CHAPTER 69 Electrical Stimulation for Spinal Fusion
Electrical stimulation therapies have been used for the past 35 years to enhance spinal fusions. Three types of electrical stimulation are currently employed. These three types are direct current (DC), capacitive coupling (CC), and inductive coupling. Inductive coupling includes the mechanisms of pulsed electromagnetic fields (PEMFs) and combined magnetic fields (CMFs).1–3 Clinical data demonstrated an enhancement in fusion rates with electrical stimulation even before the true mechanisms of action of these technologies were well understood. Recent studies have provided insight into the distinct mechanisms of action of these different electrical therapies and support the validity of the clinical data previously published. This chapter reviews the history of electrical stimulation and provides a review of the current concepts of mechanism of action including the upregulation of several growth factors.
History of Electrical Stimulation
The earliest use of electricity was in 1841 for the purpose of healing long bone fractures. Hartshorne reported on a patient with a tibial nonunion.4 In 1850 Lente reported successful use of galvanic current in the treatment of patients with delayed union or nonunions.5 The observations of Wolff in 1892 are the basis for the modern theories of electrical stimulation. He described the phenomenon that bone is formed in response to stress.6
Yasuda, Bassett, and Becker in the 1950s further characterized the electrical events occurring in healing bone callus and described the electrical potentials arising in long bones from mechanical stress.7–9 Those authors reported that the areas of bone under compression were electronegative and those under tension were electropositive. These electrical potentials were subsequently found to be related to stress and strain rates. The authors proposed and demonstrated that electricity applied to a fractured bone could impart healing.7–9 These observations were further confirmed by the work of Shamos, Friedenburg, and Brighton, who characterized the bioelectric or steady-state potential of living bone.10–12
Methods of Electrical Stimulation
Direct Current Electrical Stimulation
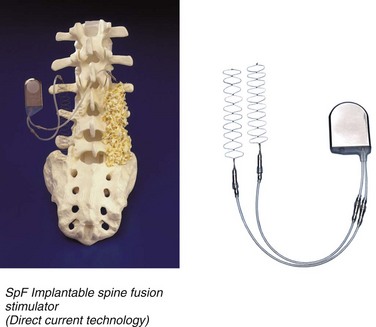
DC stimulation involves the surgical implantation of electrodes connected to a battery (Fig. 69–1). The cathodes are in direct contact with the exposed fusion bed. The cathode’s effective stimulation distance is 5 to 8 mm. The batteries deliver a constant DC for 6 to 9 months. The surgical implantation of the device obviates the need for patient compliance. However, there are disadvantages to the placement of the device because the manufacturer recommends removing the battery in 6 to 9 months, which entails a second surgical procedure. Also, there is a rare but reported risk of seeding the battery from systemic infection.
Basic Science Research Using Direct Current Stimulation
Recent studies have demonstrated that DC application enhances the production of a number of osteoinductive factors that are normal regulators of bone matrix formation. This mechanism of action was discovered using Boden’s animal model of spinal fusion.13 This model uses a New Zealand White rabbit intertransverse process fusion model for spinal fusion using autogenous bone graft. The model involves a surgical procedure similar to that performed in humans and has a similar nonunion rate to that of autograft.13 Morone and colleagues14 studied this model and showed that within the developing fusion mass, there is a distinct temporal and anatomic location for the production of bone morphogenic proteins (BMPs) and other factors that are necessary to achieve a solid fusion.
Fredericks and colleagues15 used this animal model to study the effects of DC on the temporal expression of growth factors in the developing fusion mass. They demonstrated that there was an upregulation of the production of BMP 2, 6, and 7 relative to controls. In addition, the use of DC stimulation avoided the potential complications seen with the application of a single high dose of growth factor to achieve fusion. Complications of ectopic bone formation, bone resorption, or antibody formation against the single growth factor have been reported with the application of a single growth factor.15
Clinical Studies of Direct Current Electrical Stimulation
The clinical use of DC current stimulation began as early as 1974 by Dwyer, who demonstrated clinical success in 11 out of 12 patients who received an implanted bone stimulator.16–17 Kane reported the results of a multicenter trial involving 84 patients who had an implantable DC current stimulator. This group was compared with a historical control group of 159 patients.18 The experimental group using the DC current stimulator had a higher percentage of patients who had previous surgery and nonunion. Despite this bias favoring the control group, there was a significant increase in successful fusion in the DC stimulated group: 91% versus 83% in controls. In 1988 Kane published an additional study of “difficult” patients undergoing posterior spinal fusion.18 These patients were deemed difficult because they (1) had one or more previous failed spinal fusions, (2) had grade II or worse spondylolisthesis, (3) required extensive bone grafting necessary for a multilevel fusion, or (4) had other risk factors such as obesity. He found that there was a statistically significant improvement in fusion rate of 81% in the electrically stimulated group versus 54% of controls.
In 1994 Meril reported a 93% fusion rate in patients who had undergone anterior lumbar or posterior lumbar interbody fusion with DC current stimulation compared with a 75% fusion rate in the control group.19 In 1996 Rogozinski published a study that analyzed the use of DC stimulation in patients undergoing posterior lumbar spinal fusion with pedicle screw instrumentation and autograft.20 The electrical stimulation group had a 96% successful fusion rate as compared with 85% in the control group.20 The higher rate of fusion in the Rogozinski study as compared with that in the Kane study may be related to the use of spinal instrumentation.
Kucharzyk21 reported the outcome of a controlled prospective study in a high-risk fusion population. The study involved two groups of 65 patients with similar diagnoses. All patients underwent posterolateral fusions with pedicle screw instrumentation and the use of autologous bone graft. They were divided into two groups: those with or without the use of DC electrical stimulation. The average follow-up was 3.8 years. That study reported a 95% successful fusion in the stimulated group versus 79% in the control group.21
In 1996 Tejano and colleagues22 evaluated a series of patients undergoing posterolateral intertransverse process fusion and facet fusions, either as a primary procedure or as a pseudarthrosis repair. He reported a 91.5% fusion rate in the primary surgery group and an 80% fusion in the pseudarthrosis group.
Capacitive Coupling Electrical Stimulation
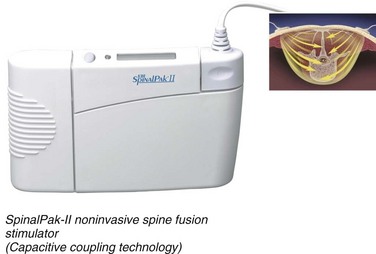
CC is a noninvasive electrical stimulation technology used to enhance spinal fusions (Fig. 69–2). This device consists of electrodes placed approximately 10 cm apart on the skin, over the area of spine undergoing fusion. The pair of external plates produce electric fields when an electric current is applied by the alternating current signal generator. The device is worn continuously for 6 to 9 months. The success of this technology, however, depends on the compliance of the patient. Brighton first used CC electrical stimulation to treat recalcitrant nonunions in 1986.23
Basic Science Research Using Capacitive Coupling Electrical Stimulation
CC perturbs bone cell membrane potentials, which then activate membrane proteins and affect healing. Osteopromotive factors are upregulated by CC electrical stimulation. Using Boden’s rabbit model for posterolateral spinal fusions,13 Fredericks and colleagues24 found that the normal physiologic expression of the following growth factors is upregulated by CC stimulation: BMP-2, BMP-4, BMP-6, BMP-7, transforming growth factor TGF-β, fibroblast growth factor (FGF)-2, and VEGF.
Lorich and colleagues25 studied the response of bone cells to capacitive coupling. They found that CC stimulation involves transmembrane calcium translocation using voltage-gated calcium channels. This mechanism increased intracellular calcium concentration and calmodulin activation. Calmodulin has been shown to enhance bone cell proliferation.
Zhuang and colleagues26 further documented that this enhancement of calcium and calmodulin also promotes the synthesis of TGF-β. Brighton and colleagues27 studied the signal transduction in electrically stimulated bone cells using capacitive coupling. These studies demonstrated a dose-response effect, showing increased cell proliferation with longer treatment times.27
Clinical Studies of Capacitive Coupling Electrical Stimulation
Goodwin and colleagues28 performed a multicenter, randomized, double-blind study of CC stimulation. Patients underwent either anterior interbody or posterolateral lumbar fusions. Clinical and radiographic results were assessed, and the capacitive coupling stimulation had a higher success rate of 84.7% versus 64.9% for the control group, although the surgeries varied in terms of use of instrumentation and type of bone graft material.
Pulsed Electromagnetic Fields
The PEMF coils must be worn across the area of the spinal fusion for approximately 6 to 8 hours daily for 3 to 6 months. Many clinical trials have shown that PEMF devices help heal nonunions.29 As with CC stimulation, the efficacy of the PEMF device depends on patient compliance.
Clinical Studies of Pulsed Electromagnetic Fields
In one study, 13 patients with documented pseudarthrosis following posterior lumbar interbody fusion (PLIF) procedures achieved fusion after being treated with PEMF. Lee reported a 67% success rate for treatment of posterior pseudarthrosis with PEMF.30 A multicenter clinical study of 195 patients undergoing primary anterior or posterior interbody fusions performed with PEMF reported 92% fusion with PEMF compared with 65% fusion in controls, although the radiographic criteria for fusion required only 50% graft incorporation.31 Another study, which evaluated 61 patients with discogenic low back pain undergoing fusion with or without PEMF, reported a 97% fusion rate with PEMF compared with 53% fusion in controls.32
One study that evaluated CMFs in 201 patients who underwent uninstrumented posterior lumbar fusions reported a 64% fusion rate in the CMF treated group compared with 43% in controls.32
Conclusions/Future Research
The clinical benefits of electrical stimulation in spinal fusion surgery have been well recognized. Many studies demonstrate a consistent enhancement in fusion success with DC stimulation for posterolateral and interbody fusions. PEMF studies, although limited, also show some enhancement in fusion results. Some recent studies have demonstrated that electrical stimulation upregulates the genes that express proteins which are synergistically required for bone healing. This is also consistent with the work of Morone and colleagues, who demonstrated that spinal fusion involves a specific spatial and temporal expression of such growth factors.14 This mechanism of action explains the enhancement in healing previously documented by the clinical studies.
In order to achieve fusions today, many surgeons use a BMP.33 Unfortunately, the current high cost of this material is a limiting factor in its use, particularly in multilevel fusions such as with spinal deformity. In addition, there are risks associated with BMP including ectopic bone formation and antibody formation. Site-specific complications such as prevertebral swelling in the neck, causing postoperative airway obstruction, and seroma formation in the lumbar spine have been reported. Future studies should evaluate whether smaller doses of BMP in conjunction with electrical stimulation may achieve similar rates of fusion as seen with higher doses of BMP alone. This may be a cost-effective way to achieve reliable fusion rates in patients requiring multilevel fusions, particularly for those patients at risk for developing pseudarthrosis.
Key Points
1 Kahanovitz N. The use of adjunctive electrical stimulation to enhance the healing of spine fusions. Spine. 1996;21:2523-2525.
2 Fredericks DC, Smucker J, Petersen EB, et al. Effects of direct current electrical stimulation on gene expression of osteopromotive factors in a posterolateral spinal fusion model. Spine (Phila Pa 1976). 2007;32:174-181.
1 Bush JL, Vaccaro AR. Electrical Stimulation in lumbar spinal fusion. Orthopedics. 2000;23:737-743.
2 Oishi M, Onesti ST. Electrical bone graft stimulation for spinal fusion: a review. Neurosurgery. 2000;47:1041-1056.
3 Kahanovitz N. The use of adjunctive electrical stimulation to enhance the healing of spine fusions. Spine. 1996;21:2523-2525.
4 Hartshorne E. On the causes and treatment of pseudarthrosis and especially that form of it sometimes called supernumerary joint. Am J Med. 1841;1:121-156.
5 Lente RW. Cases of ununited fracture treated by electricity. NY State J Med. 1850;5:317-319.
6 Wolff J. Das Gaetz der Transformation. Transformation der Knocken. Berlin: Hirschwald; 1892.
7 Yasuda I. Electrical callus. Journal of Kyoto Medical Society. 1953;4:395.
8 Yasuda I, Noguchi K, Stat T. Dynamic callus and electrical callus. Journal of Bone and Joint Surgery Am. 1955;37:1292-1293.
9 Bassett CAL, Becker RO. Generation of electrical potentials by bone in response to electromagnetic stress. Science. 1962;137:1063-1064.
10 Shamos MH, Lavine LS, Shamos MI. Piezoelectric effect in bone. Nature. 1963;197:81.
11 Shamos MH, Lavine LS. Physical basis for bioelectric effects in mineralized tissues. Clin Orthop. 1964;35:177-188.
12 Friedenberg ZB, Brighton CT. Bioelectric potentials in bone. J Bone Joint Surg Am. 1966;48A:915-923.
13 Boden SD, Schimandle JH, Hutton WC. An experimental lumbar intertransverse process spinal fusion model. Radiographic, histologic, and biomechanical healing characteristics. Spine. 1995;20:412-420.
14 Morone MA, Boden SD, Hair G, et al. Hutton WC Gene expression during allograft lumbar spine fusion and the effect of bone morphogenetic protein 2. Clin Orthop. 1998;351:252-265.
15 Fredericks DC, Smucker J, Petersen EB, et al. Effects of direct current electrical stimulation on gene expression of osteopromotive factors in a posterolateral spinal fusion model. Spine (Phila Pa 1976). 2007;32:174-181.
16 Dwyer AF, Wickham CG. Direct current stimulation in spinal fusion. Med J Aust. 1974;1:73-75.
17 Dwyer AF. The use of electrical current stimulation in spinal fusion. Orthop Clin N Am. 1975;6:265-279.
18 Kane WJ. Direct current electrical bone growth stimulation for spinal fusion. Spine. 1988;13:363-365.
19 Meril AJ. Direct current stimulation of allograft in anterior and posterior lumbar interbody fusions. Spine. 1994;19:2393-2398.
20 Rogozinski A, Rogozinski C. Efficacy of implanted bone growth stimulation in instrumented lumbosacral spinal fusion. Spine. 1996;21:2479-2483.
21 Kucharzyk DW. A controlled prospective outcome study of implantable electrical stimulation with spinal instrumentation in a high-risk spinal fusion population. Spine. 1999;24:465-469.
22 Tejano NA, Puno R, Ignacio JMF. The use of implantable direct current stimulation in multilevel spinal fusion without instrumentation: A prospective clinical and radiographic evaluation with long-term follow-up. Spine. 1996;21:1904-1908.
23 Brighton CT, Pollack SR. Treatment of recalcitrant non-union with a capacitively coupled electrical field. A preliminary report. Journal of Bone and Joint Surgery Am. 1985;67:577-585.
24 Fredericks D, Petersen E, Bobst J, et al. Effects of capacitive coupling electrical stimulation on expression of growth factors in a rabbit posterolateral spine fusion model. Chicago: North American Spine Society; 2004.
25 Lorich DG, Brighton CT, Gupta R, et al. Biochemical pathway mediating the response of bone cells to capacitive coupling. Clin Orthop. 1998;350:246-256.
26 Zhuang H, Wang W, Seldes RM, et al. Electrical stimulation induces the level of TGF-b1 mRNA in osteoblastic cells by a mechanism involving calcium/calmodulin pathway. Biochem Biophys Res Commun. 1997;237:225-229.
27 Brighton CT, Wang W, Seldes R, et al. Signal transduction in electrically stimulated bone cells. J Bone Joint Surg Am. 2001;83:1514-1523.
28 Goodwin CB, Brighton CT, Guyer RD, et al. A double-blind study of capacitively coupled electrical stimulation as an adjunct to lumbar spinal fusions. Spine. 1999;24:1349-1357.
29 Bassett CAL. The development and application of pulsed electromagnetic fields (PEMFs) for ununited fractures and arthrodeses. Orthop Clin N Am. 1984;15(1):61-87.
30 Lee K. Clinical investigation of the spinal stem system, open trial phase: pseudarthrosis stratum. Las Vegas: American Academy of Orthopaedic Surgeons; 1989.
31 Mooney V. A randomized double-blind prospective study of the efficacy of pulsed electromagnetic fields for interbody lumbar fusions. Spine. 1990;15:708-712.
32 Marks RA. Spine fusion for discogenic low back pain: outcomes in patients treated with or without pulsed electromagnetic field stimulation. Adv Ther. 2000;17:57-67.
33 McKay B, Sandhu HS. Use of recombinant human bone morphogenetic protein-2 in spinal fusion applications. Spine. 2002;27(16 Suppl 1):S66-S85.