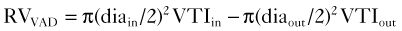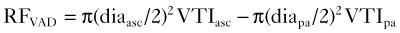12 Echocardiographic Evaluation of Ventricular Support Devices
Background
| Examples | Type |
|---|---|
| First Generation | |
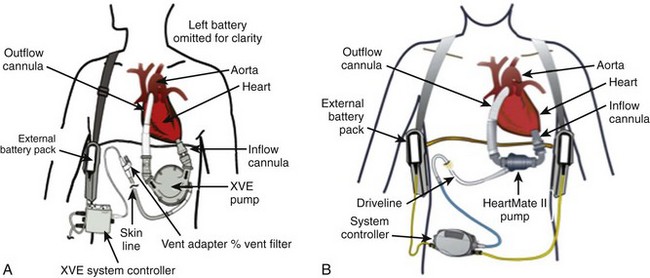
| HeartMate XVE (Thoratec Corp.) | Pulsatile |
| Novacor pumps (WorldHeart Corp.) | Pulsatile |
| Second Generation | |
| HeartMate II (Thoratec Corp.) | Axial Flow |
| Jarvik 2000 (Jarvik Heart Corp.) | Axial Flow |
| Micromed DeBakey (Micromed Cardiovascular Inc.) | Axial Flow |
| Third Generation | |
| HeartWare HVAD (HeartWare Inc.) | Centrifugal |
| Duraheart (Terumo Heart Inc.) | Centrifugal |
| Levacor (WorldHeart Corp.) | Centrifugal |
| Total Artificial Heart | |
| Abiocor (Abiomed Corp.) | |
| Cardiowest (Syncardia Inc.) |
Overview of Echocardiographic Approach (Table 12-2)
Echocardiography-Guided LVAD Optimization
Percutaneous VADs, IABP, and ECMO
Preimplantation Echocardiographic Assessment
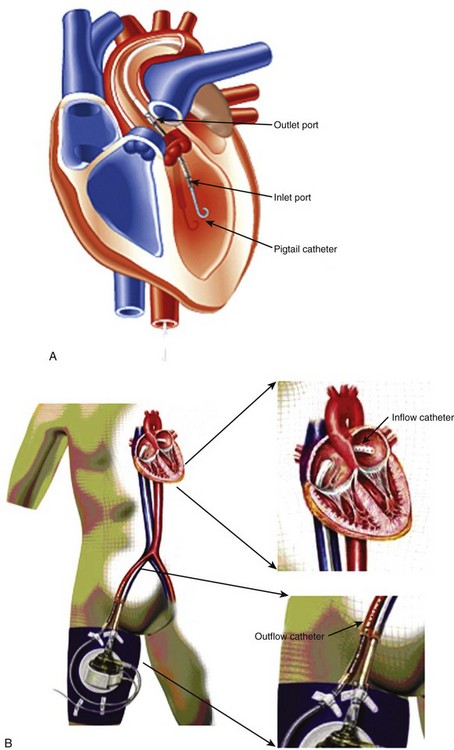
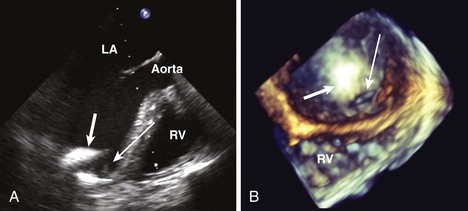
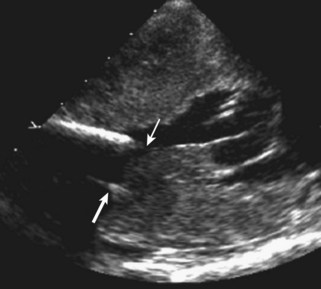
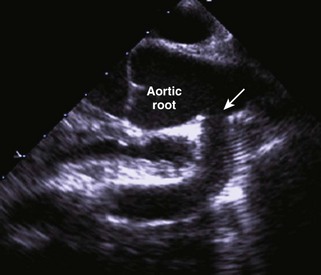
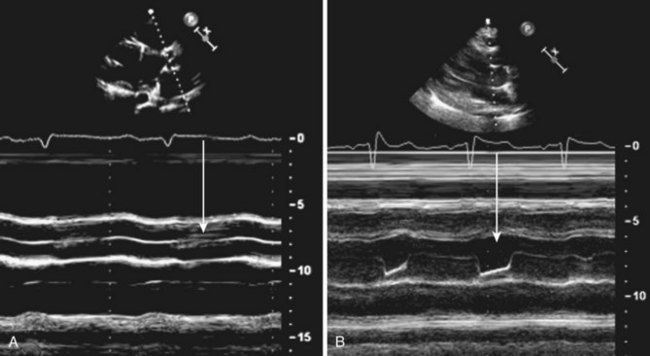
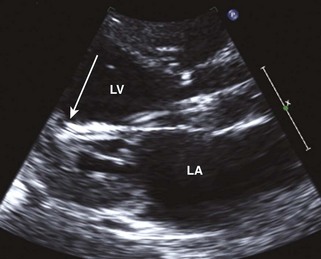
Anatomic Imaging
Acquisition
Analysis
| Echocardiographic Finding | Measurement |
|---|---|
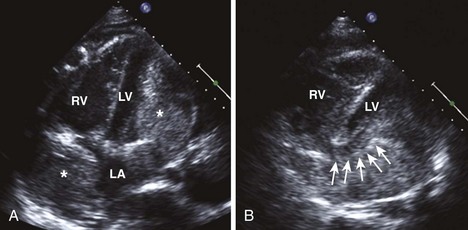
| RV enlargement |
RVEDD, right ventricular end-diastolic diameter; RVEDV, right ventricular end-diastolic volume; RVESV, right ventricular end-systolic volume; TAPSE, tricuspid annular plane systolic excursion.
Pitfalls (Box 12-1)
Box 12-1 Pitfalls in VAD Imaging
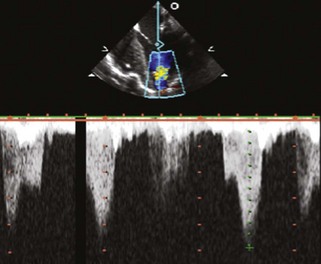
Physiologic Data
Pitfalls (see Box 12-1)
Alternative Approaches
Key Points
Implantation/Intraoperative Echocardiographic Assessment
Anatomic Imaging (Box 12-2)
Diagnosis of the Cause of LVAD Dysfunction
Anatomic Imaging
Acquisition
Analysis
Pitfalls (see Box 12-1)
Physiologic Data
Acquisition
Analysis
Pitfalls (see Box 12-1)
Alternative Approaches
Key Points
Echocardiography-Guided LVAD Optimization and Weaning
Anatomic Imaging
Acquisition
Analysis
Physiologic Data
Acquisition
Pitfalls (see Box 12-1)
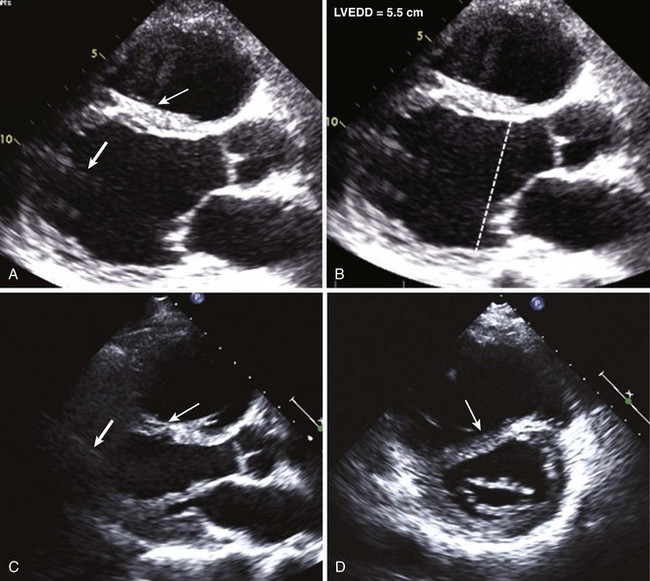
Percutaneous Ventricular Assist Devices
Anatomic Imaging
Acquisition
Analysis
Pitfalls (see Box 12-1)
Physiologic Data
Pitfalls (see Box 12-1)
Key Points
1 Catena E, Milazzo F. Echocardiography and cardiac assist devices. Minerva Cardiolangiol. 2005;55:247-265.
2 Catena E, Milazzo F, Pittella G, et al. Echocardiographic approach in a new left ventricular assist device: Impella Recover 100. J Am Soc Echocardiogr. 2004;17:470-473.
This paper provides an overview of the early experience with the Impella device.
3 Catena E, Milazzo F, Montorsi E, et al. Left ventricular support by axial flow pump: The echocardiographic approach to device malfunction. J Am Soc Echocardiogr. 2005;18:1422e7-1422e13.
4 Chumnanvej S, Wood MJ, MacGillivray TE, Vidal Melo MF. Perioperative echocardiographic examination for ventricular assist device implantation. Anesth Analg. 2007;105:583-601.
5 Horton SC, Khodaverdian R, Chatelain P, et al. Left ventricular assist device malfunction: An approach to diagnosis by echocardiography. J Am Coll Cardiol. 2005;45:1435-1440.
6 John R, Mantz K, Eckman P, Rose A, May-Newman K. Aortic valve pathophysiology during left ventricular assist device support. J Heart Lung Transplant. 2010;29:1321-1329.
7 Kirkpatrick JN, Wiegers SE, Lang RM. Left ventricular assist devices and other devices for end-stage heart failure: Utility of echocardiography. Curr Cardiol Reports. 2010;12:257-264.
8 Scalia GM, McCarthy PM, Savage RM, Smedira NG, Thomas JD. Clinical utility of echocardiography in the management of implantable ventricular assist device. J Am Soc Echocardiogr. 2000;13:754-763.