28. Eisenmenger Syndrome
Definition
Eisenmenger syndrome (ES) is the presence of a bidirectional or exclusively right-to-left cardiac shunt in conjunction with pulmonary hypertension and a patent heart defect.
Incidence
Congenital heart defects occur in approximately 1% of all live births. Out of this group, approximately 8% overall, and 11% of those with a left-to-right shunt, will eventually develop ES.
Etiology
ES is a rare disorder that affects both the respiratory and cardiac systems. This disorder is very insidious and sinister. Fulminate signs and symptoms of the disorder do not typically manifest until the third, fourth, or fifth decade of life.
The patient with ES has a congenital heart defect that has never been surgically repaired; for example, an atrial or ventricular septal defect. The blood shunted from the left side of the heart through the right side increases the stroke volume of the right side of the heart. The increased stroke volume produces shear forces within the pulmonary microvasculature. The shear forces combine with the increased stroke volume to produce irreversible damage to the walls of the pulmonary vessels. The damage to these vessel walls scars the vessels, making them less compliant.
Eventually the pulmonary vascular resistance (PVR) becomes equal to and finally exceeds the systemic vascular resistance (SVR). The shunt becomes bidirectional and eventually reverses to an exclusively right-to-left shunt, mixing deoxygenated blood with oxygenated blood, resulting in chronic hypoxemia.
Signs and Symptoms
• Angina
• Clubbing of digits
• Cyanosis
• Dyspnea on exertion
• Fatigue
• Hemoptysis
• Palpitations
• Polycythemia
• Syncope
Medical Management
The primary focus of current medical management of ES is on reducing the PVR and, by extension, the right-to-left shunting and cyanosis. Medications and treatments that have detrimental effects must be avoided. For example, calcium channel–blocking agents must be avoided; even though these medications produce reductions in the PVR, they also produce unacceptably greater reductions in the SVR, effectively exacerbating the right-to-left shunting.
Sildenafil and l-arginine are effective in decreasing or reducing the degree of pulmonary hypertension. l-Arginine is converted to nitric oxide (NO) in vivo, which activates guanylate cyclase. Guanylate cyclase increases the level of cyclic guanosine monophosphate (cGMP), which is instrumental in relaxing the smooth muscles of the pulmonary blood vessels. Sildenafil inhibits the degradation of cGMP, thus fostering greater cGMP availability. Sildenafil works synergistically with inhaled NO to enhance the effects of both in the NO-cGMP pathway. Combining sildenafil with l-arginine enhances the NO-cGMP pathway in an effort to effectively reduce the PVR.
Two other medications are used to treat ES patients: epoprostenol and bosentan. Epoprostenol is a prostacyclin analogue that has two primary pharmacologic actions: direct vasodilation of pulmonary and systemic vascular beds and inhibition of platelet aggregation. It also contributes to bronchodilation. This medication must be administered via a permanent central venous access.
Bosentan is an endothelin antagonist. It binds two endothelin-1 receptors, ET A and ET B, found in the endothelium and vascular smooth muscles. By binding to both receptors, the PVR may be reduced by as much as 25%. This reduction in the PVR by bosentan improves both the functional status and oxygenation of the patient with ES.
Finally, the patient with ES eventually develops polycythemia in an effort to counteract the chronic hypoxemia from the right-to-left shunt. Hematocrit values of 60% to 75% are not unheard of with these patients. The patient who manifests symptoms such as headaches, hemoptysis, or increased dyspnea, is treated by therapeutic phlebotomy. The phlebotomy must be carefully and strictly controlled and must be accompanied by a careful equal-volume fluid replacement of the blood that is removed in order to avoid decreasing the SVR and exacerbating the right-to-left shunt. The goal of the therapeutic phlebotomy is to reduce the red cell mass and viscosity of the blood while maintaining the SVR throughout the procedure. Therapeutic phlebotomy typically consists of removing approximately 500 mL of blood with volume-for-volume replacement with salt-free albumin, fresh frozen plasma, dextran, or isotonic saline.
Complications
• Azotemia
• Cerebrovascular accident (CVA)
• Cholelithiasis
• Decreased glomerular filtration rate
• Gout
• Hemoptysis
• Hypertrophic osteoarthropathy
• Nephrotic syndrome
• Polycythemia
• Pulmonary vasculature friability
• Renal dysfunction
• Sudden death
Anesthesia Implications
The patient with ES presents a unique challenge. The right-to-left shunt already present is easily exacerbated. This patient is very sick, often debilitated. The patient with ES is typically very anxious when faced with the possibility of surgery and anesthesia. The initial step in relieving at least a part of this anxiety is the appearance of a poised, calm, unhurried anesthesia professional equipped and ready to deal with any situation that may be presented. The attempt to obtain intravenous access will be closely scrutinized by the patient as well as family member(s). It is critically important that all air must be flushed from the administration tubing with the same degree of care and concern that would be given to the IV access of a neonate. Even the smallest air bubble within the pulmonary microvasculature of the patient with ES may become a significant embolism. The chief anesthetic concern mirrors the chief concern in the medical management of ES: avoiding worsening of the right-to-left shunt. The anesthetist must strive to minimize, if not avoid outright, decreases in the SVR. Whether regional or general anesthesia is the technique chosen, very nearly every anesthesia medication—inhaled volatiles, intravenous, or local anesthetics—may result in decreases in the patient’s SVR.
Induction of general anesthesia can best be achieved using ketamine rather than thiopental, propofol, or etomidate, which result in unacceptably large drops in the SVR. Intubation can be facilitated using a nondepolarizing muscle relaxant (NDMR). However, if the patient is receiving epoprostenol and/or bosentan, metabolism of steroid-based NDMRs, such as rocuronium, may be prolonged. The newest volatile agents, desflurane and sevoflurane, are the more appropriate choice because of the rapidity with which the depth of anesthesia can be altered with either agent. Of these two agents, desflurane may be more supportive of the SVR because of the mild sympathetic stimulation it can produce.
The goal of mechanical ventilation with the patient with ES is more to reduce the carbon dioxide (CO 2) concentration than to increase levels of oxygen saturation. Elevated CO 2 concentration results in acidosis, which increases the PVR and exacerbates the right-to-left shunting.
Pain control is vitally important for the patient with ES, both perioperatively and postoperatively. Pain produces increases in PVR, SVR, and cardiac oxygen requirements. Changes in any one of these parameters may be greatly magnified in the patient with ES.
An alternative to general anesthesia is total intravenous anesthesia (TIVA). Induction with ketamine remains the best choice and may be accompanied by a remifentanil infusion. Direct laryngoscopy should be facilitated using an NDMR as described earlier. After induction and intubation, an infusion of propofol may be initiated. The infusion rate should be based on, and adjusted by, the patient’s blood pressure and the method of awareness monitoring of the anesthetist’s or facility’s choice.
Regional anesthesia is a viable alternative to general anesthesia. Once again, the greatest concern centers on maintaining the SVR at levels as nearly normal as possible. Adequate fluid loading before instillation of the regional anesthetic is vitally important. Epidural anesthesia or continuous subarachnoid infusion are both effective. Both are superior to the usual single-injection method of subarachnoid blockade. Both continuous subarachnoid infusion and epidural anesthesia allow the onset and level of the blockade to be adjusted more gradually, which allows the patient to adjust physiologically and the anesthetist to aid that adjustment by administering additional fluids and/or appropriate vasoactive medication as needed.
Patient monitoring is necessarily more extensive with the patient with ES. Central venous pressure (CVP) and radial artery blood pressure monitoring are integral parameters for estimating the patient’s SVR and PVR. Pulmonary artery balloon flotation catheter use is somewhat controversial owing to the potential difficulty in achieving proper terminal positioning and the inconsistent measurements that may be obtained from dilated, relatively noncompliant pulmonary vessels. Transesophageal echocardiography is particularly pertinent in the patient with ES undergoing large and/or lengthy surgical procedures, where greater fluid shifts and/or administrations are anticipated. Also, owing to the precarious fluid balance of the patient with ES, the period without oral intake, particularly clear liquids, should be limited—possibly to no more than 4 hours. Once again, the careful approach is similar to that taken with a neonate being prepared for surgery.
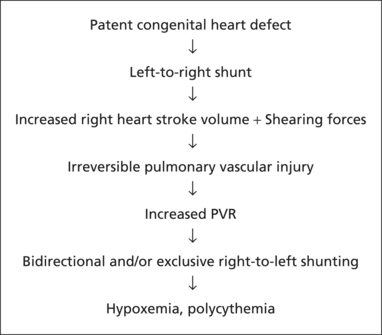 |
| Pathophysiology of Eisenmenger Syndrome. |
Finally, blood loss is always a concern for the anesthetist. In the patient with ES, surgical blood loss must be strictly assessed. The blood lost in the surgical field must be treated as if the patient is undergoing a therapeutic phlebotomy; that is, blood must be replaced volume-for-volume with one of the appropriate solutions noted earlier. The anesthetist must be vigilant and act quickly in anticipation because the blood lost from the surgical field does not occur in the relatively controlled manner of a therapeutic phlebotomy.