7 Electrical Signaling by Neurons
Neurons share many properties with other cells, including their complement of organelles, an electrical potential across their surface membranes, and an ability to secrete various substances. What distinguishes neurons is the ways in which they have adapted these common properties for their roles as information-processing and information-conveying devices. For example, neurons have specialized configurations of organelles to support their extended anatomy (see Chapter 1). Similarly, they have adapted secretory processes to communicate with each other rapidly and precisely (see Chapter 8), and individual neurons use alterations in their membrane potentials to convey information between their various parts (this chapter).
A Lipid/Protein Membrane Separates Intracellular and Extracellular Fluids
The surface membrane of neurons, like that of other cells, is a double layer of lipid molecules with proteins embedded in it. Just as oil and water don’t mix very well, the lipid part of the membrane is impermeable to water-soluble substances, prominently including the ions whose movement is central to electrical signaling. Subsets of the proteins embedded in the lipid bilayer are specialized to allow or even facilitate the movement of ions across the membrane. Some are hollow ion channels with a central, aqueous pore whose size and charged lining determines which kinds of ions can pass through; others are ion pumps that use metabolic energy to move specific ions across the membrane.
The Resting Membrane Potential of Typical Neurons Is Heavily Influenced, but Not Completely Determined, by the Potassium Concentration Gradient
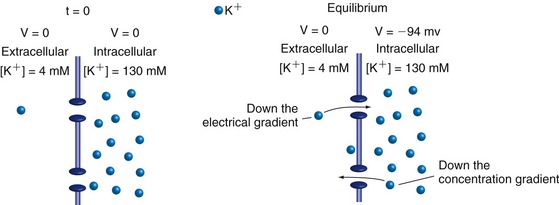
The K+ concentration inside neurons is much higher than that outside (because of ion pumps described a little later), and their surface membranes contain numerous K+ channels that are usually in the open state. If these were the only ion channels in the membrane, the following scenario would develop. The concentration gradient would drive K+ ions outward through the channels, creating a deficit of positive charges inside the cell. Opposite charges attract each other, so after a very small number of K+ ions had left, the resulting intracellular negativity would pull K+ ions back into the cell. At some point the concentration gradient and the electrical gradient would exactly counterbalance each other, and K+ ions would enter and leave at equal rates (Fig. 7-1). The system would be in equilibrium, with no net movement of K+ in either direction and no energy requirement to stay that way. The transmembrane potential at which this occurs, the potassium equilibrium potential or VK, is a logarithmic function of the concentration gradient and is specified by the Nernst equation (THB6 Appendix 7B, p. 176). At body temperature, a tenfold change in the K+ concentration on one side of the membrane causes a 62 mv change in VK.
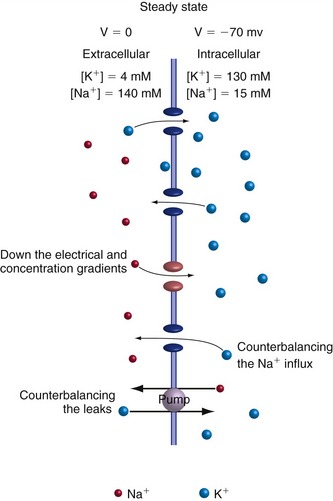
In reality, however, the K+ channels are not 100% selective for K+, and not all the channels in a resting membrane are K+ channels. As a result, Na+ ions are also able to flow across the membrane. The Na+ concentration outside neurons is much higher than that inside, so Na+ moves into the cell because of both the concentration gradient and the intracellular negativity. The result is competing ion flows (Fig. 7-2)—inward Na+ flow trying to move the membrane potential to VNa and outward K+ flow trying to move the membrane potential to VK—and a steady state is reached at a potential somewhere between VNa and VK. Just where the steady state is reached is determined by which ion the membrane is more permeable to (THB6 Appendix 7B, p. 176). Hence, VNa and VK are boundary values for the membrane potential, and changes in membrane permeability to Na+ or K+ will cause the membrane potential to move closer to one or the other; this is the usual basis for electrical signaling by neurons. The membranes of most neurons most of the time are much more permeable to K+ than to Na+, so the resting membrane potential is close to VK.
Concentration Gradients Are Maintained by Membrane Proteins That Pump Ions
A major difference between the theoretical K+-selective membrane and the real-life membrane permeable to both K+ and Na+ is that energy is required to maintain the concentration gradients across real-life membranes. If K+ continually flowed out and Na+ continually flowed in, the ionic concentration gradients across the membrane would fade away and the membrane potential would decline toward zero. This is averted by another class of membrane-spanning proteins, ion pumps, that use the energy liberated by hydrolysis of ATP to pump ions in the opposite direction and so maintain concentration gradients. The best known example is the Na+/K+ exchange pump that pumps Na+ out and K+ in, compensating for the steady-state leakage through channels.
Inputs to Neurons Cause Slow, Local Potential Changes
Inputs to neurons, whether at synapses (see Chapter 8) or in the receptive portion of sensory receptors (see Chapter 9), cause ion channels to open or close for milliseconds or longer. As a result, current flow through the channels increases or decreases and the membrane potential at the input site moves toward VNa (depolarization) or VK (hyperpolarization). The current flows and voltage changes spread passively through nearby parts of the neuron in a way that depends on the electrical properties of those parts.
Action Potentials Convey Information over Long Distances
Length constants are not very long, and graded electrical signals spreading passively in even the largest axons and dendrites would die out in a few millimeters. Neurons commonly need to convey messages over distances equal to hundreds or thousands of length constants and use action potentials (also called spikes or nerve impulses) to do so. Action potentials are fundamentally different from graded postsynaptic potentials and receptor potentials in several ways: They are large, brief, and always depolarizing; have a threshold; and are propagated actively along axons rather than dying out.
Opening and Closing of Voltage-Gated Sodium and Potassium Channels Underlies the Action Potential
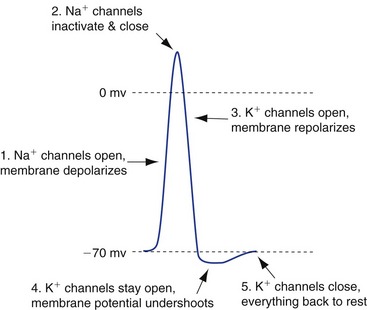
Action potentials are produced by the coordinated activity of special voltage-gated Na+ channels and K+ channels (Fig. 7-3), and areas of membrane that have sufficient quantities of these channels are electrically excitable (i.e., are able to produce action potentials). Depolarization of such an area of membrane (e.g., by depolarizing current flow from a nearby synapse) causes the voltage-gated Na+ channels to begin opening, which depolarizes the membrane even more. If enough channels are opened by the initial depolarization (i.e., the depolarization reaches threshold), this Na+ channel opening builds on itself explosively until most of the channels are open, Na+ permeability is greater than K+ permeability, and the membrane potential moves close to VNa. This depolarization is short-lived, however, because the Na+ channels spontaneously enter a closed, inactivated state and cannot reopen until they are “reset” (deinactivated) by having the membrane potential return to near the resting level.
Action Potentials Are Propagated without Decrement along Axons
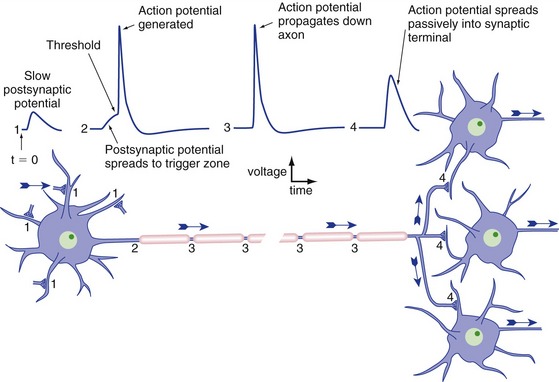
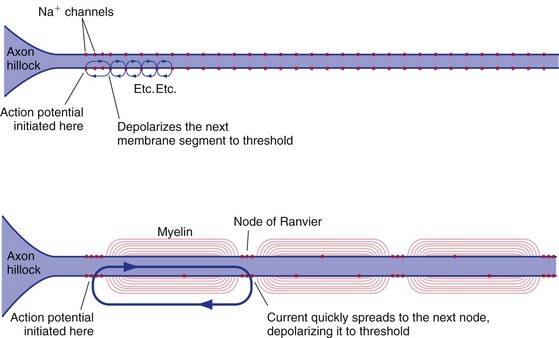
Neurons typically have a trigger zone with a low threshold, near where the axon emerges from the cell body, where action potentials are initiated (Fig. 7-4). Inputs to the dendrites and cell body sum temporally and spatially, spread passively (electrotonically) to the trigger zone, and initiate one or more action potentials if threshold is reached. Once initiated, an action potential spreads passively both down the axon and back into the cell body (THB6 Figure 7-13, p. 162). Cell bodies usually lack sufficient numbers of voltage-gated Na+ channels to make action potentials, so that’s the end of the story in that direction. The axon, on the other hand, has lots of them, so spread of the action potential in that direction brings neighboring regions to threshold and the action potential propagates down the axon. Once the action potential reaches the end of the axon, it cannot turn around and propagate back toward the cell body because the regions just traversed are refractory.
Unmyelinated axons have voltage-gated Na+ channels distributed all along them, and the action potential spreads down these axons by having each successive area of membrane depolarize to threshold (Fig. 7-5). It takes time for the channels to open, so the conduction velocity of action potentials in unmyelinated axons is limited by how far down the axon a depolarizing current spreads (i.e., by the length constant of the axon). Hence, the larger the diameter of an unmyelinated axon, the faster the conduction velocity. Even so, our largest unmyelinated axons are pretty slow, conducting at only about 2.5 m/sec. Myelinated axons conduct much more rapidly because the insulating myelin internodes prevent current from leaking out. Instead, it flows very rapidly from one node of Ranvier to the next. The voltage-gated Na+ channels of myelinated axons are concentrated at the nodes, so each successive node is depolarized to threshold and its channels open (the time-consuming step). Hence, the action potential skips from one node to the next (saltatory conduction), and conduction can be as rapid as 100 m/sec. The longer length constant of large-diameter axons allows their nodes to be spaced farther apart, so here too larger axons conduct more rapidly.