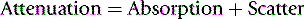Chapter 10 Effects of radiation
 A beam of X-ray photons is gradually attenuated as it passes through matter by being absorbed and scattered.
A beam of X-ray photons is gradually attenuated as it passes through matter by being absorbed and scattered. The most important interactions that result in attenuation are photoelectric absorption and Compton scatter.
The most important interactions that result in attenuation are photoelectric absorption and Compton scatter. Photoelectric absorption is more likely to occur in dense materials with atoms of high proton number.
Photoelectric absorption is more likely to occur in dense materials with atoms of high proton number. Photoelectric absorption is responsible for differential absorption in the body’s tissues that results in a radiographic image.
Photoelectric absorption is responsible for differential absorption in the body’s tissues that results in a radiographic image. Compton scatter occurs alongside photoelectric absorption and is the dominant process above a setting of 75 kV.
Compton scatter occurs alongside photoelectric absorption and is the dominant process above a setting of 75 kV. Attenuation in the body’s tissues with the transfer of energy and subsequent ionisation may result in deterministic or stochastic biological effects.
Attenuation in the body’s tissues with the transfer of energy and subsequent ionisation may result in deterministic or stochastic biological effects. Radiation protection regulations aim to reduce the risk of stochastic effects and avoid deterministic effects.
Radiation protection regulations aim to reduce the risk of stochastic effects and avoid deterministic effects. Effective dose measured in sieverts (Sv) takes into account different types of radiation and their damaging potential on different tissues and organs in the body.
Effective dose measured in sieverts (Sv) takes into account different types of radiation and their damaging potential on different tissues and organs in the body. X-ray photons produce fluorescence in phosphors, a property utilised in some image recording systems.
X-ray photons produce fluorescence in phosphors, a property utilised in some image recording systems.ATTENUATION
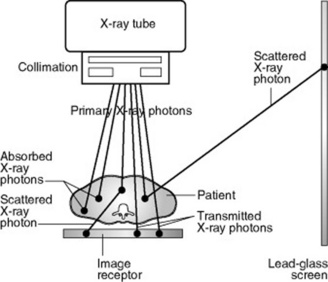
Attenuation is partly due to some X-ray photons being totally absorbed and partly to the energy of some X-ray photons being partially absorbed while the remainder is scattered in various directions (Fig. 10.1). Some X-ray photons are transmitted through the material unchanged, without interacting with any atoms.
ATTENUATION AND THICKNESS OF MATERIAL
The number of X-ray photons transmitted compared to the number attenuated in any particular type of material depends on the thickness of that material. In general, the thicker the material, the greater the attenuation. However, it is not a linear relationship where the same numbers of X-ray photons are attenuated in an equal thickness of material, but an equal percentage is attenuated in equal thickness. For example, 20% of photons may be attenuated in the first centimetre of material, then 20% of what is left in the second centimetre, and 20% of the remainder in the third centimetre, etc. This is called an exponential relationship and the percentage attenuated in each thickness is known as the linear attenuation coefficient (LAC or μ) for the specific material. This relationship is used in practice during quality control checks on X-ray equipment to measure the half value thickness/layer (HVT or HVL) of an X-ray unit. The measurement gives the thickness of aluminium that will attenuate 50% of the X-ray photons at a specific kV setting. This gives an indication of the penetrating power of the beam: the thicker the aluminium required to attenuate half of the beam, the more penetrating it is and it can be related (using published tables) to the total filtration present in the beam (see pp. 100, 107).
PHOTOELECTRIC ABSORPTION
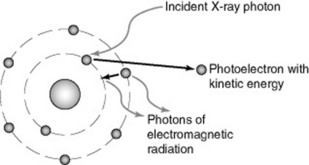
Photoelectric (PE) absorption occurs when an X-ray photon interacts with a bound electron, usually in the inner shell of an atom, when its energy exceeds the binding energy of the electron (Fig. 10.2). The atom may be in the patient (an atom of calcium in bone, for example) or it might be an atom of carbon in the carbon-fibre tabletop; or an atom of lead in the lead-glass screen.
THE IMPLICATIONS OF PHOTOELECTRIC ABSORPTION IN PRACTICE
The main implications to consider are:
The radiographic image
Bone has a fairly high density and contains atoms of calcium and phosphorus, giving an effective proton number (Zeff) of approximately 12. Soft tissues, such as muscle and fat, are lower in density and contain atoms of lower proton number (particularly carbon, hydrogen and oxygen), giving an effective proton number of approximately 7. Air is primarily nitrogen and oxygen, giving a similar effective proton number to soft tissue, but has very low density (Table 10.1).
| Material | Density (kg m−3) | Effective proton number |
|---|---|---|
| Bone | 1700 | 12.3 |
| Soft tissue (muscle) | 1000 | 7.6 |
| Soft tissue (fat) | 900 | 6.5 |
| Air | 1 | 7.8 |
All of these elements found in tissue have low binding energies, so typical mean X-ray photon energies of 25 keV used in radiographic imaging (corresponding to a setting of 75 kV; see p. 113) are likely to undergo PE absorption. If all these elements attenuated X-ray photons equally, we would not be able to tell the difference between bone and muscle or bone and air on an X-ray image. But the bone tissue with higher proton number and higher density will experience relatively more PE absorptions – at least eight times more than soft tissue – and air will barely experience any attenuation due to its very low density, leading to what is called differential absorption.
X-ray tube filtration
In atoms such as aluminium (Z = 13), with low K shell binding energies, low X-ray photon energies are preferentially absorbed. The quality of the X-ray beam produced from an X-ray tube is increased by attenuating the low energy X-ray photons in a filter made of aluminium (see p. 100).
Absorption edges
Photoelectric absorption is more likely to occur with a bound electron whose binding energy is just below the X-ray photon’s energy, so there will be situations where some bound electrons have binding energies higher than those of the interacting X-ray photons. These electrons cannot contribute to the attenuation of the beam by PE absorption. For example, in lead the binding energy of the K shell electrons is 88 keV and of the L shell 15 keV, so a beam produced at 80 kV will not contain any X-ray photons of sufficient energy to interact with the K shell electrons, only those of the L shell and beyond. If the kV is increased to 90, the beam will contain photons of 90 keV able to interact with K shell electrons. This leads to a large increase in photoelectric absorption of the X-ray photons with energy greater than 88 keV and is known as an absorption edge. Photons with slightly lower energies than 88 keV are much less likely to be attenuated and therefore more likely to be transmitted. This means shielding materials using lead may be slightly less efficient at attenuating photons of this energy. Some manufacturers promote the use of protective aprons made of a composite of materials to give increased attenuation below the 88 keV absorption edge for lead.
COMPTON SCATTER
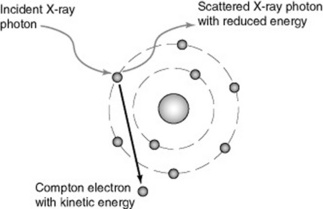
The X-ray photon transfers some of its energy to the electron and retains the remainder: the photon may retain virtually all of its initial energy and be very slightly deviated or scattered from its track. This is called forward scatter and occurs more frequently with photons of higher energy. Alternatively, lower energy X-ray photons may be scattered out to the side retaining less energy (side scatter) or backwards retaining least energy (backscatter). Scattered X-ray photons always have less energy than the original photon (Fig. 10.3). In all cases, an electron escapes from the atom as a Compton electron, carrying the transferred energy as kinetic energy. Electrons will rearrange in the shells with the emission of electromagnetic radiation as before.
FACTORS AFFECTING COMPTON SCATTER
Compton scatter is more likely to occur where the X-ray photon energy is much higher than the binding energy of the electron with which it interacts. It dominates for X-ray photon energies greater than 25 keV. As photon energy increases, the proportion of forward scatter increases whilst back and side scatter decrease. Compton scatter is also more likely to occur in materials containing a higher proportion of hydrogen, such as water, a major component of the body’s soft tissues.
THE IMPLICATIONS OF COMPTON SCATTER
BIOLOGICAL EFFECTS OF X-RAY PHOTONS
IONISATION DAMAGE RESULTING IN CELL DEATH: DETERMINISTIC EFFECTS
Sometimes, particularly if both DNA strands are broken, the damage may not be repairable. In this case, the cell will die either immediately, or when the cell attempts division, or of old age, which may be days or even years later. The death of a single cell among millions in a particular tissue does not cause any signs or symptoms. However, higher doses of radiation that might occur following certain lengthy procedures using fluoroscopy, such as angiography/angioplasty or lithotripsy, could damage a significant number of cells and lead to an observable clinical effect.
RADIATION PROTECTION
In the UK, the Health and Safety Executive enforces regulations relating to ionising radiation (see p. 13). These are in two parts: the current Ionising Radiations Regulations 1999 apply to employers using ionising radiation, such as hospital Trusts, and requires them to protect all employees by ensuring any exposures are as low as reasonably achievable and do not exceed specified dose limits. This is achieved by ensuring equipment, shielding and working practices are safe and by monitoring effective doses received by employees during their work.
HOW DO WE QUANTIFY ‘RADIATION DOSE’?
When we use the terms ‘dose of ionising radiation’ or just ‘radiation dose’, we mean how much energy has been deposited in the organs and tissues of the body following attenuation of a beam of ionising radiation. This is called the absorbed dose and is measured in units of gray (Gy), where 1 Gy equates to 1 joule of energy deposited per kilogram mass.
Effective doses for the average-sized person can be approximated for radiographic imaging of all parts of the body and can be compared to the effective dose received from natural background radiation over a period of time. The average background dose in the UK is 2.2 mSv per year (Table 10.2).
Table 10.2 Typical effective doses for diagnostic medical exposures2
| X-ray examination | Typical effective doses (mSv) | Equivalent period of natural background radiation |
|---|---|---|
| Limbs and joints (except hip) | <0.01 | <1.5 days |
| Teeth (single bitewing) | <0.01 | <1.5 days |
| PA chest | 0.02 | 3 days |
| Skull | 0.07 | 11 days |
| Cervical spine | 0.08 | 2 weeks |
| Hip | 0.3 | 7 weeks |
| Thoracic spine | 0.7 | 4 months |
| Pelvis/abdomen | 0.7 | 4 months |
| Lumbar spine | 1.3 | 7 months |
| Barium swallow | 1.5 | 8 months |
| IVU | 2.5 | 14 months |
| Barium meal | 3.0 | 16 months |
| Barium enema | 7.0 | 3.2 years |
| CT head | 2.0 | 1 year |
| CT chest | 8.0 | 3.6 years |
| CT abdomen/pelvis | 10.0 | 4.5 years |
CT, computed tomography; PA, posteroanterior; IVU, intravenous urogram
In practice, effective dose is a complicated calculation for the individual patient, so if the X-ray set is fitted with a DAP (dose–area product) ionisation chamber (see pp. 126–127), a DAP reading can be recorded. This is the radiation dose to air multiplied by the area exposed, giving a reading in Gy cm2. Again, it is not easily related to effective dose, but the accumulated DAP reading can be recorded for each examination and used to compare relative exposures. If other factors such as source–image distance (SID) and kV are recorded, effective dose can be calculated retrospectively if required.
FLUORESCENCE
Some materials, called phosphors, convert X-ray photons into light photons. This means they glow when exposed to an X-ray beam. As the energy of an X-ray photon is much greater than that of a light photon, one X-ray photon may be converted into hundreds of light photons. The colour of the light emitted depends on the type of phosphor. In certain phosphors this effect is instantaneous and is known as fluorescence. Fluorescent phosphors such as gadolinium oxysulphide are utilised in the intensifying screens used in conventional film-screen imaging systems (see p. 140).
Ball J, Moore AD. Essential physics for radiographers, 3rd edn. Oxford: Blackwell Science, 1997.
SC Bushong. Radiologic science for technologists, 8th edn. St Louis: Mosby. 2004.
Be aware that subsequent chapters refer to radiation protection legislation in the United States and although the principles are the same anywhere in the world, the regulations are not!
The Ionising Radiations Regulations 1999 (SI No. 3232). London: HMSO.
The Ionising Radiations (Medical Exposure) Regulations 2000 (SI No. 1059) London: HMSO.