons with definite generalized myasthenia gravis, and in 31% with mild myasthenia gravis.80 Repetitive nerve stimulation of a proximal muscle increases the sensitivity to 89% for definite and 68% for mild myasthenia gravis. Single-fiber EMG is the most sensitive test for myasthenia gravis. In persons with generalized myasthenia gravis, the sensitivity of single-fiber EMG performed on the extensor digitorum communis muscle was 92%. In patients with ocular myasthenia gravis, 78% had abnormal jitter in the extensor digitorum communis, and 92% had increased jitter with a facial muscle study (frontalis). Single-fiber EMG is a nonspecific test unfortunately, and increased jitter can also be seen in ALS, peripheral neuropathies, and some myopathies.
LEMS is a unique condition characterized by weakness and fatigability of proximal limb muscles with sparing of ocular muscles. Dry mouth is often reported, and the patients demonstrate hyporeflexia and normal sensation.80 There is a strong association with malignancy, most commonly oat cell carcinoma of the lung. There is reduced release of acetylcholine quanta from the presynaptic nerve terminal. A unique feature of this disorder is that with rapid repetitive nerve stimulation (20 Hz) or a brief voluntary maximal contraction, a postactivation increase in CMAP of more than 200% is seen. Although such potentiation can be seen in myasthenia gravis and botulism, it is usually to a much lesser degree than that seen in LEMS. This profound potentiation is the hallmark electrodiagnostic finding in LEMS. Other features of LEMS that are similar to those found in myasthenia gravis include normal sensory conductions, decrementing response to 2-Hz stimulation, and increased jitter on single-fiber EMG (see Chapter 11).93
Botulism is a rare disorder caused by the potent toxin of the bacterium Clostridium botulinum, through both oral ingestion or wound infection.80 A rapid-onset paralysis of the eye muscles, followed by rapid spread to other parts of the body, is seen. The toxin irreversibly blocks acetylcholine release from presynaptic nerve terminals. Electrodiagnostic findings reveal low CMAPs, with decrementing responses on 2-Hz repetitive nerve stimulation. The incremental increase in CMAP amplitude with exercise is less dramatic than in LEMS but should be greater than 40%.75 With severe involvement, the neuromuscular junction is completely blocked and no facilitation with rapid stimulation is seen. In these cases, the end plates break down and fibrillation potentials are seen.
In this group of disorders the SNAPs are normal. The CMAPs are normal or of low amplitude, particularly in LEMS and botulism. Needle EMG reveals normal or polyphasic MUAPs with low amplitudes and short durations, similar to those found in myopathies. With EMG, MUAP variation in size can be seen as well. Fibrillation potentials are seen only in severe disease with complete disintegration of the neuromuscular junction (see Table 10-13). Stimulated single-fiber EMG offers advantages over conventional single-fiber EMG. Stimulated single-fiber EMG can be performed in patients who cannot maintain a voluntary contraction or in children and infants.26 Stimulated single-fiber EMG is less time-consuming and, by controlling the rate of stimulation, better quantification of the neuromuscular junction disorder can be obtained.26
Ertas and etal.59 reported the use of concentric needle electrodes for single-fiber EMG. They showed that when the low-frequency filter is set at 2 kHz, concentric needle electrodes are comparable with single-fiber EMG electrodes and yield the same jitter values for normal individuals and for persons with myasthenia gravis. Additional advantages include that they are disposable. Other investigators have confirmed the usefulness and comparability of single-fiber EMG using a concentric electrode in the evaluation of persons with suspected neuromuscular disorders, and the concentric needle may be even less painful than the conventional single-fiber electrode.62,91,121 Because of a larger recording area, somewhat more single muscle fiber discharges were identified with concentric needle electrodes than with standard single-fiber EMG electrodes.59
Motor Neuron Disease
Motor neuron disease will be discussed in the context of the most common type in adults: ALS. This is a progressive motor system disease with upper motor neuron findings from spinal white matter involvement, and lower motor neuron findings on EMG from anterior horn cell loss.52,94 There are different classifications based on the predominance of these characteristics. From an electrodiagnostic standpoint, EMG is the most helpful part of the study. To electrodiagnostically confirm ALS in the appropriate clinical circumstances, the patient must demonstrate the following findings:
Motor unit morphology depends on the rate of denervation and reinnervation. Reduced recruitment of MUAPs is the primary EMG finding in ALS (see Chapter 46).34 In a rapidly progressive ALS, there might be little motor unit remodeling.101
Makki and Benatar101 examined the accuracy of the El Escorial criteria for the diagnosis of ALS. The El Escorial criteria divide the body into four segments: cranial, cervical, thoracic, and lumbosacral. Evidence of lower motor dysfunction in the cervical and lumbosacral segments is defined by the presence of both active denervation (fibrillations and PSWs) and chronic reinnervation (large MUAPs, reduced interference pattern, or unstable motor units) in at least two muscles. Active denervation and chronic denervation in a single cranial or thoracic muscle is sufficient to declare these segments abnormal. If three of the four segments are abnormal by EMG, this is considered supportive evidence of definite ALS. If two segments are abnormal, this suggests possible ALS.101 Their study was supportive of optimizing the sensitivity and specificity by requiring EMG changes in two muscles in the arm (cervical) or leg (lumbar) segments. These two muscles should demonstrate lower motor neuron findings.101 These findings support the validity of the El Escorial EMG criteria.
In examining cranial nerves, the trapezius is a high-yield muscle125 for establishing the presence of cranial segment involvement. The thoracic segment can be examined by means of EMG of the thoracic paraspinal muscles or by testing the rectus abdominus.141
Accurate identification of persons with ALS is important not only for instituting appropriate counseling and multidisciplinary care, but in minimizing unnecessary surgical interventions. In one retrospective study, 21% of persons with ALS had surgeries within the previous 5 years of diagnosis.126 It was estimated that 61% of these surgeries were inappropriate and likely related to early manifestations of ALS.126 These were surgeries of the knee and spine despite the absence of pain or parasthesias.126
Final Electrodiagnostic Conclusions and Report
The electrodiagnostic report is a critical document that conveys the findings and conclusions derived from testing. The electrodiagnostic report should include a brief history regarding the presenting complaint, a focused physical examination, the tabulated electrodiagnostic findings, and the final assessment and conclusions. There should be internal consistency within the report, such that the conclusions are supported by the electrophysiologic data, independent of the investigator’s clinical impression. The electrodiagnostician can then comment on how these electrodiagnostic findings correlate with the clinical impression, the limitations of the test, and any other observations made. This then gives a more complete clinical picture. In electrodiagnostic reports, the referring question(s) should be addressed. Conditions that were excluded can also be listed. It is best to state that there is “no electrodiagnostic evidence of” a particular disorder if the testing for that disorder was normal. This reflects that electrodiagnostic testing can be normal in persons with the particular condition that the referring physicians suspect. Electrodiagnostic testing is not as sensitive, yet more specific, for many conditions, and for this reason quite helpful when positive.
1. Adamova B., Vohanka S., Dusek L. Differential diagnostics in patients with mild lumbar spinal stenosis: the contributions and limits of various tests. Eur Spine J. 2003;12:190-196.
2. Alan T.A., Chaudhry V., Cornblath D.R. Electrophysiological studies in the Guillain-Barré syndrome: distinguishing subtypes by published criteria. Muscle Nerve. 1998;21:1275-1279.
3. Albers J.W., Kelly J.J.Jr. Acquired inflammatory demyelinating polyneuropathies: clinical and electrodiagnostic features. Muscle Nerve. 1989;12:435-451.
4. American Association of Electrodiagnostic Medicine. Guidelines for establishing a quality assurance program in an electrodiagnostic laboratory. Muscle Nerve. 1999;Suppl 8:S33-S39.
5. American Association of Electrodiagnostic Medicine. Guidelines in electrodiagnostic medicine. Muscle Nerve Suppl 8. 1999:S1-S300.
6. American Association of Electrodiagnostic Medicine. Guidelines in electrodiagnostic medicine. Risks in electrodiagnostic medicine. Muscle Nerve. 1999;Suppl 8::S53-S58.
7. American Association of Electrodiagnostic Medicine. Guidelines in electrodiagnostic medicine: the electrodiagnostic medicine consultation. Muscle Nerve, 1999;Suppl 8:S73-S90.
8. American Association of Neuromuscular & Electrodiagnostic Medicine. Needle EMG in certain uncommon clinical contexts. Muscle Nerve. 2005;31:398-399.
9. American Association of Neuromuscular & Electrodiagnostic Medicine (AANEM). Proper performance and interpretation of electrodiagnostic studies. Muscle Nerve. 2006;33:436-439.
10. Becker M.H., Lassner F., Bahm J., et al. The cervical rib: a predisposing factor for obstetric brachial plexus lesions. J Bone Joint Surg Br. 2002;84(5):740-743.
11. Bennett R. The fibrositis-fibromyalgia syndrome. In: Schumacher R., Klippel J., Robinson L.R., editors. Primer on the rheumatic diseases. Atlanta: Arthritis Foundation, 1988.
12. Bird S.J., Brown M.J., Spino C., et al. Value of repeated measures of nerve conduction and quantitative sensory testing in a diabetic neuropathy trial. Muscle Nerve. 2006;34:214-224.
13. Boden S.D., McCowin P.R., Davis D.O., et al. Abnormal magnetic-resonance scans of the cervical spine in asymptomatic subjects: a prospective investigation. J Bone Joint Surg Am. 1990;72(8):1178-1184.
14. Bragg J.A., Benatar M.G. Sensory nerve conduction slowing is a specific marker for CIDP. Muscle Nerve. 2008;38:1599-1603.
15. Bralliar F. Electromyography: its use and misuse in peripheral nerve injuries. Orthop Clin North Am. 1981;12(2):229-238.
16. Buschbacher R.M. Median nerve F-wave latencies recorded from the abductor pollicis brevis. Am J Phys Med Rehabil. 1999;78:S32-S37.
17. Buschbacher R.M. Normal range for H-reflex recording from the calf muscles. Am J Phys Med Rehabil. 1999;78:S75-S79.
18. Buschbacher R.M. Peroneal nerve F-wave latencies recorded from the extensor digitorum brevis. Am J Phys Med Rehabil. 1999;78:S48-S52.
19. Buschbacher R.M. Tibial nerve F-waves recorded from the abductor hallucis. Am J Phys Med Rehabil. 1999;78:S43-S47.
20. Buschbacher R.M. Ulnar nerve F-wave latencies recorded from the abductor digiti minimi. Am J Phys Med Rehabil. 1999;78:S38-S42.
21. Campbell W.W., Pridgeon R.M., Singh Sahni K. Short segment incremental studies in the evaluation of the ulnar neuropathy at the elbow. Muscle Nerve. 1992;15(9):1050-1054.
22. Cannon D.E., Dillingham T.R., Miao H., et al. Musculoskeletal disorders in referrals for suspected cervical radiculopathy. Arch Phys Med Rehabil. 2007;88:1256-1259.
23. Cannon D.E., Dillingham T.R., Miao H., et al. Musculoskeletal disorders in referrals for suspected lumbosacral radiculopathy. Am J Phys Med Rehabil. 2007;86:957-961.
24. Caress J.B., Rutkove S.B., Carlin M., et al. Paraspinal muscle hematoma after electromyography. Neurology. 1996;47(1):269-272.
25. Chaudhry V., Cornblath D.R. Wallerian degeneration in human nerves: serial electrophysiological studies. Muscle Nerve. 1992;15(6):687-693.
26. Chaudhry V., Crawford T.O. Stimulation single-fiber EMG in infant botulism. Muscle Nerve. 1999;22:1698-1703.
27. Chiodo A., Haig A.J., Yamakawa K.S., et al. Needle EMG has a lower false positive rate than MRI in asymptomatic older adults being evaluated for lumbar spinal stenosis. Clin Neurophysiol. 2007;118(4):751-756.
28. Chiou-Tan F.Y., Kemp K.Jr., Elfenbaum M., et al. Lumbosacral plexopathy in gunshot wounds and motor vehicle accidents: comparison of electrophysiologic findings. Am J Phys Med Rehabil. 2001;80(4):280-285.
29. Chuang T.Y., Chiou-Tan F.Y., Vennix M.J. Brachial plexopathy in gunshot wounds and motor vehicle accidents: comparison of electrophysiologic findings. Arch Phys Med Rehabil. 1998;79(2):201-204.
30. Cinotti G., Postacchini F., Weinstein J.N. Lumbar spinal stenosis and diabetes. Outcome of surgical decompression. J Bone Joint Surg Br. 1994;76(2):215-219.
31. Cornblath D.R., Sladky J.T., Sumner A.J. Clinical electrophysiology of infantile botulism. Muscle Nerve. 1983;6:448-452.
32. Cupka B., Zaza D., Dillingham T.R. Traumatic nerve injuries: outcome prediction using clinical and diagnostic findings. Muscle Nerve. 2006. (in review)
33. Dasher K.J., Dillingham T.R. The lumbosacral electromyographic screen: revisiting a classic paper. Clin Neurophysiol. 2000;111(12):2219-2222.
34. Daube J.R. AAEE minimonograph #18: EMG in motor neuron diseases. Rochester, NY: American Association of Electrodiagnostic Medicine; 1982.
35. Daube J.R. AAEM mini-monograph #11: needle examination in clinical electromyography. Rochester, NY: American Association of Electrodiagnostic Medicine; 1991.
36. Daube J.R., Rubin D.I. Needle electromyography. Muscle Nerve. 2009;39:244-270.
37. Denys E.H. AAEM minimonograph #14: the influence of temperature in clinical neurophysiology. Rochester: American Association of Electrodiagnostic Medicine; 1991.
38. Dillingham T.R. Approach to trauma of the peripheral nerves. American Association of Electrodiagnostic Medicine Course Proceedings. Orlando, FL: AAEM Annual Scientific Meeting; 1998.
39. Dillingham T.R., Lauder T.D., Andary M., et al. Identifying lumbosacral radiculopathies: an optimal electromyographic screen. Am J Phys Med Rehabil. 2000;79(6):496-503.
40. Dillingham T.R., Lauder T.D., Andary M., et al. Identification of cervical radiculopathies: optimizing the electromyographic screen. Am J Phys Med Rehabil. 2001;80(2):84-91.
41. Dillingham T.R., Pezzin L.E. Under-recognition of polyneuropathy in persons with diabetes by non-physician electrodiagnostic services providers. Am J Phys Med Rehabil. 2005;84(6):339-406.
42. Dillingham T.R., Pezzin L.E., Lauder T.D. Cervical paraspinal muscle abnormalities and symptom duration: a multivariate analysis. Muscle Nerve. 1998;21(5):640-642.
43. Dillingham T.R., Pezzin L.E., Lauder T.D. Relationship between muscle abnormalities and symptom duration in lumbosacral radiculopathies. Am J Phys Med Rehabil. 1998;77(2):103-107.
44. Dillingham T.R., Pezzin L.E., Lauder T.D., et al. Symptom duration and spontaneous activity in lumbosacral radiculopathy. Am J Phys Med Rehabil. 2000;79(2):124-132.
45. Dillingham T.R., Pezzin L.E., Rice B. Electrodiagnostic services in the United States. Muscle Nerve. 2004;29(2):198-204.
46. Donnelly V., Foran A., Murphy J., et al. Neonatal brachial plexus palsy: an unpredictable injury. Am J Obstet Gynecol. 2002;187(5):1209-1212.
47. Donofrio P., Albers J. AAEM minimonograph #34: polyneuropathy: classification by nerve conduction studies and electromyography. Muscle Nerve. 1990;13:889-903.
48. Dorfman L.J. Quantitative clinical electrophysiology in the evaluation of nerve injury and regeneration. Muscle Nerve. 1990;13(9):822-828.
49. Dorfman L.J., Robinson L.R. AAEM minimonograph #47: normative data in electrodiagnostic medicine. Muscle Nerve. 1997;20:4-14.
50. Dumitru D. Focal peripheral neuropathies. In Dumitru D., editor: Electrodiagnostic medicine, ed 2, Philadelphia: Hanley & Belfus, 2002.
51. Dumitru D. Generalized peripheral neuropathies. In: Dumitru D., editor. Electrodiagnostic medicine. Philadelphia: Hanley & Belfus, 1995.
52. Myopathies Dumitru D. In: Dumitru D., editor. Electrodiagnostic medicine. Philadelphia: Hanley & Belfus, 1995.
53. Dumitru D. Needle electromyography. In: Dumitru D., editor. Electrodiagnostic medicine. Philadelphia: Hanley & Belfus, 1995.
54 Dumitru D. Reaction of the peripheral nervous system to injury. In: Dumitru D., editor. Electrodiagnostic medicine. Philadelphia: Hanley & Belfus, 1995.
55. Dumitru D., Diaz C.A.J., King J.C. Prevalence of denervation in paraspinal and foot intrinsic musculature. Am J Phys Med Rehabil. 2001;80(7):482-490.
56. Dumitru D., Santa Maria D.L. Positive sharp wave origin: evidence supporting the electrode initiation hypothesis. Muscle Nerve. 2007;36:349-356.
57. El-Salem K., Shakhatreh M. Prospective double-blind crossover trial of ibuprofen in reducing EMG pain. Muscle Nerve. 2008;38:1016-1020.
58. England J.D., Gronseth G.S., Franklin G., et al. Distal symmetrical polyneuropathy: definition for clinical research. Muscle Nerve. 2005;31:113-123.
59. Ertas M., Baslo M.B., Yildiz N., et al. Concentric needle electrode for neuromuscular jitter analysis. Muscle Nerve. 2000;23:715-719.
60. Falco F.J., Hennessey W.J., Braddom R.L., et al. Standardized nerve conduction studies in the upper limb of the healthy elderly. Am J Phys Med Rehabil. 1992;71:263-271.
61. Falco F.J., Hennessey W.J., Goldberg G., et al. Standardized nerve conduction studies in the lower limb of the healthy elderly. Am J Phys Med Rehabil. 1994;73:168-174.
62. Farrugia M.E., Weir A.I., Cleary M., et al. Concentric and single fiber needle electrodes yield comparable jitter results in myasthenia gravis. Muscle Nerve. 2009;39:579-585.
63. Fraser J.L., Olney R.K. The relative diagnostic sensitivity of different F-wave parameters in various polyneuropathies. Muscle Nerve. 1992;15:912-918. (see comments)
64. Frykman G.K., Wolf A., Coyle T. An algorithm for management of peripheral nerve injuries. Orthop Clin North Am. 1981;12(2):239-244.
65. Gilliatt R.W., Hjorth R.J. Nerve conduction during Wallerian degeneration in the baboon. J Neurol Neurosurg Psychiatry. 1972;35:335-341.
66. Goldkamp O. Electromyography and nerve conduction studies in 116 patients with hemiplegia. Arch Phys Med Rehabil. 1967;48:59-63.
67. Gruis K.L., Little A.A., Zebarah V.A., et al. Survey of electrodiagnostic laboratories regarding hemorrhagic complications from needle electromyography. Muscle Nerve. 2006;34:356-358.
68. Haig A.J. Clinical experience with paraspinal mapping. II. A simplified technique that eliminates three-fourths of needle insertions. Arch Phys Med Rehabil. 1997;78(11):1185-1190.
69. Haig A.J., LeBreck D.B., Powley S.G. Paraspinal mapping. Quantified needle electromyography of the paraspinal muscles in persons without low back pain. Spine. 1995;20(6):715-721.
70. Haig A.J., Talley C., Grobler L.J., et al. Paraspinal mapping: quantified needle electromyography in lumbar radiculopathy. Muscle Nerve. 1993;16(5):477-484.
71. Haig A.J., Tzeng H.M., LeBreck D.B. The value of electrodiagnostic consultation for patients with upper extremity nerve complaints: a prospective comparison with the history and physical examination. Arch Phys Med Rehabil. 1999;80(10):1273-1281.
72. Harris M.I. Diabetes in America: epidemiology and scope of the problem. Diabetes Care. 1998;21(suppl 3):C11-C14. C11-14
73. Hong C.Z., Lee S., Lum P. Cervical radiculopathy: clinical, radiographic and EMG findings. Orthop Rev. 1986;15(7):433-439.
74. Jablecki C.K. AAEM case report #3: myasthenia gravis. Rochester, NY: American Association of Electrodiagnostic Medicine; 1981.
75. Jablecki C.K., Busis N.A., Brandstater M.A., et al. Reporting the results of needle EMG and nerve conduction studies: an educational report. Muscle Nerve. 2005;32:682-685.
76. Jensen M.C., Brant-Zawadzki M.N., Obuchowski N., et al. Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med. 1994;331(2):69-73.
77. Johnson E.W., Denny S.T., Kelley J.P. Sequence of electromyographic abnormalities in stroke syndrome. Arch Phys Med Rehabil. 1975;56:468-473.
78. Jones H.R.Jr., Herbison G.J., Jacobs S.R., et al. Intrauterine onset of a mononeuropathy: peroneal neuropathy in a newborn with electromyographic findings at age one day compatible with prenatal onset. Muscle Nerve. 1996;19(1):88-91.
79. Kaplan B.J., Gravenstein D., Friedman W.A. Intraoperative electrophysiology in treatment of peripheral nerve injuries. J Fla Med Assoc. 1984;71(6):400-403.
80. Keesey J.A.A.E.M. minimonograph #33: electrodiagnostic approach to defects of neuromuscular transmission. Muscle Nerve. 1989;12:613-626.
81. Kendall R., Werner R.A. Interrater reliability of the needle examination in lumbosacral radiculopathy. Muscle Nerve. 2006;34:238-241.
82. Kerman K., Shahani B. Pediatric electromyography. Indian J Pediatr. 1990;57:469-479.
83. Khatri B.O., Baruah J., McQuillen M.P. Correlation of electromyography with computed tomography in evaluation of lower back pain. Arch Neurol. 1984;41(6):594-597.
84. Polyneuropathies Kimura J. In: Kimura J., editor. Electrodiagnosis in diseases of nerve and muscle: principles and practice, ed 2, Philadelphia: FA Davis, 1989.
85. Kimura J. Techniques in normal findings. In Kimura J., editor: Electrodiagnosis in diseases of nerve and muscle: principles and practice, ed 2, Philadelphia: FA Davis, 1989.
86. Kirshblum S., Lim S., Garstang S., et al. Electrodiagnostic changes of the lower limbs in subjects with chronic complete cervical spinal cord injury. Arch Phys Med Rehabil. 2001;82:604-607.
87. Kline D.G. Physiological and clinical factors contributing to the timing of nerve repair. Clin Neurosurg. 1977;24:425-455.
88. Kline D.G. Surgical repair of peripheral nerve injury. Muscle Nerve. 1990;13(9):843-852.
89. Kline D.G., Hackett E.R., Happel L.H. Surgery for lesions of the brachial plexus. Arch Neurol. 1986;43(2):170-181.
90. Knutsson B. Comparative value of electromyographic, myelographic, and clinical-neurological examinations in diagnosis of lumbar root compression syndrome. Acta Orthop Scand. 1961;(Suppl 49):1-23.
91. Kouyoumdjian J.A., Stalberg E.V. Reference jitter values for concentric needle electrodes in voluntarily activated extensor digitorum communis and orbicularis oculi muscles. Muscle Nerve. 2008;37:694-699.
92. Kraft G.H. Fibrillation potential amplitude and muscle atrophy following peripheral nerve injury. Muscle Nerve. 1990;13:814-821.
93. Krivickas L. Electrodiagnosis in neuromuscular diseases. Phys Med Rehabil Clin N Am. 1998;9(1):83-114.
94. Kuncl R.W., Cornblath D.R., Griffin J.W. Assessment of thoracic paraspinal muscles in the diagnosis of ALS. Muscle Nerve. 1988;11:484-492.
95. Kuruoglu R., Oh S.J., Thompson B. Clinical and electromyographic correlations of lumbosacral radiculopathy. Muscle Nerve. 1994;17(2):250-251.
96. Lauder T.D., Dillingham T.R. The cervical radiculopathy screen: optimizing the number of muscles studied. Muscle Nerve. 1996;19(5):662-665.
97. Lauder T.D., Dillingham T.R., Huston C.W., et al. Lumbosacral radiculopathy screen. Optimizing the number of muscles studied. Am J Phys Med Rehabil. 1994;73(6):394-402.
98. Linden D., Berlit P. Comparison of late responses, EMG studies, and motor evoked potentials (MEPs) in acute lumbosacral radiculopathies. Muscle Nerve. 1995;18:1205-1207.
99. Mackin G.A., Horowitz S.H., Leonard J.A.Jr., et al. American Association of Neuromuscular & Electrodiagnostic Medicine: guidelines for ethical behavior relating to clinical practice issues in electrodiagnostic medicine. Muscle Nerve. 2005;31:400-405.
100. MacKinnon S.E., Dellon A.L. Surgery of the peripheral nerve. New York: Thieme Medical Publishers; 1988.
101. Makki A.A., Benatar M. The electromyographic diagnosis of amyotrophic lateral sclerosis: does the evidence support the El Escorial criteria? Muscle Nerve. 2007;35:614-619.
102. Marin R., Dillingham T.R., Chang A., et al. Extensor digitorum brevis reflex in normals and patients with L5 and S1 radiculopathies. Muscle Nerve. 1995;18(1):52-59.
103. Mateen F.J., Grant I.A., Sorenson E.J. Needlestick injuries among electromyographers. Muscle Nerve. 2008;38:1541-1545.
104. Meekins G.D., So Y., Quan D. American Association of Neuromuscular & Electrodiagnostic Medicine evidenced-based review: use of surface electromyography in the diagnosis and study of neuromuscular disorders. Muscle Nerve. 2008;38:1219-1224.
105. Miledi R., Slater C.R. On the degeneration of rat neuromuscular junctions after nerve section. J Physiol. 1970;207:507-528.
106. Miller R.G. AAEE minimonograph #28: injury to peripheral motor nerves. Muscle Nerve. 1987;10:698-710.
107. Miller R.G., Peterson G.W., Daube J.R., et al. Prognostic value of electrodiagnosis in Guillain-Barré syndrome. Muscle Nerve. 1988;11:769-774.
108. Olney R.K., Aminoff M.J. Electrodiagnostic features of the Guillain-Barré syndrome: the relative sensitivity of different techniques. Neurology. 1990;40:471-475.
109. Parano E., Uncini A., DeVivo D.C., et al. Electrophysiologic correlates of peripheral nervous system maturation in infancy and childhood. J Child Neurol. 1993;8(4):336-338.
110. Parry G.J. Electrodiagnostic studies in the evaluation of peripheral nerve and brachial plexus injuries. Neurol Clin. 1992;10(4):921-934.
111. Parry G.J. AAEM case report #30: multifocal motor neuropathy. Muscle Nerve. 1996;19:269-276.
112. Partanen J.V., Danner R. Fibrillation potentials after muscle injury in humans. Muscle Nerve. 1982;5(suppl 9):S70-S73.
113. Pastore C., Izura V., Geijo-Barrientos E., et al. A comparison of electrophysiological tests for the early diagnosis of diabetic neuropathy. Muscle Nerve. 1999;22:1667-1673.
114. Pestronk A. Multifocal motor neuropathy: diagnosis and treatment. Neurology. 1998;51:S22-S24.
115. Pezzin L.E., Dillingham T.R., Lauder T.D., et al. Cervical radiculopathies: relationship between symptom duration and spontments EMG activity. Muscle Nerve. 1999;22(10):1412-1418.
116. Robinson L. AAEM case report #22: polymyositis. Muscle Nerve. 1991;14:310-315.
117. Robinson L.R. Electromyography, magnetic resonance imaging, and radiculopathy: it’s time to focus on specificity. Muscle Nerve. 1999;22(2):149-150.
118. Rondinelli R.D., Robinson L.R., Hassanein K.M., et al. Further studies on the electrodiagnosis of diabetic peripheral polyneuropathy using discriminant function analysis. Am J Phys Med Rehabil. 1994;73:116-123.
119. Sabbahi M.A., Khalil M. Segmental H-reflex studies in upper and lower limbs of patients with radiculopathy. Arch Phys Med Rehabil. 1990;71(3):223-227.
120. Sanders D., Stalberg E. AAEM minimonograph #25: single fiber electromyography. Muscle Nerve. 1996;19:1069-1083.
121. Sarrigiannis P.G., Kennett R.P., Read S., et al. Single-fiber EMG with a concentric needle electrode: validation in myasthenia gravis. Muscle Nerve. 2006;33:61-65.
122. Scelsa S.N., Herskovitz S., Berger A.R. The diagnostic utility of F waves in L5/S1 radiculopathy. Muscle Nerve. 1995;18(12):1496-1497.
123. Schoeck A.P., Mellion M.L., Gilchrist J.M., et al. Safety of nerve conduction studies in patients with implanted cardiac devices. Muscle Nerve. 2007;35:521-524.
124. Shields R. AAEE case report #10: alcoholic polyneuropathy. Muscle Nerve. 1985;8:183-187.
125. Sonoo M., Kuwabara S., Shimizu T., et al. Utility of trapezius EMG for diagnosis of amyotrophic lateral sclerosis. Muscle Nerve. 2009;39:63-70.
126. Srinivasan J., Scala S., Jones H.R., et al. Inappropriate surgeries resulting from misdiagnosis of early amyotrophic lateral sclerosis. Muscle Nerve. 2006;34:359-360.
127. Stevens J.C. AAEM minimonograph 26: The electrodiagnosis of carpal tunnel syndrome. Muscle Nerve. 1997;20:1977-1986.
128. Swoboda K.J., Edelbol-eeg-olofsson K., Harmon R.L., et al. Pediatric electromyography. In: Jones H., et al, editors. Neuromuscular disorders of infancy, childhood and adolescence. Oxford: Butterworth-Heinemann, 2002.
129. Tackmann W., Radu E.W. Observations of the application of electrophysiological methods in the diagnosis of cervical root compressions. Eur Neurol. 1983;22(6):397-404.
130. Taylor B.V., Wright R.A., Harper C.M., et al. Natural history of 46 patients with multifocal motor neuropathy with conduction block. Muscle Nerve. 2000;23:900-908.
131. Taylor R.G., Kewalramani L.S., Fowler W.M.Jr. Electromyographic findings in lower extremities of patients with high spinal cord injury. Arch Phys Med Rehabil. 1974;55:16-23.
132. Tong H.C., Haig A.J., Yamakawa K.S., et al. Specificity of needle electromyography for lumbar radiculopathy and plexopathy in 55- to 79-year-old asymptomatic subjects. Am J Phys Med Rehabil. 2006;85:908-912. quiz 913-915, 934
133. Tullberg T., Svanborg E., Isacsson J., et al. A preoperative and postoperative study of the accuracy and value of electrodiagnosis in patients with lumbosacral disc herniation. Spine. 1993;18(7):837-842.
134. Turk M.A. Pediatric electrodiagnostic medicine. In: Dumitru D., editor. Electrodiagnostic medicine. Philadelphia: Hanley & Belfus, 1995.
135. Valls-Canals J., Montero J., Pradas J. Stimulated single fiber EMG of the frontalis muscle in the diagnosis of ocular myasthenia. Muscle Nerve. 2000;23:779-783.
136. Weber F., Albert U. Electrodiagnostic examination of lumbosacral radiculopathies. Electromyogr Clin Neurophysiol. 2000;40(4):231-236.
137. Weinberg D.H. AAEM case report 4: Guillain-Barré syndrome. American Association of Electrodiagnostic Medicine. Muscle Nerve. 1999;22:271-281.
138. Wiechers D.O., Johnson E.W. Diffuse abnormal electromyographic insertional activity: a preliminary report. Arch Phys Med Rehabil. 1979;60:419-422.
139. Wilbourn A.J. Electrodiagnostic testing of neurologic injuries in athletes. Clin Sports Med. 1990;9(2):229-245.
140. Wilbourn A.J., Aminoff M.J. AAEM minimonograph 32: the electrodiagnostic examination in patients with radiculopathies. Muscle Nerve. 1998;21:1612-1631.
141. Xu Y., Zheng J., Zhang S., et al. Needle electromyography of the rectus abdominis in patients with amyotrophic lateral sclerosis. Muscle Nerve. 2007;35:383-385.