Chapter 21 Electrical Stimulation
History
Electricity has been applied to human disease for centuries. As early as 400 BC, torpedo fish possessing paired organs capable of generating up to 220 V of electricity, and amber, a fossilized resin that produces electrical current when rubbed, were used to treat a wide variety of conditions ranging from headaches to hemorrhoids.15,23,86 “Electricity” was derived from the Latin word for amber, “electrica.” In 1744 Kratzenstein, a German physician, reported the first use of static electricity to treat paralysis.65 Beginning in 1745 with the invention of the Leyden jar, a precursor to the capacitor, technological advances provided a growing capacity to harness, store and modulate electricity. In 1831 Faraday constructed an electromagnetic machine, a forerunner of the electric generator and many electrotherapeutic devices that are currently in use.65 Because of ongoing technological development and the invention of devices such as the battery, the latter half of the nineteenth century became an era where electrotherapeutic treatments expanded in a largely unregulated fashion, possibly contributing to a backlash of skepticism occurring later. Around 1840 electrical stimulation of muscle began its development as a diagnostic tool. Although electrical changes corresponding to the human heartbeat had been demonstrated decades earlier, Einthoven (inventor of the string galvanometer) in 1903 began electrical recordings of the human heart and coined the term “electrocardiogram.” Zoll demonstrated that an artificial pacemaker could sustain a human heartbeat in 1952, and in 1958 the first human heart was paced for 96 days. Liberson et al.58 reported the first practical application of electrical stimulation in rehabilitation in 1961. He described the application of external current to the peroneal nerve to correct foot drop in a hemiparetic patient. Today there are three electrical stimulation systems to correct foot drop that are commercially available in the United States. A wide variety of electrical stimulation applications have been clinically implemented to address many rehabilitation issues, and many more are currently in development.
Physiology
With NMES the point of stimulation can occur anywhere along the axon of the lower motor neuron including the motor points or the terminal branches of the nerve. Stimulation-induced muscle activation requires an intact motor unit—that is, the lower motor neuron (α-motor neuron), neuromuscular junction and corresponding muscle fibers. The threshold for muscle fiber stimulation is 100 to 1000 times higher than that for nerve fiber stimulation.69 For clinical purposes, direct stimulation of muscle fibers to induce contraction cannot be safely performed with available technology. Consequently NMES cannot induce muscle contraction in denervated muscle in clinical applications, and is generally best suited for upper motor neuron diseases to induce muscle activation in paralyzed muscle where the motor unit remains intact.
The generation of an action potential in the α-motor neuron follows the “all-or-none” principle for NMES, just as it does for normal physiologic excitation. The order of recruitment of motor units with NMES is reversed, however, in comparison with normal physiologic excitation. This is because the threshold for excitation with an externally applied current is inversely proportional to the nerve fiber diameter.92 During normal volitional muscle contraction, motor units with low conduction velocity, slow twitch force, long contraction time, low force generation, and greater resistance to fatigue are recruited first. With increasing volitional force generation, motor units with higher conduction velocity, greater force generation, and less resistance to fatigue are recruited.7,41,42 The orderly recruitment of motor units during normal volitional muscle contraction facilitates gradations in muscle force generation, smooth movement, and resistance to fatigue. With NMES the motor units containing larger nerve fibers are recruited first, followed by motor units containing smaller nerve fibers. As a result, NMES first recruits motor units with higher conduction velocities, greater force generation, and lower resistance to fatigue, followed by motor units with slower conduction velocities, lower force generation, and greater resistance to fatigue.92 NMES-induced recruitment, the reverse of normal recruitment, can pose challenges in clinical applications such as abrupt force generation and rapid onset of fatigue that can be only partially compensated for by adjusting stimulation parameters to modulate the delivery of the external current. For example, a longer stimulus ramp-on time can induce more gradual force generation, and lower stimulus frequencies can minimize muscle fatigue. The modulation of stimulation parameters relevant to the clinical use of NMES is discussed in subsequent sections.
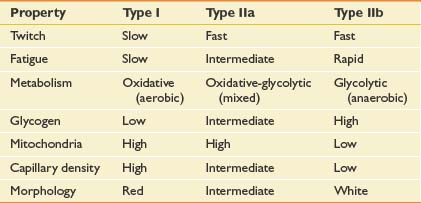
Muscle fiber types can change in response to NMES. Although most data come from studies in mammalian animal models, the consistency of the results suggests that the data can be generalized to all mammals including humans. Skeletal muscle can be classified into types I, IIa, and IIb based on metabolic, histochemical, and functional characteristics (Table 21-1).64 All muscle fibers are histologically identical within a motor unit, but muscle fiber types vary within a muscle and convey unique metabolic and contractile characteristics.14 Continuous (24 hr/day) low-frequency (10 Hz) stimulation results in the transformation of fast-twitch type IIb fibers into slow-twitch type I fibers. Histologic changes are seen within 2 to 4 days of stimulation onset, beginning in the sarcoplasmic reticulum.40 Fiber type conversion is completed within 8 weeks.77
The timing and sequence of histochemical and ultrastructural changes suggest that the regulation of fiber type transformation occurs at the level of gene transcription. The fiber type changes are accompanied by associated metabolic changes from the glycolytic pathway of type IIb fibers to the Krebs cycle pathway of type I fibers. Capillary density and oxygen utilization are increased.13,45 The fiber type transformation is accompanied by characteristic functional changes, including greater resistance to fatigue.44 A reduction in daily stimulation time (noncontinuous, 8 hr/day) results in similar but delayed changes in muscle fiber type.78 When the electrical stimulation is discontinued, muscle fibers return to their prestimulation type in a first-in, last-out sequence. Functional characteristics such as contractile properties return within 6 weeks, and metabolic changes are reversed within 12 weeks, but changes in capillary density may persist for several months.87
Electrical stimulation delivered at higher frequencies (40 to 60 Hz) results in similar fiber type changes if the total number of stimuli remains constant.46,93 An increase in frequency (100 Hz) and number of stimuli has been shown to result in conversion of slow-twitch to fast-twitch fiber in denervated rodent muscle, but similar effects have not been demonstrated in innervated muscle.60
Stimulation Variables and Parameters
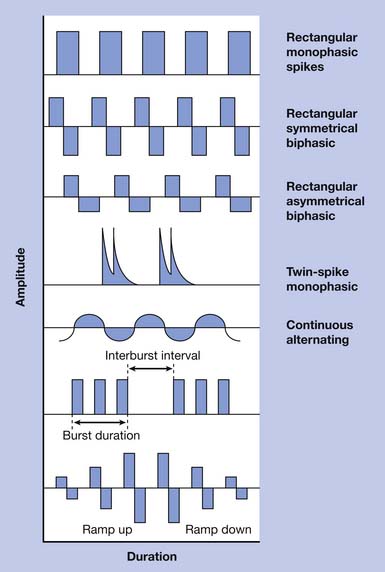
Externally applied electrical current can be characterized by a number of parameters including waveform, amplitude, pulse width, frequency, and duty cycle. Electrical current can be direct (DC), alternating (AC), or pulsed. DC current involves a continuous, unidirectional flow of charge that is generally not applicable to NMES. However, stimulation of the brain cortex with DC current has been investigated for treatment of depression and various cognitive impairments.9,53,61 AC current involves a continuous, bidirectional flow of charge. Pulsed current is most commonly used in NMES and involves a pulsed, interrupted flow of charge that can take on a variety of waveforms. Pulsed waveforms can be monophasic or biphasic. Biphasic waveforms can be symmetric or asymmetric with respect to baseline. Waveform asymmetry can be related to any aspect of the waveform shape including current amplitude, duration, rise, and decay. Electrical current delivered via implanted electrodes requires special waveform considerations to avoid charge accumulation that can result in electrode corrosion, tissue injury, or both.69 Consequently devices designed to deliver transcutaneous stimulation might not be safely used with percutaneous or other implanted electrodes. Pulsed or AC current can be delivered in trains or bursts, where the frequency of bursts is known as the carrier frequency. Waveforms have been studied extensively with respect to stimulation-induced pain and tolerance because transcutaneous stimulation, despite preferential recruitment of larger nerve fibers, tends to stimulate smaller cutaneous pain fibers because of their proximity. Results have varied, however, and no consensus has been reached regarding the most tolerable waveform. Electrical stimulation delivered via implanted electrodes can bypass cutaneous nociceptive nerve fibers and results in significantly less discomfort.105 Waveform examples are shown in Figure 21-1.
Stimulation intensity is typically regulated via current amplitude, voltage, or pulse width. Increasing these parameters generates greater contractile force with associated stimulation-induced pain.12,43,57 Stimulators are either current or voltage regulated. Current-regulated stimulation provides (adjustable) constant current despite changing resistance that can be because of reduced contact between electrode and skin interface or variable impedance resulting from tissue movement. Current-regulated stimulators have safety mechanisms that prevent the delivery of excessively high voltage when excessive impedance is encountered. Increasing stimulation frequency also results in greater contractile force but is associated with greater muscle fatigue and stimulation-induced pain.11,49 The lowest stimulation intensity and frequency that achieves the desired physiologic or functional effect is generally recommended. The lowest stimulation frequency that results in fused muscle contraction can be as low as 12 Hz. Frequencies of 12 to 16 Hz for upper limb applications and 18 to 25 Hz for lower limb applications have been recommended.89 The duty cycle refers to “on” and “off” time during cyclic stimulation. The duty cycle can be described by on:off time (e.g., 10 seconds:10 seconds) or as a percentage of on time (e.g., 50%.) Stimulation is typically ramped on by linear increases in stimulus intensity, with effects on graduated force generation. Stimulation can also be ramped off.
System Components
Although implanted systems are expanding rapidly, transcutaneous NMES systems are more commonly used in clinical practice because of their availability, lower cost, safety, and noninvasive implementation. Optimal clinical use of transcutaneous NMES requires experience and knowledge beyond the scope of this chapter. Clinicians who use transcutaneous NMES in clinical practice are referred to a practical guide developed at Rancho Los Amigos National Rehabilitation Center.7 A number of factors determine whether current delivered through a transcutaneous electrode will be adequate to cause neural excitation and subsequent muscle activation. The neural excitation will occur if the accumulation of charge (charge density) at the target neural structure reaches a threshold for depolarization that initiates an action potential (stimulus threshold.) The sum of the resistive and capacitive elements (i.e., electrode or tissue impedance) that oppose the flow of current affects charge density at the target neural structure and can be influenced by electrode size, orientation, configuration, and location. Current flows in a path of least resistance (Kirchoff’s principle). Most of the applied current bypasses the target nerve and dissipates through interstitial fluids. Humans are composed of heterogenous tissues with variable electrical resistance and capacitance (i.e., impedance). Tissue impedance is inversely proportional to water content. The water content of muscle, fat, epidermis or bone are approximately 75%, 15%, and 5% to 16%, respectively.91 Even a given type of tissue such as muscle is nonhomogenous with respect to tissue impedance. Current conducts about four times better in parallel to muscle fiber orientation compared with transversely across fibers. The clinical implications of this for electrode orientation and configuration are most significant when stimulating larger muscles.34,91
Transcutaneous electrodes come in a variety of sizes and shapes. The impedance within the water-based gel on the conducting surface of the electrode is much less than that of epidermis and subcutaneous fat. Electrode size, shape, and orientation influence the electrical field as it enters the body. The cathode is the negative electrode. Positively charged ions are drawn to the cathode, creating a negatively charged environment external to the neuron that results in a reduction in the transmembrane potential. If the stimulus threshold is reached, explosive opening of voltage-dependent sodium ion (Na+) channels results in the generation of an action potential. The relatively greater neuromuscular activation at the cathode compared with the anode should be considered in clinical applications. For example, during transcutaneous NMES to reduce shoulder subluxation in hemiplegia, the cathode is applied to the posterior deltoid where the greatest muscle activation is desired. The anode is applied over the supraspinatus fossa to minimize activation of the upper trapezius muscle, which lies superficial to the supraspinatus muscle, thereby minimizing uncomfortable shoulder shrugging. Electrode configuration is often referred to as monopolar or bipolar. In a monopolar configuration, the cathode (“active”) is placed over the target stimulation site and the anode (“inactive”) is placed remotely, usually on the muscle tendon. Current density is high near the cathode, and volumetric dispersion is large, with only a fraction of the current returning through the anode. In a bipolar configuration, where the anode and cathode are placed in close proximity (typically a few centimeters), the current density between electrodes is maximized.39 Maximizing current density to the target neural structure is desirable to minimize the overall current delivered, because muscle activation will occur with less pain. When the target neural structure is deep, current dispersion over a larger volume might be needed, requiring a greater distance between electrodes.
Clinical Applications for the Upper Limb
Neuroprostheses
An advanced, surgically implanted neuroprosthesis has been developed for spinal cord–injured individuals with C5–C6 motor levels, or more precisely, groups 0, 1, and 2 of the International Classification for Surgery of the Hand in Tetraplegia.59 These are individuals who retain sufficient volitional movement of the shoulder and elbow (often with posterior deltoid to triceps tendon transfer to improve elbow extension) but who lack volitional control of muscles below the elbow to benefit from tendon transfer surgery to improve prehensile function. The system, formerly known as the Freehand System (NeuroControl Corp, Valley View, OH), was commercially available in the United States and Europe for several years (Figure 21-2). The Freehand system included eight channels of stimulation to provide grasp and release in palmar prehension for larger objects, such as a drinking glass, and lateral pinch for smaller objects such as an eating utensil. The electrodes, receiver-stimulator, and leads were surgically implanted. A radiofrequency inductive link to the receiver-stimulator provided power and real-time proportional control of prehensile function. The system was controlled by the user through a device worn on the contralateral shoulder that could detect various shoulder movements. A multicenter clinical trial of 50 subjects implanted with the neuroprosthesis demonstrated significant improvement in activities, regular use of the device in the community, and greater than 90% satisfaction among users.74 Satisfaction and patterns of use persisted beyond 4 years for most users.104 There were no reported cases of neuroprosthesis failure. Lead failure and infection rates were less than 1% and 2%, respectively.89 The Freehand was commercially available for several years, but eventually was removed from the market by the manufacturer for many reasons that need to be understood by those who seek clinical implementation of such devices. Suffice it to say that developing an effective electrical stimulation intervention and conducting sound science to demonstrate efficacy and safety are not enough to ensure societal benefit. Many factors beyond science and engineering affect medical utilization and commercial viability.
Upper limb neuroprostheses for high tetraplegia (C1–C4 neurologic level) have been evaluated to a much lesser extent. One case report describes a C3 tetraplegic individual who was able to feed himself using a percutaneously implanted eight-channel neuroprosthesis, a mobile arm support, and a universal cuff after setup following an 8-week program of stimulated muscle conditioning.108 Musculoskeletal modeling suggests that 24 channels might be needed, including four channels for electromyographic (EMG) control from face and neck muscles.52 The overall feasibility of an upper limb neuroprosthesis for high tetraplegia remains questionable for a number of reasons, including the need for stimulation of muscles to provide shoulder and elbow control, and the need for more stimulation channels and fewer options for user control. Further, denervation to critical myotomes is more prohibitive, compared with lower cervical neurologic levels. Traumatic spinal cord injury (SCI) often results in a zone of injury where injury to anterior horn cells results in lower motor neuron injury that can encompass three to four spinal segments adjacent to the neurologic level. Although this is a potential challenge for FES at any neurologic level, C1–C4 neurologic levels are often accompanied by an injury zone that results in denervation to critical myotomes responsible for control of the shoulder and elbow. An effective upper limb neuroprosthesis for individuals with high tetraplegia has not yet been developed.
Several upper limb neuroprostheses have been developed for survivors of hemiparetic stroke by using transcutaneous or percutaneously placed intramuscular electrodes, with suggested improvements in motor impairment and selected laboratory-based activities in small, nonblinded clinical trials.3,19,67,85 These studies provide proof-in-concept that a neuroprosthesis can be developed for stroke survivors with upper limb paresis. The barriers to developing an effective upper limb neuroprosthesis for persons with stroke need to be addressed, however, before significant improvements in community-based activities can be expected. First, synergistic patterns of muscle activation and spasticity inhibit volitional movement after stroke and tend to be exacerbated during the performance of functional tasks, particularly when greater amounts of effort are required. As a result, electrically stimulated movement tends to be more easily controlled when the limb is at rest, but abnormal muscle activation patterns can become prohibitive during neuroprosthesis-facilitated, goal-oriented movement. Traditional spasmolytic medications have not been effective adjuncts to improve targeted movement while using a neuroprosthesis.89 Focal, dose-dependent chemodenervation with botulinum toxin and other neurolytic procedures warrant further evaluation as adjuncts to the use of upper limb neuroprostheses in hemiparetic persons. Second, control strategies should not limit functional use of the neurologically intact limb. Third, the functional demands of a neuroprosthesis in the setting of unilateral (hemiplegia) as compared with bilateral (tetraplegia) upper limb paresis might be higher. At the minimum a neuroprosthesis for stroke must facilitate simple bimanual tasks. The NESS H200 (Bioness, Inc, Valencia, CA) is an exoskeletal device marketed as an upper limb neuroprosthesis (Figure 21-3). The H200 is the only commercially available device that has been shown to improve selected laboratory-based activities in stroke and tetraplegia, and bears mentioning for this reason.2,3 Transcutaneous electrodes are fastened into the exoskeleton, resulting in more reliable electrode placement and thus easier donning that facilitates repetitive stimulation to prehensile muscles in a coordinated sequence. However, the device remains subject to the clinical barriers described earlier. Available control strategies limit its utility for performing functional activities in the real world. An upper limb neuroprosthesis for stroke that improves community-based activities has yet to be developed.
Motor Recovery
Therapeutic interventions in the upper limb have primarily addressed motor recovery and shoulder pain after stroke. TES for motor recovery after stroke is discussed extensively in Chapter 50. The past decades have witnessed a paradigm shift in stroke rehabilitation. Therapeutic strategies to induce neurologic recovery are being incorporated into rehabilitation programs, which historically have been dominated by compensatory strategies that expedite functional independence but often overlook recovery of the impaired limb. This has been particularly true of the hemiparetic upper limb because many basic self-care activities can be performed with a single neurologically intact upper limb. Ambulation, however, requires both lower limbs. This paradigm shift is based on cumulative preclinical and clinical data that have led to the widespread acceptance that the adult human brain is capable of significant adaptive reorganization after acquired injury, and that these changes can be influenced by appropriate behavioral interventions. Current knowledge regarding the critical components and mechanisms of interventions that maximize recovery of motor function is incomplete. Basic tenets, however, include learned nonuse that can be overcome by forced use of the paretic limb, massed practice whereby many repetitions are needed to induce adaptive changes, and skill acquisition whereby new or lost skills must be acquired during movement tasks. Electrically stimulated movement can be formatted to follow these tenets. Further, limb movement can be electrically stimulated even when severe motor impairment after stroke precludes participation in therapies that require volitional movement.
TES for upper limb motor recovery after stroke has been studied extensively but has yet to be studied rigorously. Methodologic limitations among studies include small sample sizes, inconsistent evaluator and subject blinding, inadequate controls, and inconsistent use of intention-to-treat analyses.66,89 Available data support an effect of TES on reducing motor impairment, but the effect on clinically relevant outcomes such as activities has been less consistent.∗ Cortical mapping with functional magnetic resonance imaging or transcranial magnetic stimulation consistently demonstrates enhanced brain activity with TES interventions, supporting a mechanism of cortical plasticity.16,17,51 Effects seem to be greater in the acute phase rather than the chronic phase after stroke.95 Although post hoc subset analysis in one study suggested that TES effects seem to be greater for stroke survivors with mild to moderate as compared with severe motor impairment,80 another trial demonstrated efficacy for severely impaired subjects (but only during the acute phase of recovery).95 Six clinical trials evaluating EMG-triggered NMES reported improvement in motor impairment. Two of these that evaluated clinically relevant outcomes also reported improvement in activities. However, the only study directly comparing EMG-triggered NMES with cyclic NMES did not demonstrate a difference, but the lack of statistical significance might have been because of a lack of statistical power.26 Although few clinical trials have evaluated the therapeutic effect of a neuroprosthesis, available data suggest a therapeutic benefit with respect to improvements in motor impairment and activities.4,79 Available data make it difficult to draw definitive conclusions, but clinicians are nevertheless challenged to provide patients with stroke with the best possible care. Given a consistent effect of TES on motor impairment, neurophysiologic data supporting brain plasticity, clinical data demonstrating safety, and clinical availability of systems using transcutaneous electrodes, TES should be used selectively in clinical practice. TES should not supplant therapies requiring volitional movement, but it can be considered as an adjunct. TES should be strongly considered in motivated patients who are too impaired to benefit from therapies requiring volitional movement. TES, when appropriate, should be implemented early after stroke. When practical, TES should be EMG triggered or more closely coupled to cognitive control by the user.
Poststroke Shoulder Pain
Poststroke shoulder pain (PSP) is a common complication after stroke that inhibits functional recovery and reduces quality of life (see Chapter 50). Many causes of shoulder pain have been postulated, including shoulder subluxation, bicipital tendonitis, supraspinatus tendonitis, subacromial bursitis, impingement syndrome, rotator cuff tear, spasticity, capsulitis, and complex regional pain syndrome. The pathogenesis of PSP has yet to be studied rigorously. Among various types of pathophysiology that have been correlated with PSP, more severe motor impairment has been the most highly correlated.33,84,102 The role of shoulder subluxation as a cause of pain remains controversial, particularly as a direct cause of pain. Studies correlating shoulder subluxation with other types of pathology suggest that subluxation might also cause pain indirectly by predisposing to the development of other painful conditions such as rotator cuff tear and complex regional pain syndrome.22,29,70 Direct therapeutic goals for TES have included reduction of subluxation, reduction of pain, or both. Motor recovery has also been evaluated, but TES for PSP has not been designed to promote motor recovery. The resulting stimulated muscle activation does not occur through a functional range of motion. The mechanisms underlying pain reduction have yet to be fully elucidated, but might include sensory neuromodulation, as well as biomechanical effects that stabilize the glenohumeral joint.
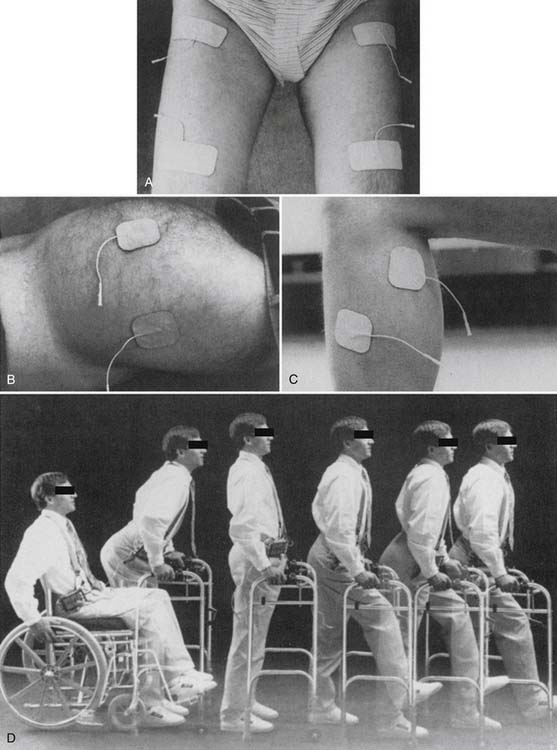
In 1986 Baker and Parker6 published the first article evaluating the use of electrical stimulation for treating poststroke shoulder subluxation and pain. An external stimulator delivered electrical current to the supraspinatus and posterior deltoid muscles via transcutaneous electrodes for 6 hours daily over 6 weeks. Several additional studies using similar protocols have been published since that time. The potential clinical benefits of TES applied to the shoulder region in hemiparetic stroke survivors include reduction of shoulder pain, improvement in range of motion, reduction of subluxation, and recovery of motor function.21,30,54,100 The clinical practicality of TES for treating PSP using transcutaneous electrodes, however, has been questioned. Transcutaneous electrical stimulation in the shoulder region can be painful because of stimulation of cutaneous nociceptors, and can limit tolerance and compliance in a significant number of cases.54 Although methods to enhance tolerance of TES for treating PSP that include gradual increases in stimulation time and intensity have been developed, stimulation-induced pain continues to preclude clinical use in a significant number of cases.47 Further, stimulation of deeper muscles might be limited by unwanted stimulation of more superficial muscles that are positioned between the transcutaneous electrode and target muscle. Lastly, many patients have difficulty applying TES in the home environment because of technical difficulty with electrode placement and proper adjustment of stimulation parameters. This is a particularly important consideration for treating PSP because studies suggest that hours of daily treatment over weeks might be required to achieve clinical benefit (necessitating home-based treatment). For these reasons, even the first investigators of TES for PSP recognized the need for implanted systems. They stated in their first publication in 1986 that “until implanted electrode systems become available, however, long-term use of surface electrical stimulation can be managed by only a few patients with hemiparesis and their families.”6
Partially and fully implanted electrical stimulation systems have been developed to address the limitations of systems that use transcutaneous electrodes. None, however, are commercially available at the time of this writing. The efficacy of implanted TES for treating PSP and associated activities limitations has been demonstrated in an evaluator-blinded, randomized clinical trial of 61 subjects.106 Pain reduction persists for at least 12 months after completing treatment.20 In this trial, intramuscular electrodes with percutaneous leads were placed near supraspinatus, posterior deltoid, middle deltoid, and upper trapezius motor points. Electrical stimulation was delivered for 6 hours daily over 6 weeks. There was no clear plateau in pain reduction that would suggest that treatment over a longer period could reduce pain further. Adverse events using intramuscular electrodes with percutaneous leads include accidental displacement, infection, and foreign body reaction. Despite data supporting efficacy, this partially implanted system was not commercialized because of concerns regarding adverse events relating to the intramuscular electrodes with percutaneous leads. Systems using fully implanted microstimulators powered by an external power source via a radiofrequency link90 and using fully implanted microstimulators containing an internal battery105 are currently under evaluation.
Because of the existence of multiple studies with low statistical power, attempts to pool data and provide clinical recommendations have been published. A Cochrane review concluded that electrical stimulation increases pain-free passive range of motion, a pain measure used in some studies, and reduces glenohumeral subluxation. There did not appear to be any negative effects of electrical stimulation. Effects on spasticity and motor recovery were not supported.81 In a meta-analysis evaluating TES on shoulder subluxation, the effects were greater for prevention of subluxation in the acute phase rather than with treatment in the chronic phase after stroke.1 In terms of practical recommendations for clinical use, only external systems using transcutaneous electrodes are available at this time. They are safe and relatively inexpensive.
Clinical Applications for the Lower Limb
In contrast to electrical stimulation for the upper limb, where clinical applications fall into three categories focused on neuroprosthetic effects, motor recovery, or hemiparetic shoulder pain, lower limb applications have predominately focused on neuroprosthetic effects to enhance standing and walking. Although motor recovery resulting from use of a lower limb neuroprosthesis has been reported frequently, formal experimentation to evaluate this therapeutic effect is in the early stages. The evolution of lower limb neuroprostheses has followed an interesting path. In 1961 Liberson et al.58 reported the first electrical stimulation application in neurologic rehabilitation—stimulation of the peroneal nerve to correct foot drop in hemiplegia. A great deal of interest in electrical stimulation was generated soon after, but was directed largely toward restoration of walking in paraplegia (a seductive clinical problem with deceptively evident solutions because of the stereotyped movement patterns of bipedal locomotion). Because the restoration of gait in paraplegia met with numerous barriers, the pendulum seems to have swung back toward simpler applications including standing in paraplegia, restoration of gait in hemiplegia, and correction of foot drop. A number of electrical stimulation applications are under investigation for transfers, standing, walking, and motor recovery in stroke and SCI, but the following section focuses on devices that may be relevant to current clinical practice.
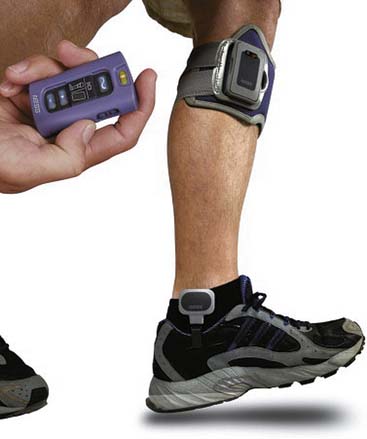
Presently three external FES systems for correction of foot drop are commercially available in the United States: the Odstock Drop Foot Stimulator (Odstock Medical Ltd, Wiltshire, England), the WalkAide (Innovative Neurotronics, Inc, Austin, TX) (Figure 21-4), and the Bioness L300 (Bioness, Inc) (Figure 21-5). Implanted systems that are available in Europe include the StimUStep (Finetech Medical, Welwyn Garden City, England) and the Acti-Gate (Neurodan, Aalborg SV, Denmark). All systems are based on electrical stimulation of the peroneal nerve or its branches, including motor points of the ankle dorsiflexors and evertors. By clinical necessity, these are closed loop systems where the device must be able to sense the gait cycle to stimulate during the swing phase of gait. Sensors include pressure sensors worn under the heel and tilt sensors (accelerometers) worn below the knee. The design features of each device convey unique clinical benefits. The WalkAide uses a tilt sensor worn below the knee that removes the need for a heel sensor for individuals with sufficient knee flexion during swing phase, allowing users to walk barefoot or with a wider range of shoe types. Excessive extensor hypertonia and certain compensatory strategies such as hip circumduction, however, might render the tilt sensor ineffective. In these cases the WalkAide can be used with a pressure sensor worn under the heel connected by external leads to the stimulator worn below the knee. The L300 uses a heel pressure sensor but does not require external connection with the stimulator because it uses a radiofrequency link. The L300 also features a garment for the stimulator and electrodes that conforms anatomically to the patella, potentially making the device easier to don and improving the reliability of electrode placement and the resulting muscle activation. Programming features are also different for the various devices, making some better for wider variations in terrain and activity. A number of small clinical trials have demonstrated improvement in gait parameters with use of various FES devices to correct foot drop. Large, well-controlled clinical trials comparing FES with the standard of care (the ankle-foot orthosis) have yet to be reported. For many patients, it seems that a personal trial of an FES foot drop device compared with an ankle-foot orthosis might be more compelling than scientific data.
Several transcutaneous and implanted FES systems have been developed with the goal of restoration of walking for persons with thoracic level SCI. Work in this area began in the 1970s with systems using transcutaneous electrodes, where standing is achieved with bilateral quadriceps activation in conjunction with support from the upper limbs. While unilateral quadriceps activation is maintained, a flexion withdrawal reflex is induced in the contralateral lower limb to initiate a step. Activation of the knee extensors in the swing phase limb completes the stride.5,56,99 This sequence of stimulated movement is used in the Parastep (Sigmedics, Inc, Fairborn, OH), which is commercially available in the United States (Figure 21-6). This sequence of stimulated movement commonly encounters several barriers. First, undesirable hip flexion caused by rectus femoris activation adversely affects posture during standing. Second, the flexion withdrawal reflex can be inadequate even after conditioning. Third, the latter half of swing phase is difficult to control because of the mass flexion response. Moreover, this strategy is subject to excessive energy costs and early muscle fatigue because of preferential recruitment of fast-fatiguing type II fibers that complicate all FES applications for walking in SCI. It has been estimated that FES walking systems require up to 75% of maximum aerobic capacity, a level of energy consumption that might rival the use of mechanical orthoses that require up to ninefold the energy consumption of normal walking.23,63 To overcome barriers associated with activation of a flexion withdrawal reflex, individual muscles can be activated, a strategy that requires a larger number of stimulation channels. The practicality of transcutaneous stimulation declines with the need for more channels, leading to the development of a number of implanted FES systems for walking. The implanted systems have grown in their complexity and have yielded incremental improvements in walking as well as standing and transfers. Because of the limitations described above, however, FES for ambulation in SCI remains elusive for functional distances and is unlikely to replace the mobility conferred by wheelchair use in the immediate future.10
FES cycle ergometry is distinct from the neuroprosthetic applications discussed above, but bears mentioning because of its current clinical relevance. Several systems are commercially available. Some combine upper limb with lower limb training. One system uses transcutaneous electrodes placed on the quadriceps, hamstrings, and gluteal muscles bilaterally to maintain cycling against resistance at 35 to 50 rpm for spinal cord–injured persons. A number of benefits have been shown including reversal of muscle atrophy, changes in muscle function, increased strength, improved vascular supply, increased lean body mass, and improved aerobic capacity.∗ Clinicians are encouraged to consider FES cycle ergometry as a means for individuals with central nervous system injury to enjoy the multitude of benefits that exercise involving large muscle groups can provide for able bodied persons.
Clinical Applications for the Bladder
Sacral nerve stimulation can produce effective micturition in persons with suprasacral SCI.48 A surgically implanted system is clinically available in the United States and in Europe. Electrodes are placed on anterior sacral nerve roots or on mixed sacral nerves after exposure by lumbar or sacral laminectomy. Subcutaneous leads connect the electrodes to a subcutaneous receiver-stimulator in the abdomen or chest. Stimulation results in co-contraction of detrusor and external sphincter muscles. The somatic sphincter relaxes faster than the detrusor, however, resulting in micturition when pulsed trains of stimulation are applied at appropriate intervals. A dorsal rhizotomy is often performed concomitantly to abolish reflex detrusor activity that can cause incontinence, but this also eliminates reflex erections. The system is powered and controlled by a hand-held remote control. More than 2000 individuals worldwide use this device.96 Urinary continence occurs in greater than 85% of users.97 Additional benefits include improvement in bowel evacuation, decreased incidence of symptomatic urinary tract infection, decreased incidence of upper and lower urinary tract complications and fewer episodes of autonomic dysreflexia.62,96 The greatest clinical benefit is conveyed to users who can independently transfer to the toilet, have sufficient hand function to operate the remote control, and are not affected by the loss of reflex erections. Use of this system including dorsal rhizotomy has been shown to be cost-effective after 5 years compared with customary care.24,25
Electroejaculation
Ejaculation induced by electrical stimulation via a rectal probe is one of several standard techniques to collect semen in spinal cord–injured persons with ejaculatory failure. This technique does not adversely affect sperm motility, but retrograde and bidirectional ejaculation can occur.101 In these cases the ejaculate can be retrieved from the bladder with a catheter. Electroejaculation appears safe from the standpoint of mucosal injury and autonomic dysreflexia, but might not be well tolerated in persons with preserved rectal sensation.75,88
Clinical Applications for Respiration
Surgically implanted PNP systems are commercially available and have been applied to more than 1200 individuals with cervical SCI worldwide.36,37,94 PNP is typically done bilaterally. Unilateral PNP combined with intercostal nerve stimulation can be considered, however, if only one phrenic nerve is intact.28 Electrodes can be implanted onto the phrenic nerves through a cervical or transthoracic approach. Although the latter requires a thoracotomy and is technically more difficult, it is preferable because the cervical approach can be hampered by inadequate nerve stimulation, unwanted stimulation of other nerves, and neck movement that can affect implanted components.27,31,98 The electrodes are wired to a radiofrequency receiver that is typically placed subcutaneously in the anterior chest wall. Stimulated diaphragm conditioning is required to improve strength and endurance after device implantation.38 In a cohort study of 14 tetraplegic PNP users, the average duration of successful ventilation by PNP was 7.6 years, and the longest use was 15 years. Although invasive mechanical ventilation has been associated with significant morbidity and mortality, data directly comparing invasive mechanical ventilation with PNP are not currently available.
Other Effects and Applications
A number of additional interventions have not become standard in clinical practice but warrant mentioning. Electrical stimulation has been evaluated to enhance healing of wounds including pressure ulcers, diabetic ulcers, and vascular ulcers. Although the efficacy data have been equivocal, selection of appropriate stimulation parameters is likely to be important and has varied widely. A placebo-controlled randomized trial evaluating low-intensity pulsed current (0.3 to 0.6 mA) demonstrated significant healing of pressure ulcers.103 NMES to the distal lower limb has been evaluated for deep venous thrombosis prevention, with demonstrated reduction in venous stasis and therapeutic benefit in conjunction with low-dose unfractionated heparin.50,68 Studies comparing adjunctive NMES with sequential compression plus low-molecular-weight heparins (that have become the standard of care for acute SCI and many orthopedic procedures) have yet to be reported. Although the benefits of electrically induced muscle conditioning after central nervous system injury have been elucidated, similar benefits in neurologically intact individuals capable of volitional exercise remain questionable.73 The reversal of normal physiologic motor unit recruitment and muscle fiber type changes with NMES must be considered carefully. Under most conditions, neurologically intact humans are capable of volitional maximal motor unit recruitment and changes in muscle fiber types in response to functional demands. NMES-induced muscle conditioning might have a role in conditions that prohibit volitional exercise, as occurs during pain inhibition or limb immobilization as a result of orthopedic conditions.
1. Ada L., Foonghchomcheay A. Efficacy of electrical stimulation in preventing or reducing subluxation of the shoulder after stroke: a meta-analysis. Aust J Physiother. 2002;48:257-267.
2. Alon G., McBride K. Persons with C5 or C6 tetraplegia achieve selected functional gains using a neuroprosthesis. Arch Phys Med Rehabil. 2003;84(1):119-124.
3. Alon G., McBride K., Ring H. Improving selected hand functions using a noninvasive neuroprosthesis in persons with chronic stroke. J Stroke Cerebrovasc Dis. 2002;11(2):99-106.
4. Alon G., Sunnerhagen K.S., Geurts A.C., et al. A home-based, self-administered stimulation program to improve selected hand functions of chronic stroke. NeuroRehabilitation. 2003;18(3):215-225.
5. Bajd T., Kralj A., Turk R. Standing-up of a healthy subject and a paraplegic patient. J Biomech. 1982;15(1):1-10.
6. Baker L.L., Parker K. Neuromuscular electrical stimulation of the muscles surrounding the shoulder. Phys Ther. 1986;66(12):1930-1937.
7. Baker L.L., Wederich C.L., McNeal D.R., et al. Neuromuscular electrical stimulation: a practical guide, ed 4, Downey, CA. Rancho Los Amigos National Rehabilitation Center; 2000.
8. Baldi J.C., Jackson R.D., Moraille R., et al. Muscle atrophy is prevented in patients with acute spinal cord injury using functional electrical stimulation. Spinal Cord. 1998;36(7):463-469.
9. Berlim M.T., Dias Neto V., Turecki G. [Transcranial direct current stimulation: a promising alternative for the treatment of major depression?] [Portuguese]. Rev Bras Psiquiatr. 2009;31(suppl 1):S34-S38.
10. Bhadra N., Chae J. Implantable neuroprosthetic technology. NeuroRehabilitation. 2009;25(1):69-83.
11. Binder-Macleod S.A., Guerin T. Preservation of force output through progressive reduction of stimulation frequency in human quadriceps femoris muscle. Phys Ther. 1990;70(10):619-625.
12. Bowman B.R., Baker L.L. Effects of waveform parameters on comfort during transcutaneous neuromuscular electrical stimulation. Ann Biomed Eng. 1985;13(1):59-74.
13. Brown M.D., Cotter M.A., Hudlicka O., et al. The effects of different patterns of muscle activity on capillary density, mechanical properties and structure of slow and fast rabbit muscles. Pflugers Arch. 1976;361(3):241-250.
14. Burke R.E., Levine D.N., Tsairis P., et al. Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol. 1973;234(3):723-748.
15. Cameron T., Loeb G.E., Peck R.A., et al. Micromodular implants to provide electrical stimulation of paralyzed muscles and limbs. IEEE Trans Biomed Eng. 1997;44(9):781-790.
16. Cauraugh J., Light K., Kim S., et al. Chronic motor dysfunction after stroke: recovering wrist and finger extension by electromyography-triggered neuromuscular stimulation. Stroke. 2000;31(6):1360-1364.
17. Cauraugh J.H., Kim S. Two coupled motor recovery protocols are better than one: electromyogram-triggered neuromuscular stimulation and bilateral movements. Stroke. 2002;33(6):1589-1594.
18. Chae J., Bethoux F., Bohine T., et al. Neuromuscular stimulation for upper extremity motor and functional recovery in acute hemiplegia. Stroke. 1998;29(5):975-979.
19. Chae J., Hart R. Intramuscular hand neuroprosthesis for chronic stroke survivors. Neurorehabil Neural Repair. 2003;17(2):109-117.
20. Chae J., Mascarenhas D., Yu D.T., et al. Poststroke shoulder pain: its relationship to motor impairment, activity limitation, and quality of life. Arch Phys Med Rehabil. 2007;88(3):298-301.
21. Chantraine A., Baribeault A., Uebelhart D., et al. Shoulder pain and dysfunction in hemiplegia: effects of functional electrical stimulation. Arch Phys Med Rehabil. 1999;80:328-331.
22. Chino N. Electrophysiological investigation on shoulder subluxation in hemiplegics. Scand J Rehabil Med. 1981;13:17-21.
23. Clinkingbeard J.R., Gersten J.W., Hoehn D. Energy cost of ambulation in the traumatic paraplegic. Am J Phys Med. 1964;43:157-165.
24. Creasey G.H., Dahlberg J.E. Economic consequences of an implanted neuroprosthesis for bladder and bowel management. Arch Phys Med Rehabil. 2001;82(11):1520-1525.
25. Creasey G.H., Kilgore K.L., Brown-Triolo D.L., et al. Reduction of costs of disability using neuroprostheses. Assist Technol. 2000;12(1):67-75.
26. de Kroon J.R., MJ, IJ. Electrical stimulation of the upper extremity in stroke: cyclic versus EMG-triggered stimulation. Clin Rehabil. 2008;22(8):690-697.
27. DiMarco A.F. Restoration of respiratory muscle function following spinal cord injury. Review of electrical and magnetic stimulation techniques. Respir Physiol Neurobiol. 2005;147(2-3):273-287.
28. DiMarco A.F., Takaoka Y., Kowalski K.E. Combined intercostal and diaphragm pacing to provide artificial ventilation in patients with tetraplegia. Arch Phys Med Rehabil. 2005;86(6):1200-1207.
29. Dursun E., Dursun N., Ural C.E., et al. Glenohumeral joint subluxation and reflex sympathetic dystrophy in hemiplegic patients. Arch Phys Med Rehabil. 2000;81(7):944-946.
30. Faghri P.D., Rodgers M.M., Glaser R.M., et al. The effects of functional electrical stimulation on shoulder subluxation, arm function recovery, and shoulder pain in hemiplegic stroke patients. Arch Phys Med Rehabil. 1994;75:73-79.
31. Fodstad H. The Swedish experience in phrenic nerve stimulation. Pacing Clin Electrophysiol. 1987;10(1 Pt 2):246-251.
32. Francisco G., Chae J., Chawla H., et al. Electromyogram-triggered neuromuscular stimulation for improving the arm function of acute stroke survivors: a randomized pilot study. Arch Phys Med Rehabil. 1998;79(5):570-575.
33. Gamble G.E., Barberan E., Laasch H.U., et al. Poststroke shoulder pain: a prospective study of the association and risk factors in 152 patients from a consecutive cohort of 205 patients presenting with stroke. Eur J Pain. 2002;6:467-474.
34. Gedes L.A., Baker L.E., editors. Principles of applied biomedical instrumentation, ed 3, New York: John Wiley and Sons, 1989.
35. Glanz M., Klawansky S., Stason W., et al. Functional electrostimulation in poststroke rehabilitation: a meta-analysis of the randomized controlled trials. Arch Phys Med Rehabil. 1996;77(6):549-553.
36. Glenn W.W., Hogan J.F., Loke J.S., et al. Ventilatory support by pacing of the conditioned diaphragm in quadriplegia. N Engl J Med. 1984;310(18):1150-1155.
37. Glenn W.W., Hogan J.F., Phelps M.L. Ventilatory support of the quadriplegic patient with respiratory paralysis by diaphragm pacing. Surg Clin North Am. 1980;60(5):1055-1078.
38. Glenn W.W., Phelps M.L. Diaphragm pacing by electrical stimulation of the phrenic nerve. Neurosurgery. 1985;17(6):974-984.
39. Grandjean P.A., Mortimer J.T. Recruitment properties of monopolar and bipolar epimysial electrodes. Ann Biomed Eng. 1986;14(1):53-66.
40. Heilman C., Muller W., Pette D. Correlation between ultrastructural and functional changes in sarcoplasmic reticulum during chronic stimulation of fast muscle. J Membr Biol. 1981;59(2):143-149.
41. Henneman E., Somjen G., Carpenter D.O. Excitability and inhibitability of motoneurons of different sizes. J Neurophysiol. 1965;28(3):599-620.
42. Henneman E., Somjen G., Carpenter D.O. Functional significance of cell size in spinal motoneurons. J Neurophysiol. 1965;28(3):560-580.
43. Howson D.C. Peripheral neural excitability. Implications for transcutaneous electrical nerve stimulation. Phys Ther. 1978;58(12):1467-1473.
44. Hudlicka O., Brown M., Cotter M., et al. The effect of long-term stimulation of fast muscles on their blood flow, metabolism and ability to withstand fatigue. Pflugers Arch. 1977;369(2):141-149.
45. Hudlicka O., Dodd L., Renkin E.M., et al. Early changes in fiber profile and capillary density in long-term stimulated muscles. Am J Physiol. 1982;243(4):H528-H535.
46. Hudlicka O., Tyler K.R., Srihari T., et al. The effect of different patterns of long-term stimulation on contractile properties and myosin light chains in rabbit fast muscles. Pflugers Arch. 1982;393(2):164-170.
47. Ikai T., Tei K., Yoshida K., et al. Evaluation and treatment of shoulder subluxation in hemiplegia: relationship between subluxation and pain. Am J Phys Med Rehabil. 1998;77(5):421-426.
48. Jezernik S., Craggs M., Grill W.M., et al. Electrical stimulation for the treatment of bladder dysfunction: current status and future possibilities. Neurol Res. 2002;24(5):413-430.
49. Jones D.A., Bigland-Ritchie B., Edwards R.H. Excitation frequency and muscle fatigue: mechanical responses during voluntary and stimulated contractions. Exp Neurol. 1979;64(2):401-413.
50. Kaplan R.E., Czyrny J.J., Fung T.S., et al. Electrical foot stimulation and implications for the prevention of venous thromboembolic disease. Thromb Haemost. 2002;88(2):200-204.
51. Kimberley T.J., Lewis S.M., Auerbach E.J., et al. Electrical stimulation driving functional improvements and cortical changes in subjects with stroke. Exp Brain Res. 2004;154(4):450-460.
52. Kirsch R. Development of a neuroprosthesis for restoring arm and hand function via functional electrical stimulation following high cervical spinal cord injury. Conf Proc IEEE Eng Med Biol Soc. 2005;4:4142-4144.
53. Ko M.H., Han S.H., Park S.H., et al. Improvement of visual scanning after DC brain polarization of parietal cortex in stroke patients with spatial neglect. Neurosci Lett. 2008;448(2):171-174.
54. Kobayashi H., Ihashi K., Matsumura Y., et al. The effects of therapeutic electrical stimulation on subluxation and function of the shoulder joint after CVA. Burnaby, BC, Canada: Proceedings of the Second Annual IFESS Conference and Neural Prosthesis: Motor Systems 5; 1997.
55. Kraft G.H., Fitts S.S., Hammond M.C. Techniques to improve function of the arm and hand in chronic hemiplegia. Arch Phys Med Rehabil. 1992;73(3):220-227.
56. Kralj A., Bajd T., editors. Functional electrical stimulation: standing and walking after spinal cord injury. Boca Raton: CRC Press, 1989.
57. Lake D.A. Neuromuscular electrical stimulation. An overview and its application in the treatment of sports injuries. Sports Med. 1992;13(5):320-336.
58. Liberson W.T., Holmquest H.J., Scot D. Functional electrotherapy: stimulation of the peroneal nerve synchronized with the swing phase of gait of hemiplegic patients. Arch Phys Med Rehabil. 1961;42:101-105.
59. Lin V.W., editor. Spinal cord medicine: principles and practice. New York: Demos, 2003.
60. Lomo T., Westgaard R.H., Dahl H.A. Contractile properties of muscle: control by pattern of muscle activity in the rat. Proc R Soc Lond B Biol Sci. 1974;187(1086):99-103.
61. Loo C.K., Sachdev P., Martin D., et al. A double-blind, sham-controlled trial of transcranial direct current stimulation for the treatment of depression. Psychopharmocology. 2010;13(1):61-69.
62. MacDonagh R.P., Sun W.M., Smallwood R., et al. Control of defecation in patients with spinal injuries by stimulation of sacral anterior nerve roots. BMJ. 1990;300(6738):1494-1497.
63. Marsolais E.B., Edwards B.G. Energy costs of walking and standing with functional neuromuscular stimulation and long leg braces. Arch Phys Med Rehabil. 1988;69(4):243-249.
64. McDonagh J.C., Binder M.D., Reinking R.M., et al. Tetrapartite classification of motor units of cat tibialis posterior. J Neurophysiol. 1980;44(4):696-712.
65. McNeal D.R. 2000 years of electrical stimulation. In: Hambrecht F.T., Reswick J.B., editors. Functional electrical stimulation: applications in neural prostheses. New York: Dekker; 1977:3-35.
66. Meilink A., Hemmen B., Seelen H.A., et al. Impact of EMG-triggered neuromuscular stimulation of the wrist and finger extensors of the paretic hand after stroke: a systematic review of the literature. Clin Rehabil. 2008;22(4):291-305.
67. Merletti R., Acimovic R., Grobelnik S., et al. Electrophysiological orthosis for the upper extremity in hemiplegia: feasibility study. Arch Phys Med Rehabil. 1975;56(12):507-513.
68. Merli G.J., Crabbe S., Paluzzi R.G., et al. Etiology, incidence, and prevention of deep vein thrombosis in acute spinal cord injury. Arch Phys Med Rehabil. 1993;74(11):1199-1205.
69. Mortimer J.T., editor. Motor prostheses. Bethesda: American Physiologic Society, 1981.
70. Najenson T., Yacubovich E., Pikielni S.S. Rotator cuff injury in shoulder joints of hemiplegic patients. Scand J Rehabil Med. 1971;3:131-137.
71. Nash M.S., Bilsker S., Marcillo A.E., et al. Reversal of adaptive left ventricular atrophy following electrically-stimulated exercise training in human tetraplegics. Paraplegia. 1991;29(9):590-599.
72. Nash M.S., Montalvo B.M., Applegate B. Lower extremity blood flow and responses to occlusion ischemia differ in exercise-trained and sedentary tetraplegic persons. Arch Phys Med Rehabil. 1996;77(12):1260-1265.
73. Paillard T., Noe F., Passelergue P., et al. Electrical stimulation superimposed onto voluntary muscular contraction. Sports Med. 2005;35(11):951-966.
74. Peckham P.H., Keith M.W., Kilgore K.L., et al. Efficacy of an implanted neuroprosthesis for restoring hand grasp in tetraplegia: a multicenter study. Arch Phys Med Rehabil. 2001;82(10):1380-1388.
75. Perkash I., Martin D.E., Warner H., et al. Electroejaculation in spinal cord injury patients: simplified new equipment and technique. J Urol. 1990;143(2):305-307.
76. Petrofsky J.S., Phillips C.A. The use of functional electrical stimulation for rehabilitation of spinal cord injured patients. Cent Nerv Syst Trauma. 1984;1(1):57-74.
77. Pette D., Muller W., Leisner E., et al. Time dependent effects on contractile properties, fibre population, myosin light chains and enzymes of energy metabolism in intermittently and continuously stimulated fast twitch muscles of the rabbit. Pflugers Arch. 1976;364(2):103-112.
78. Pette D., Ramirez B.U., Muller W., et al. Influence of intermittent long-term stimulation on contractile, histochemical and metabolic properties of fibre populations in fast and slow rabbit muscles. Pflugers Arch. 1975;361(1):1-7.
79. Popovic D.B., Popovic M.B., Sinkjaer T., et al. Therapy of paretic arm in hemiplegic subjects augmented with a neural prosthesis: a cross-over study. Can J Physiol Pharmacol. 2004;82(8-9):749-756.
80. Powell J., Pandyan A.D., Granat M., et al. Electrical stimulation of wrist extensors in poststroke hemiplegia. Stroke. 1999;30(7):1384-1389.
81. Price C.I.M., Pandyan A.D. Electrical stimulation for preventing and treating post-stroke shoulder pain: a systematic Cochrane review. Clin Rehabil. 2001;15:5-19.
82. Ragnarsson K.T. Physiologic effects of functional electrical stimulation-induced exercises in spinal cord-injured individuals. Clin Orthop Relat Res. 1988;(233):53-63.
83. Ragnarsson K.T., Pollack S., O’Daniel W.Jr., et al. Clinical evaluation of computerized functional electrical stimulation after spinal cord injury: a multicenter pilot study. Arch Phys Med Rehabil. 1988;69(9):672-677.
84. Ratnasabapathy Y., Broad J., Baskett J., et al. Shoulder pain in people with a stroke: a population-based study. Clin Rehabil. 2003;17:304-311.
85. Rebersek S., Vodovnik L. Proportionally controlled functional electrical stimulation of hand. Arch Phys Med Rehabil. 1973;54(8):378-382.
86. Reswick J.B., editor. A brief history of functional electrical stimulation. New York: Intercontinental Medical Book, 1973.
87. Salmons S., Sreter F.A. Significance of impulse activity in the transformation of skeletal muscle type. Nature. 1976;263(5572):30-34.
88. Seager S.W., Halstead L.S. Fertility options and success after spinal cord injury. Urol Clin North Am. 1993;20(3):543-548.
89. Sheffler L.R., Chae J. Neuromuscular electrical stimulation in neurorehabilitation. Muscle Nerve. 2007;35(5):562-590.
90. Shimada Y., Davis R., Matsunaga T., et al. Electrical stimulation using implantable radiofrequency microstimulators to relieve pain associated with shoulder subluxation in chronic hemiplegic stroke. Neuromodulation. 2006;9(1):234-238.
91. Shribner W.J., editor. A manual of electrotherapy, ed 4, Philadelphia: Lea and Febiger, 1975.
92. Solomonow M. External control of the neuromuscular system. IEEE Trans Biomed Eng. 1984;31(12):752-763.
93. Sreter F.A., Pinter K., Jolesz F., et al. Fast to slow transformation of fast muscles in response to long-term phasic stimulation. Exp Neurol. 1982;75(1):95-102.
94. Thoma H., Gerner H., Holle J., et al. The phrenic pacemaker. Substitution of paralyzed functions in tetraplegia. ASAIO Trans. 1987;33(3):472-479.
95. Thrasher T.A., Zivanovic V., McIlroy W., et al. Rehabilitation of reaching and grasping function in severe hemiplegic patients using functional electrical stimulation therapy. Neurorehabil Neural Repair. 2008;22(6):706-714.
96. Van Kerrebroeck P.E., Koldewijn E.L., Debruyne F.M. Worldwide experience with the Finetech-Brindley sacral anterior root stimulator. Neurourol Urodyn. 1993;12(5):497-503.
97. Van Kerrebroeck P.E., Koldewijn E.L., Rosier P.F., et al. Results of the treatment of neurogenic bladder dysfunction in spinal cord injury by sacral posterior root rhizotomy and anterior sacral root stimulation. J Urol. 1996;155(4):1378-1381.
98. Vanderlinden R.G., Epstein S.W., Hyland R.H., et al. Management of chronic ventilatory insufficiency with electrical diaphragm pacing. Can J Neurol Sci. 1988;15(1):63-67.
99. Veltink P.H., Franken H.M., Van Alste J.A., et al. Modelling the optimal control of cyclical leg movements induced by functional electrical stimulation. Int J Artif Organs. 1992;15(12):746-755.
100. Wang Y., Wu Y., Chung K., et al. Functional electrical stimulation for treatment of shoulder subluxation of stroke patients [abstract]. Arch Phys Med Rehabil. 1996;77:977.
101. Wang Y.H., Chiang H.S., Wu C.H., et al. Electroejaculation in spinal cord injured males. J Formos Med Assoc. 1992;91(4):413-418.
102. Wanklyn P., Forster A., Young J. Hemiplegic shoulder pain (HSP): natural history and investigation of associated features. Disabil Rehabil. 1996;18(10):497-501.
103. Wood J.M., Evans P.E.III, Schallreuter K.U., et al. A multicenter study on the use of pulsed low-intensity direct current for healing chronic stage II and stage III decubitus ulcers. Arch Dermatol. 1993;129(8):999-1009.
104. Wuolle K.S., Bryden A.M., Peckham P.H., et al. Satisfaction with upper-extremity surgery in individuals with tetraplegia. Arch Phys Med Rehabil. 2003;84(8):1145-1149.
105. Yu D.T., Chae J., Walker M.E., et al. Pain associated with percutaneous versus surface neuromuscular electrical stimulation for treating shoulder dysfunction in hemiplegia [abstract]. Arch Phys Med Rehabil. 1998;79(9):1151.
106. Yu D.T., Chae J., Walker M.E., et al. Intramuscular neuromuscular electric stimulation for poststroke shoulder pain: a multicenter randomized clinical trial. Arch Phys Med Rehabil. 2004;85(5):695-704.
107. Yu D.T., Friedman A.S., Rosenfeld E.L. Electrical stimulation for treating chronic post-stroke shoulder pain using a fully-implanted microstimulator with internal battery: a case report. Am J Phys Med Rehabil. 2010;89(5):423-428.
108. Yu D.T., Kirsch R.F., Bryden A.M., et al. A neuroprosthesis for high tetraplegia. J Spinal Cord Med. 2001;24(2):109-113.