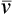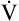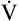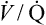Effects of Aging on the Cardiopulmonary System
After reading this chapter you will be able to:
• Explain why health care of elderly people has taken on increasingly greater importance in the twenty-first century
• Explain why older people are more prone to falls and how this phenomenon is related to pulmonary health
• Explain why elderly people are more predisposed to infections than younger people
• Describe the nature of major age-related physiological changes of the respiratory and cardiovascular systems
• Describe the nature of the body’s compensatory responses to age-related cardiopulmonary changes
• Explain why the PaO2 of a healthy elderly person is lower than the PaO2 of a younger person when both individuals are breathing room air
• Explain why an elderly person has a lower maximum cardiac output and oxygen consumption during exercise than a younger person
• Explain the major benefits of an exercise program for elderly individuals
Implications of an Aging Population
People are increasingly surviving to older ages than they did in previous generations, owing to modern advances in medicine that affect old age mortality and because of improved diet and exercise habits. In addition, with the aging of baby boomers (individuals born in the post World War II era, generally between 1946 and 1964), increasingly greater numbers of people in the United States are entering the 65 years and older age bracket. According to the U.S. Census Bureau, the elderly population (≥65 years old) increased more than 10-fold between 1900 and 2000 (from 3.1 million to 35 million), whereas the general population only tripled in the same time span.1 Elderly people account for about 12% of the U.S. population. In 1900, the median age (half of the population younger and half older) was 23 years; in 2000, the median age was 35 years. The elderly population as a whole is aging; the fastest growing age group in the United States as well as the world is the oldest-old—80 years old and older.1,2 It is predicted that by 2050, the number of people in the world 60 years old or older will exceed, for the first time in history, the number of people younger than age 15.2
The aging population has significantly influenced the focus of health care.3 Health of older people typically worsens with increasing age because of increased vulnerability to accidents and disease. The demand for long-term health care facilities and home-based health care is ever increasing.
The clinician must be able to distinguish between the effects of normal aging and the effects of disease to treat elderly patients effectively; effects of aging and disease are often combined. It is difficult to sort and assess the subtle effects of other factors such as smoking history, environmental pollution, and physical activity. Separating the effects of normal aging from the effects of disease is complicated because age increases susceptibility to disease. Increased propensity for developing disease, especially more severe disease, is the most important physiological change of aging.3 The lung function of a healthy 70-year-old is about half that of a 30-year-old; renal function declines by about the same amount. Although decreased reserve capacity does not affect activities of daily living, it greatly affects the ability of older people to recover from severe illnesses.3
Neuromuscular systems that maintain upright posture become less effective with age; this leads to postural instability and a predisposition to falls, which are more likely to produce fractures because of bone mass loss and greater bone fragility.3 Danger of falling is exacerbated by sensory impairments such as poor eyesight, hearing loss, and balance disorders. In addition, loss of muscular mass and strength, arthritis, orthopedic problems, blood pressure instability, and medications contribute to the tendency to fall. Bone fractures lead to immobility, and when combined with an impaired gag reflex and a less effective mucociliary clearance mechanism, the susceptibility to respiratory infection and pneumonia increases.3
Effects of Aging on the Respiratory System
Structural Changes
Aging changes the compliance of the lungs and chest wall. The chest wall progressively stiffens as the thoracic cage joints and attachments calcify and become less mobile.3,4 With advancing age, vertebral column deformities produce some degree of kyphoscoliosis (abnormal front-to-back and side-to-side curvature of the spine).5 Simultaneously, intercostal and diaphragmatic muscles atrophy, losing strength and endurance. These changes combine to decrease upper and lower rib cage and abdominal expansion.5,6
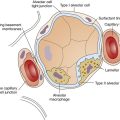
In contrast to the chest wall, the lung becomes more compliant with age as it loses elastic tissue and recoil force. As a result, the chest wall expands slightly, which mildly increases lung volumes at end-tidal exhalation (functional residual capacity [FRC]) and at maximal effort exhalation (residual volume [RV]). These changes lead to slight hyperinflation and a mildly increased anterior-posterior chest diameter (barrel chest).3,4
Loss of elastic recoil forces also allows small noncartilaginous airways to remain at larger diameters after normal expiration, increasing the anatomical dead space volume and the dead space-to-tidal volume ratio (VD/VT); in addition, alveolar ducts and alveoli become wider, shallower, and less complex in shape, resulting in a marked decrease in gas-exchange surface area.4 These changes are suggestive of emphysema—hence the misleading term senile emphysema. Anatomical changes are much more extensive and debilitating in clinically significant pulmonary emphysema than they are in the healthy aged lung.
Functional Changes
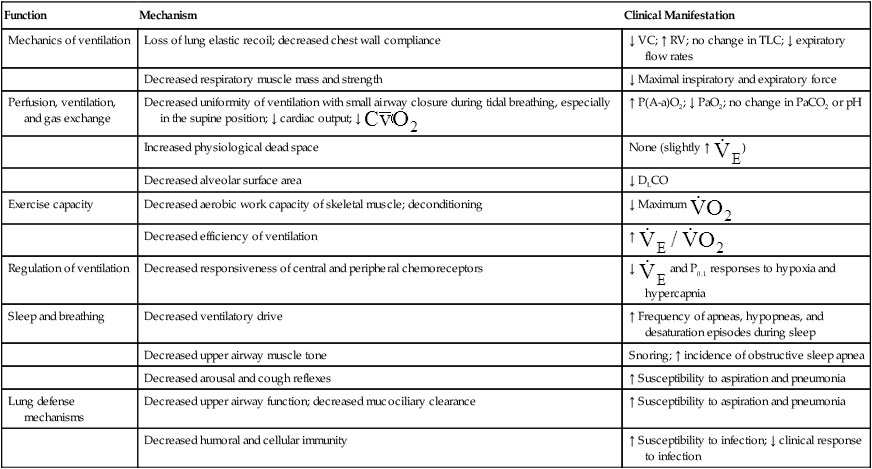
Table 24-1 summarizes functional respiratory changes with aging. Loss of elastic tissue with subsequent loss of alveolar complexity decreases gas-exchange surface area and diffusion capacity. Small noncartilaginous airways become more prone to compression and collapse during forced expiration as they lose the tethering effect of surrounding elastic fibers. This tendency toward collapse decreases maximum expiratory flow rates, especially in effort-independent portions of the forced vital capacity (FVC). Expiratory flow limitation is the most consistent effect of aging during moderate and heavy exercise.4 As the lung ages, total lung capacity remains relatively constant, while residual volume increases; this causes vital capacity to decrease by about 30 mL per year after age 30 years.7
TABLE 24-1
Changes in Respiratory Function with Aging
| Function | Mechanism | Clinical Manifestation |
| Mechanics of ventilation | Loss of lung elastic recoil; decreased chest wall compliance | ↓ VC; ↑ RV; no change in TLC; ↓ expiratory flow rates |
| Decreased respiratory muscle mass and strength | ↓ Maximal inspiratory and expiratory force | |
| Perfusion, ventilation, and gas exchange | Decreased uniformity of ventilation with small airway closure during tidal breathing, especially in the supine position; ↓ cardiac output; ↓ < ?xml:namespace prefix = "mml" />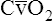 |
↑ P(A-a)O2; ↓ PaO2; no change in PaCO2 or pH |
| Increased physiological dead space | None (slightly ↑ 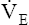 ) ) |
|
| Decreased alveolar surface area | ↓ DLCO | |
| Exercise capacity | Decreased aerobic work capacity of skeletal muscle; deconditioning | ↓ Maximum 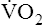 |
| Decreased efficiency of ventilation | ↑ 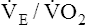 |
|
| Regulation of ventilation | Decreased responsiveness of central and peripheral chemoreceptors | ↓ 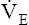 and P0.1 responses to hypoxia and hypercapnia and P0.1 responses to hypoxia and hypercapnia |
| Sleep and breathing | Decreased ventilatory drive | ↑ Frequency of apneas, hypopneas, and desaturation episodes during sleep |
| Decreased upper airway muscle tone | Snoring; ↑ incidence of obstructive sleep apnea | |
| Decreased arousal and cough reflexes | ↑ Susceptibility to aspiration and pneumonia | |
| Lung defense mechanisms | Decreased upper airway function; decreased mucociliary clearance | ↑ Susceptibility to aspiration and pneumonia |
| Decreased humoral and cellular immunity | ↑ Susceptibility to infection; ↓ clinical response to infection |
Modified from Pierson DJ, Kacmarek RM: Foundations of respiratory care, New York, 1992, Churchill Livingstone.
The volume expired in the first second of the FVC (forced expiratory volume in 1 second [FEV1]) decreases progressively and linearly with age, paralleling the decline in FVC so that FEV1/FVC changes only to a small degree in a healthy elderly person.4,8 FEV1 decreases about 30 mL per year after age 25 years.5 Apparently, habitual physical exercise and high aerobic capacity do not slow this normal deterioration in lung function9; however, cigarette smoking accelerates it. Smoking cessation brings the rate of FEV1 decline back to a more normal rate.10
In a clinical study comparing three healthy older age groups, maximal rib cage expansion, abdominal expansion, FVC, and FEV1 were measured in subjects (1) 75 to 79 years old, (2) 80 to 84 years old, and (3) 85 years old and older.5 Significant decreases in all measurements were noted between groups 1 and 2 and groups 1 and 3, but there were no differences between the two oldest groups, groups 2 and 3. The investigators attributed this finding to a “survival effect.” In other words, very old age eliminates weaker elements of the population, and further deterioration over time does not occur rapidly. The same study found a significant correlation between expansion parameters (e.g., upper rib cage, lower rib cage, and abdominal expansion) and FVC and FEV1. The authors concluded that a decrease in thoracic and abdominal expansion ability may be a simple but reliable index of ventilatory impairment and respiratory weakness in elderly persons.5
A different study showed that airway hyperresponsiveness to methacholine (a parasympathetic drug that elicits bronchospasm) may be linked to accelerated pulmonary function decline in middle-aged and older men.11 In this study, responsiveness to inhaled methacholine was a significant predictor of the FEV1 decline rate. Drugs that reduce nonspecific airway hyperresponsiveness, such as inhaled corticosteroids, may slow the progression of deteriorating lung function in older patients who have hyperreactive airways.11
The tendency for small airways to collapse during expiration leads to an increase in the closing volume (i.e., the lung volume at which small airways in gravity-dependent lung regions close [see Chapter 3]).4 At about 65 years of age, the closing capacity (closing volume plus RV) equals FRC in seated subjects. This means that during tidal ventilation, some small airways close at end-expiration, and the alveoli they supply are not ventilated continuously. Thus, at rest, older people ventilate their upper lung regions more than their lower lung regions; this is the opposite of the lung’s blood flow distribution. The resulting low 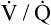 ratio in the lung bases leads to arterial hypoxemia and an increased P(A-a)O2 (see Table 24-1). This
ratio in the lung bases leads to arterial hypoxemia and an increased P(A-a)O2 (see Table 24-1). This 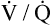 mismatch is the major mechanism whereby PaO2 declines with age.7,8 During exercise, however, increased tidal volumes improve
mismatch is the major mechanism whereby PaO2 declines with age.7,8 During exercise, however, increased tidal volumes improve 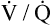 ratios of lung bases, which elevates PaO2 and decreases P(A-a)O2.8
ratios of lung bases, which elevates PaO2 and decreases P(A-a)O2.8
The overall deterioration of ventilation mechanics with age can decrease maximum voluntary ventilation and maximum achievable ventilation during heavy exercise by as much as 40% (see Table 24-1).3 Inefficient gas exchange, as manifested by an increased VD/VT ratio, increases the ventilation required for each 1 L of oxygen consumption (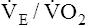 ).4 However, age decreases the maximum oxygen consumption during peak exercise mainly because the skeletal muscle mass is reduced, not because ventilatory or cardiac abnormalities exist.12 Despite decreased respiratory function in elderly persons, exercise is still limited by the heart’s maximum pumping capacity; only in habitually active elderly subjects who achieve high oxygen consumption does the respiratory system likely place some degree of limitation on exercise capacity.4
).4 However, age decreases the maximum oxygen consumption during peak exercise mainly because the skeletal muscle mass is reduced, not because ventilatory or cardiac abnormalities exist.12 Despite decreased respiratory function in elderly persons, exercise is still limited by the heart’s maximum pumping capacity; only in habitually active elderly subjects who achieve high oxygen consumption does the respiratory system likely place some degree of limitation on exercise capacity.4
The ventilatory response to hypercapnia and hypoxia decreases by as much as 50% in subjects 64 years old and older.8 This decreased responsiveness may impair the ability of older people to accommodate the effects of acute or chronic pulmonary disease.
Aging is associated with a significantly increased risk of community-acquired pneumonia and influenza.3 Overall immune competence decreases with age (see Table 24-1). Although a healthy elderly individual can mount immune responses, they are not as robust and enduring as in a younger person.3 The increased incidence of cancer in older people is further evidence of a weakened immune system.13 However, decreased immune function is probably responsible for the decreased incidence of autoimmune diseases in elderly individuals.3
Airway clearance mechanisms are less effective in older patients (see Table 24-1). Impaired respiratory mechanics and weakened respiratory muscles decrease cough effectiveness. Even in healthy older people, mucociliary clearance rates are slowed. In addition, inflammatory responses and humoral and cell-mediated immunity decrease with age.3 All of these factors place elderly persons at greater risk for developing pneumonia with any respiratory illnesses.
Effects of Aging on the Cardiovascular System
Box 24-1 outlines age-related changes in cardiovascular structure and function. Distinguishing between the normal effects of aging and cardiac disease (quite common in older people) is difficult.
Structural Changes
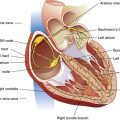
Age changes heart muscle, valvular, and vascular structures (see Box 24-1). Heart mass, primarily the left ventricle, increases gradually between 25 and 80 years of age, even in people free of cardiovascular disease or hypertension.14 This gradual hypertrophy is probably caused by the increased afterload imposed by increasing arterial wall stiffness. In addition, increased collagen deposition in the heart muscle stiffens the ventricles, making them less distensible during diastolic filling.3,14,15
With age, the heart valve leaflets thicken and calcify (see Box 24-1). Mitral and aortic valves are most commonly affected; mitral valve calcification is particularly common in older women.14 Calcification may cause mild mitral valve regurgitation (backflow) during systole, increasing atrial size and the incidence of atrial fibrillation and conduction defects.14 Atrial fibrillation is the most prevalent arrhythmia of elderly patients, occurring in approximately one third of older patients undergoing surgery.3
Age thickens the inner layer of arterial walls and increases their collagen content. In the process, the arteries stiffen, and systolic pressure increases.3 This change increases the pulse pressure.16
Functional Changes
Box 24-1 outlines age-related changes in myocardial function. The most important physiological change is the delay in ventricular filling, which can decline by 50% at age 80.3 Ventricular filling becomes much more dependent on atrial contraction, primarily because the left ventricular wall becomes thickened and stiffened. Systolic function is normally preserved.
The resting heart rate is only slightly reduced, in contrast to a more markedly reduced maximum achievable heart rate during exercise. These reductions are caused by loss of sinus node pacemaker cells (approaching 90% by age 80)3 and by decreased myocardial responsiveness to beta-adrenergic stimulation.12,17
The combination of reduced ventricular filling and a slower heart rate contributes to postural hypotension, which is common in 20% of elderly individuals.3 This phenomenon also contributes to instability and falls by making an individual susceptible to fainting. Generally, the cardiovascular system of an elderly person has a decreased capacity to adjust to position changes to maintain a constant blood pressure. Elderly people are especially prone to postural hypotension after large meals and during any volume-depleting stress, such as diarrhea or diuretic therapy.3 Volume depletion or dehydration is a very common disorder in frail elderly individuals because of decreased fluid intake.
Although vascular resistance increases with age, two mechanisms maintain an appropriate cardiac output at rest and during exercise: (1) compensatory left ventricular hypertrophy and (2) more vigorous atrial contraction, which increases ventricular filling pressure.12,18 Enhanced atrial activity increases the end-diastolic volume, ensuring an adequate stroke volume.19 The stroke volume index increases during exercise because cardiac output is sustained at a lower maximum heart rate, although at higher filling pressures.
Two factors probably contribute to the age-related slowing of ventricular filling during the early diastolic phase (see Box 24-1): (1) decreased ventricular compliance and (2) impaired ventricular relaxation after systole.15 The latter involves interference with the actin-myosin inactivation process. Age impairs the ability of the sarcoplasmic reticulum to take up Ca++ ions from the muscle fiber after contraction. The result is intracellular Ca++ overload, leading to impaired diastolic relaxation.15 Studies have shown that calcium channel–blocking drugs improve left ventricular diastolic filling in older subjects without hindering systolic function.14,15
Although healthy older people have lower maximum heart rates, they increase their cardiac outputs appropriately during exercise by increasing the preload and thus, stroke volume. To accomplish this, older people rely more heavily on atrial contraction. The major age-related impairments are (1) increased vascular resistance, (2) reduced response to beta-adrenergic stimulation, and (3) hampered ventricular relaxation. The body’s major adaptations are (1) left ventricular hypertrophy and (2) increased atrial contribution to diastolic filling. The maximum oxygen consumption is reduced in fit, older people mainly because of decreased skeletal muscle mass.12,16 Well-designed exercise programs of low to moderate intensity are extremely important in maintaining muscle mass and physical function in older people. Regardless of functional limitations, exercise programs can improve flexibility, strength, muscle mass, and mobility in older people; exercise also promotes psychological well-being and is about as effective as antidepressants in treating depression in elderly persons.20,21 A simple walking program is a safe, effective activity for many older people.