9 Echocardiographic Assessment of Treatment for Systolic Congestive Heart Failure
Echocardiographic Assessment of Systolic HF
Medications
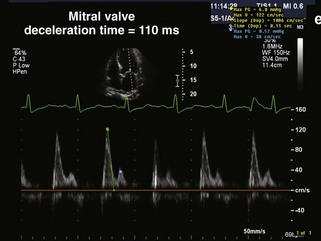
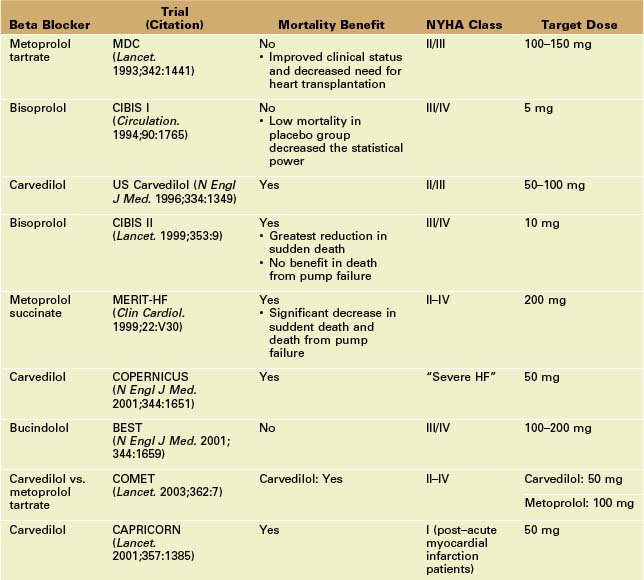
Beta Blockers
Key Points
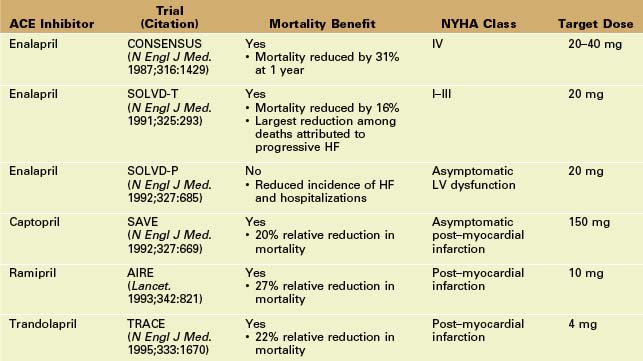
Angiotensin-Converting Enzyme (ACE) Inhibitors
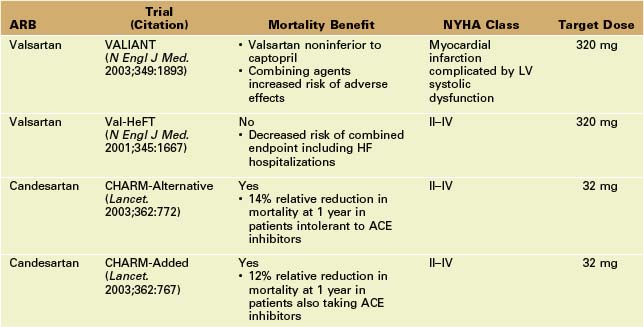
Angiotensin II Receptor Blockers (ARBs)
Key Points
Aldosterone Antagonists
Key Points
Hydralazine and Isosorbide Dinitrate
Implantable Cardioverter-Defibrillator (ICD)
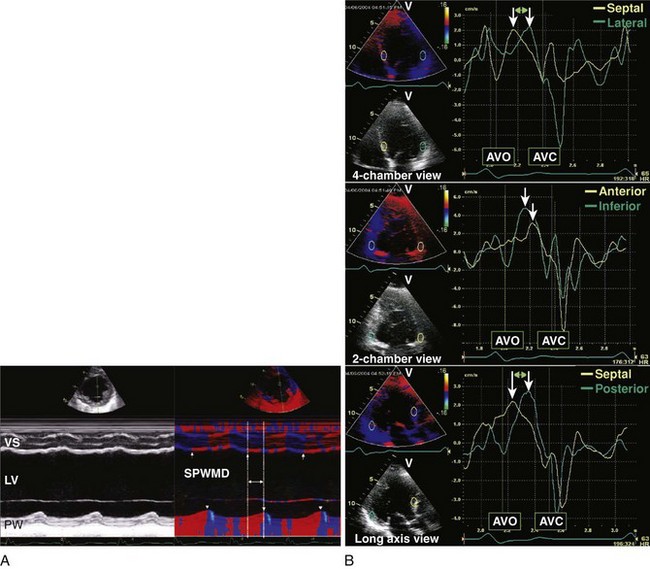
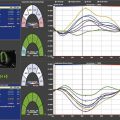
Cardiac Resynchronization Therapy (CRT)
Key Points
Novel Devices
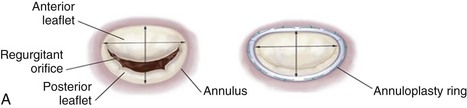
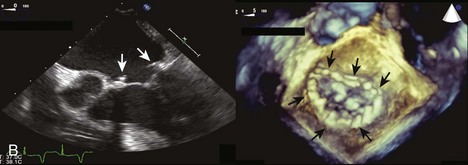
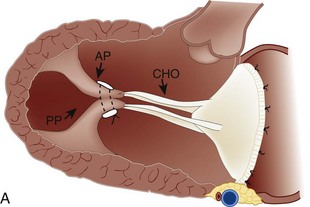
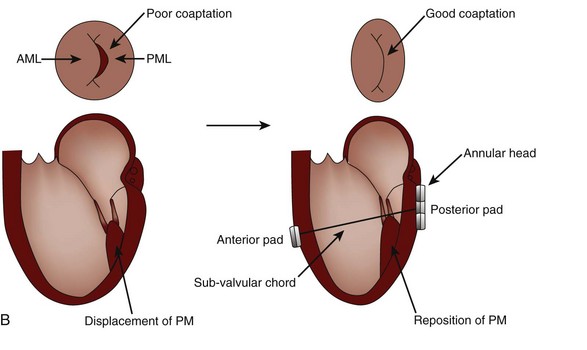
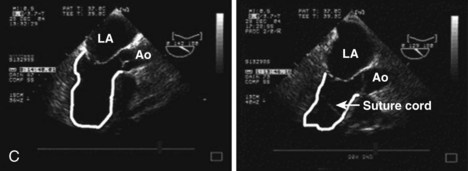
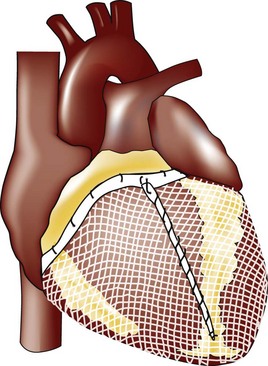
MR Reduction and Direct Ventricular Remodeling
Key Points
1 Douglas PS, Garcia MJ, Haines DE, et al. ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 appropriate use criteria for echocardiography. J Am Coll Cardiol. 2011;57:1126-1166.
2 Rihal CS, Nishimura RA, Hatle LK, Bailey KR, Tajik AJ. Systolic and diastolic dysfunction in patients with clinical diagnosis of dilated cardiomyopathy: Relation to symptoms and prognosis. Circulation. 1994;90:2772-2779.
3 Xie GY, Berk MR, Smith MD, Gurley JC, DeMaria AN. Prognostic value of Doppler transmitral flow patterns in patients with congestive heart failure. J Am Coll Cardiol. 1994;24:132-139.
4 Sugeng L, Mor-Avi V, Weinert L, et al. Quantitative assessment of left ventricular size and function: Side-by-side comparison of real-time three-dimensional echocardiography and computed tomography with magnetic resonance reference. Circulation. 2006;114:654-661.
5 Nahum J, Bensaid A, Dussault C, et al. Impact of longitudinal myocardial deformation on the prognosis of chronic heart failure patients. Circ Cardiovasc Imaging. 2010;3:249-256.
6 Hung CL, Verma A, Uno H, et al. Longitudinal and circumferential strain rate, left ventricular remodeling, and prognosis after myocardial infarction. J Am Coll Cardiol. 2010;56:1812-1822.
7 Maurer MS, Sackner-Bernstein JD, El-Khoury Rumbarger L, Yushak M, King DL, Burkhoff D. Mechanisms underlying improvements in ejection fraction with carvedilol in heart failure. Circ Heart Fail. 2009;2:189-196.
8 Pfeffer MA, Lamas GA, Vaughan DE, et al. Effect of captopril on progressive ventricular dilatation after anterior myocardial infarction. N Engl J Med. 1988;319:80-86.
9 Wong M, Staszewsky L, Latini R, et al. Valsartan benefits left ventricular structure and function in heart failure: Val-HeFT echocardiographic study. J Am Coll Cardiol. 2002;40:970-975.
10 Cicoira M, Zanolla L, Rossi A, et al. Long-term, dose-dependent effects of spironolactone on left ventricular function and exercise tolerance in patients with chronic heart failure. J Am Coll Cardiol. 2002;40:304-310.
11 Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309-1321.
12 Vizzardi E, D’Aloia A, Giubbini R, et al. Effect of spironolactone on left ventricular ejection fraction and volumes in patients with class I or II heart failure. Am J Cardiol. 2010;106:1292-1296.
13 Taylor AL, Ziesche S, Yancy C, et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med. 2004;351:2049-2057.
14 Hunt SA, Abraham WT, Chin MH, et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines; developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:e391-e479.
15 St. John Sutton MG, Keane MG. Reverse remodeling in heart failure with cardiac resynchronization therapy. Heart. 2007;93:167-171.
16 St. John Sutton MG, Plappert T, Hilpisch KE, et al. Sustained reverse left ventricular structural remodeling with cardiac resynchronization at one year is a function of etiology: Quantitative Doppler echocardiographic evidence from the Multicenter InSync Randomized Clinical Evaluation (MIRACLE). Circulation. 2006;113:266-272.
17 Cleland JG, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539-1549.
18 Moss AJ, Hall WJ, Cannom DS, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361:1329-1338.
19 Beshai JF, Grimm RA, Nagueh SF, et al. Cardiac-resynchronization therapy in heart failure with narrow QRS complexes. N Engl J Med. 2007;357:2461-2471.
20 Pitzalis MV, Iacoviello M, Romito R, et al. Ventricular asynchrony predicts a better outcome in patients with chronic heart failure receiving cardiac resynchronization therapy. J Am Coll Cardiol. 2005;45:65-69.
21 Marcus GM, Rose E, Viloria EM, et al. Septal to posterior wall motion delay fails to predict reverse remodeling or clinical improvement in patients undergoing cardiac resynchronization therapy. J Am Coll Cardiol. 2005;46:2208-2214.
22 Yu CM, Fung WH, Lin H, et al. Predictors of left ventricular reverse remodeling after cardiac resynchronization therapy for heart failure secondary to idiopathic dilated or ischemic cardiomyopathy. Am J Cardiol. 2003;91:684-688.
23 Yu CM, Fung JW, Zhang Q, et al. Tissue Doppler imaging is superior to strain rate imaging and postsystolic shortening on the prediction of reverse remodeling in both ischemic and nonischemic heart failure after cardiac resynchronization therapy. Circulation. 2004;110:66-73.
24 Bax JJ, Marwick TH, Molhoek SG, et al. Left ventricular dyssynchrony predicts benefit of cardiac resynchronization therapy in patients with end-stage heart failure before pacemaker implantation. Am J Cardiol. 2003;92:1238-1240.
25 Bax JJ, Bleeker GB, Marwick TH, et al. Left ventricular dyssynchrony predicts response and prognosis after cardiac resynchronization therapy. J Am Coll Cardiol. 2004;44:1834-1840.
26 Richardson M, Freemantle N, Calvert MJ, et al. Predictors and treatment response with cardiac resynchronization therapy in patients with heart failure characterized by dyssynchrony: A pre-defined analysis from the CARE-HF trial. Eur Heart J. 2007;28:1827-1834.
27 Chung ES, Leon AR, Tavazzi L, et al. Results of the Predictors of Response to CRT (PROSPECT) trial. Circulation. 2008;117:2608-2616.
28 Solis J, McCarty D, Levine RA, et al. Mechanism of decrease in mitral regurgitation after cardiac resynchronization therapy: Optimization of the force-balance relationship. Circ Cardiovasc Imaging. 2009;6:444-450.
29 Grigioni F, Enriquez-Sarano M, Zehr KJ, et al. Ischemic mitral regurgitation: Long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation. 2001;103:1759-1764.
30 Bouma W, van der Horst IC, Wijdh-den Hamer IJ, et al. Chronic ischaemic mitral regurgitation: Current treatment results and new mechanism-based surgical approaches. Eur J Cardiothorac Surg. 2010;37:170-185.
31 Grossi EA, Patel N, Woo YJ, et al. Outcomes of the RESTOR-MV Trial (Randomized Evaluation of a Surgical Treatment for Off-Pump Repair of the Mitral Valve). J Am Coll Cardiol. 2010;56:1984-1993.
32 Feldman T, Foster E, Glower DD, et al. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med. 2011;364:1395-1406.
33 Siminiak T, Hoppe UC, Schofer J, et al. Effectiveness and safety of percutaneous coronary sinus-based mitral valve repair in patients with dilated cardiomyopathy (from the AMADEUS trial). Am J Cardiol. 2009;104:565-570.
34 Acker MA, Bolling S, Shemin R, et al. Mitral valve surgery in heart failure: Insights from the ACORN Clinical Trial. J Thorac Cardiovasc Surg. 2006;132:568-577.
35 Jones RH, Velazquez EJ, Michler RE, et al. Coronary bypass surgery with or without surgical ventricular reconstruction. N Engl J Med. 2009;360:1705-1717.