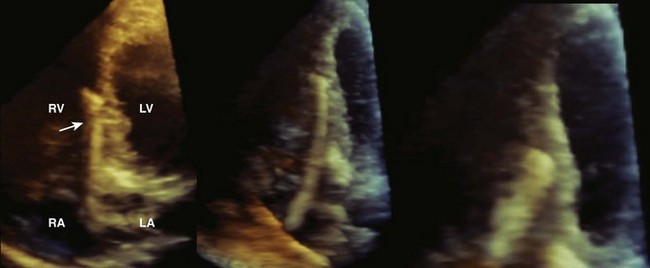
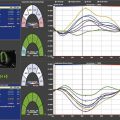
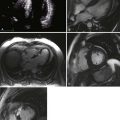
13 Echocardiography in Cardiac Transplantation
Introduction
Assessing the Appropriateness of Heart Transplantation in Acute and Chronic Heart Failure Patients
Evaluating Suitability of the Donor Heart
Step-By-Step Approach
Step 1: Assessment of Left Ventricular Hypertrophy
Key Points
Step 2: Assessment of Valvular and Congenital Abnormalities
Step 3: Careful Assessment of Ejection Fraction, and Dobutamine Challenge if Indicated
The Transplanted Heart in the Postoperative Period
Step-By-Step Approach
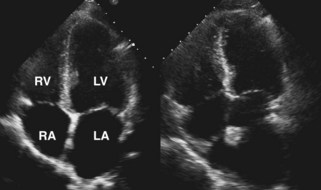
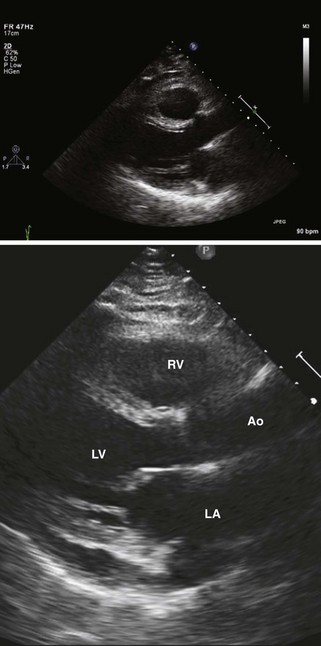
Step 1: Assessment of Atrial Structure and Function
Key Points
Step 2: Assessment of Right Ventricular Dysfunction
Key Points
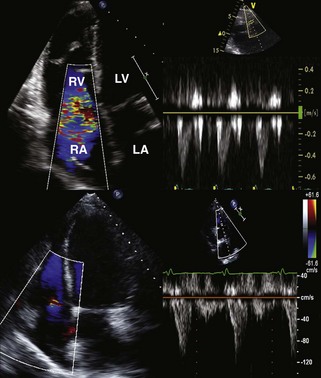
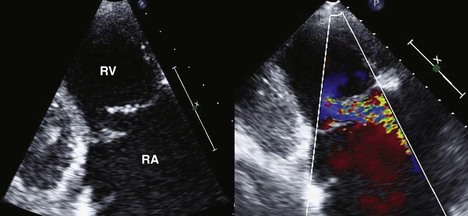
Step 3: Assessment for Presence and Mechanism of TR
Key Points
Step 4: Assessment for Presence and Etiology of Pericardial Effusions
Key Points
Step 5: Assessment of Left Ventricular Function and Mass
Key Points
Step 6: Assessment for Possible Technical Complications of Surgery
Key Points
Echocardiographic Determinants of Cardiac Rejection
Step-By-Step Approach
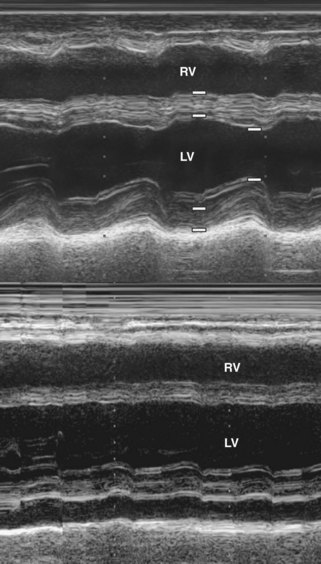
Step 1: M-mode and 2D Echocardiography of the Left Ventricle
Key Points
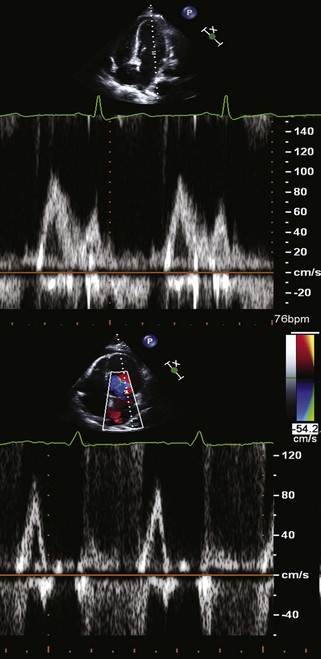
Step 2: Evaluation of Diastolic Function Using Standard Doppler Echocardiography
Key Points
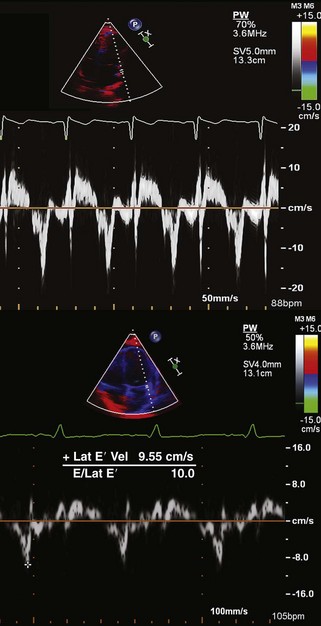
Step 3: Use of Tissue Doppler, Strain Imaging, and Advanced Techniques as Needed
Key Points
Long-Term Surveillance of Heart Transplantation Survivors
Step-By-Step Approach
Step 1: Use of Serial Resting 2D and Doppler Echocardiography
Key Points
Step 2: Use of DSE for Evaluation of CAV
Key Points
Guidance of Endomyocardial Biopsy
1 Aggarwal M, Drachenberg C, Douglass L, deFilippi C. The efficacy of real-time 3-dimensional echocardiography for right ventricular biopsy. J Am Soc Echocardiogr. 2005;18:1208-1212.
2 Akosah KO, McDaniel S, Hanrahan JS, Mohanty PK. Dobutamine stress echocardiography early after heart transplantation predicts development of allograft coronary artery disease and outcome. J Am Coll Cardiol. 1998;31:1607-1614.
3 Akosah KO, Mohanty PK, Funai JT, et al. Noninvasive detection of transplant coronary artery disease by dobutamine stress echocardiography. J Heart Lung Transplant. 1994;13:1024-1038.
4 Aranda JMJr, Weston MW, Puleo JA, Fontanet HL. Effect of loading conditions on myocardial relaxation velocities determined by Doppler tissue imaging in heart transplant recipients. J Heart Lung Transplant. 1998;17:693-697.
5 Aziz TM, Burgess MI, Rahman AN, Campbell CS, Deiraniya AK, Yonan NA. Risk factors for tricuspid valve regurgitation after orthotopic heart transplantation. Ann Thorac Surg. 1999;68:1247-1251.
6 Bacal F, Moreira L, Souza G, et al. Dobutamine stress echocardiography predicts cardiac events or death in asymptomatic patients long-term after heart transplantation: 4-year prospective evaluation. J Heart Lung Transplant. 2004;23:1238-1244.
7 Bedanova H, Necas J, Petrikovits E, et al. Echo-guided endomyocardial biopsy in heart transplant recipients. Transpl Int. 2004;17:622-625.
8 Bhatia SJ, Kirshenbaum JM, Shemin RJ, et al. Time course of resolution of pulmonary hypertension and right ventricular remodeling after orthotopic cardiac transplantation. Circulation. 1987;76:819-826.
9 Bolad IA, Robinson DR, Webb C, Hamour I, Burke MM, Banner NR. Impaired left ventricular systolic function early after heart transplantation is associated with cardiac allograft vasculopathy. Am J Transplant. 2006;6:161-168.
10 Ciliberto GR, Mascarello M, Gronda E, et al. Acute rejection after heart transplantation: Noninvasive echocardiographic evaluation. J Am Coll Cardiol. 1994;23:1156-1161.
11 Dandel M, Hummel M, Muller J, et al. Reliability of tissue Doppler wall motion monitoring after heart transplantation for replacement of invasive routine screenings by optimally timed cardiac biopsies and catheterizations. Circulation. 2001;104(suppl I):I184-I191.
12 Desruennes M, Corcos T, Cabrol A, et al. Doppler echocardiography for the diagnosis of acute cardiac allograft rejection. J Am Coll Cardiol. 1988;12:63-70.
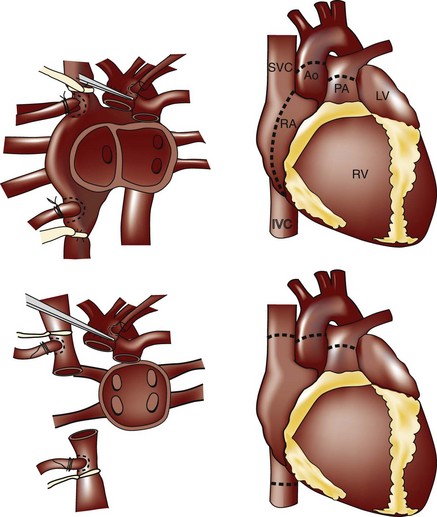
13 El Gamel A, Yonan NA, Grant S, et al. Orthotopic cardiac transplantation: A comparison of standard and bicaval Wythenshawe techniques. J Thorac Cardiovasc Surg. 1995;109:721-729. discussion 729-730
14 French JW, Popp RL, Pitlick PT. Cardiac localization of transvascular bioptome using 2-dimensional echocardiography. Am J Cardiol. 1983;51:219-223.
15 Fyfe DA, Ketchum D, Lewis R, et al. Tissue Doppler imaging detects severely abnormal myocardial velocities that identify children with pre-terminal cardiac graft failure after heart transplantation. J Heart Lung Transplant. 2006;25:510-517.
16 Grande AM, Minzioni G, Martinelli L, et al. Echo-controlled endomyocardial biopsy in orthotopic heart transplantation with bicaval anastomosis. G Ital Cardiol. 1997;27:877-880.
17 Kato TS, Oda N, Hashimura K, et al. Strain rate imaging would predict sub-clinical acute rejection in heart transplant recipients. Eur J Cardiothorac Surg. 2010;37:1104-1110.
18 Kociolek LK, Bierig SM, Herrmann SC, Labovitz AJ. Efficacy of atropine as a chronotropic agent in heart transplant patients undergoing dobutamine stress echocardiography. Echocardiography. 2006;23:383-387.
19 Kono T, Nishina T, Morita H, Hirota Y, Kawamura K, Fujiwara A. Usefulness of low-dose dobutamine stress echocardiography for evaluating reversibility of brain death-induced myocardial dysfunction. Am J Cardiol. 1999;84:578-582.
20 Leonard GTJr, Fricker FJ, Pruett D, Harker K, Williams B, Schowengerdt KOJr. Increased myocardial performance index correlates with biopsy-proven rejection in pediatric heart transplant recipients. J Heart Lung Transplant. 2006;25:61-66.
21 Lewis JF, Selman SB, Murphy JD, Mills RMJr, Geiser EA, Conti CR. Dobutamine echocardiography for prediction of ischemic events in heart transplant recipients. J Heart Lung Transplant. 1997;16:390-393.
22 Mahle WT, Cardis BM, Ketchum D, Vincent RN, Kanter KR, Fyfe DA. Reduction in initial ventricular systolic and diastolic velocities after heart transplantation in children: Improvement over time identified by tissue Doppler imaging. J Heart Lung Transplant. 2006;25:1290-1296.
23 Mankad S, Murali S, Kormos RL, Mandarino WA, Gorcsan J3rd. Evaluation of the potential role of color-coded tissue Doppler echocardiography in the detection of allograft rejection in heart transplant recipients. Am Heart J. 1999;138:721-730.
24 Mannaerts HF, Balk AH, Simoons ML, et al. Changes in left ventricular function and wall thickness in heart transplant recipients and their relation to acute rejection: An assessment by digitised M mode echocardiography. Br Heart J. 1992;68:356-364.
25 Mannaerts HF, Simoons ML, Balk AH, et al. Pulsed-wave transmitral Doppler does not diagnose moderate acute rejection after heart transplantation. J Heart Lung Transplant. 1993;12:411-421.
26 Marciniak A, Eroglu E, Marciniak M, et al. The potential clinical role of ultrasonic strain and strain rate imaging in diagnosing acute rejection after heart transplantation. Eur J Echocardiogr. 2007;8:213-221.
27 McCreery CJ, McCulloch M, Ahmad M, deFilippi CR. Real-time 3-dimensional echocardiography imaging for right ventricular endomyocardial biopsy: A comparison with fluoroscopy. J Am Soc Echocardiogr. 2001;14:927-933.
28 Mena C, Wencker D, Krumholz HM, McNamara RL. Detection of heart transplant rejection in adults by echocardiographic diastolic indices: A systematic review of the literature. J Am Soc Echocardiogr. 2006;19:1295-1300.
29 Mondillo S, Maccherini M, Galderisi M. Usefulness and limitations of transthoracic echocardiography in heart transplantation recipients. Cardiovasc Ultrasound. 2008;6:2.
30 Morgan JA, Edwards NM. Orthotopic cardiac transplantation: Comparison of outcome using biatrial, bicaval, and total techniques. J Card Surg. 2005;20:102-106.
31 Palka P, Lange A, Galbraith A, et al. The role of left and right ventricular early diastolic Doppler tissue echocardiographic indices in the evaluation of acute rejection in orthotopic heart transplant. J Am Soc Echocardiogr. 2005;18:107-115.
32 Peteiro J, Redondo F, Calvino R, Cuenca J, Pradas G, Castro Beiras A. Differences in heart transplant physiology according to surgical technique. J Thorac Cardiovasc Surg. 1996;112:584-589.
33 Pham MX, Teuteberg JJ, Kfoury AG, et al. Gene-expression profiling for rejection surveillance after cardiac transplantation. N Engl J Med. 2010;362:1890-1900.
34 Prakash A, Printz BF, Lamour JM, Addonizio LJ, Glickstein JS. Myocardial performance index in pediatric patients after cardiac transplantation. J Am Soc Echocardiogr. 2004;17:439-442.
35 Rodney RA, Johnson LL. Myocardial perfusion scintigraphy to assess heart transplant vasculopathy. J Heart Lung Transplant. 1992;11:S74-S78.
36 Roshanali F, Mandegar MH, Bagheri J, et al. Echo rejection score: New echocardiographic approach to diagnosis of heart transplant rejection. Eur J Cardiothorac Surg. 2010;38:176-180.
37 Santos-Ocampo SD, Sekarski TJ, Saffitz JE, et al. Echocardiographic characteristics of biopsy-proven cellular rejection in infant heart transplant recipients. J Heart Lung Transplant. 1996;15:25-34.
38 Scheurer M, Bandisode V, Ruff P, Atz A, Shirali G. Early experience with real-time three-dimensional echocardiographic guidance of right ventricular biopsy in children. Echocardiography. 2006;23:45-49.
39 Spes CH, Mudra H, Schnaack SD, et al. Dobutamine stress echocardiography for noninvasive diagnosis of cardiac allograft vasculopathy: A comparison with angiography and intravascular ultrasound. Am J Cardiol. 1996;78:168-174.
40 Stork S, Behr TM, Birk M, et al. Assessment of cardiac allograft vasculopathy late after heart transplantation: When is coronary angiography necessary? J Heart Lung Transplant. 2006;25:1103-1108.
41 Sun JP, Abdalla IA, Asher CR, et al. Non-invasive evaluation of orthotopic heart transplant rejection by echocardiography. J Heart Lung Transplant. 2005;24:160-165.
42 Sundereswaran L, Nagueh SF, Vardan S, et al. Estimation of left and right ventricular filling pressures after heart transplantation by tissue Doppler imaging. Am J Cardiol. 1998;82:352-357.
43 Thorn EM, de Filippi CR. Echocardiography in the cardiac transplant recipient. Heart Fail Clin. 2007;3:51-67.
44 Tona F, Caforio AL, Montisci R, et al. Coronary flow reserve by contrast-enhanced echocardiography: A new noninvasive diagnostic tool for cardiac allograft vasculopathy. Am J Transplant. 2006;6:998-1003.
45 Tona F, Caforio AL, Montisci R, et al. Coronary flow velocity pattern and coronary flow reserve by contrast-enhanced transthoracic echocardiography predict long-term outcome in heart transplantation. Circulation. 2006;114(suppl I):I49-I55.
46 Valantine HA, Fowler MB, Hunt SA, et al. Changes in Doppler echocardiographic indexes of left ventricular function as potential markers of acute cardiac rejection. Circulation. 1987;76(suppl V):V86-V92.
47 Valantine HA, Yeoh TK, Gibbons R, et al. Sensitivity and specificity of diastolic indexes for rejection surveillance: Temporal correlation with endomyocardial biopsy. J Heart Lung Transplant. 1991;10:757-765.
48 Vandenberg BF, Mohanty PK, Craddock KJ, et al. Clinical significance of pericardial effusion after heart transplantation. J Heart Transplant. 1988;7:128-134.
49 Venkateswaran RV, Townend JN, Wilson IC, Mascaro JG, Bonser RS, Steeds RP. Echocardiography in the potential heart donor. Transplantation. 2010;89:894-901.
50 Weller GE, Lu E, Csikari MM, et al. Ultrasound imaging of acute cardiac transplant rejection with microbubbles targeted to intercellular adhesion molecule-1. Circulation. 2003;108:218-224.
51 Wong RC, Abrahams Z, Hanna M, et al. Tricuspid regurgitation after cardiac transplantation: An old problem revisited. J Heart Lung Transplant. 2008;27:247-252.
52 Yankah AC, Musci M, Weng Y, et al. Tricuspid valve dysfunction and surgery after orthotopic cardiac transplantation. Eur J Cardiothorac Surg. 2000;17:343-348.
53 Zaroff JG, Rosengard BR, Armstrong WF, et al. Consensus conference report. Maximizing use of organs recovered from the cadaver donor: Cardiac recommendations. Circulation. 2002;106:836-841.