Chapter 16 EBUS-TBNA of Right Lower Paratracheal Lymph Node (Station 4R)
Case Description
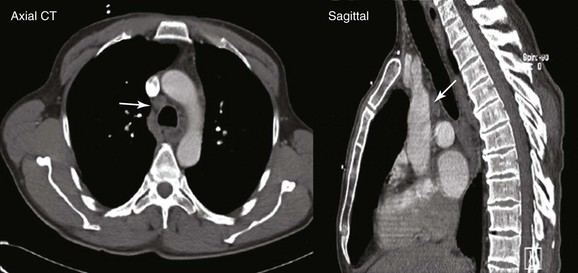
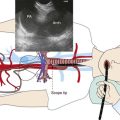
A 67-year-old Korean man with a 20–pack-year history of smoking was found to have an abnormal chest radiograph showing a right upper lobe pulmonary nodule during a routine preoperative evaluation for hernia repair. The patient had COPD (FEV1 50% of predicted) and pulmonary hypertension, presumptively related to his COPD (WHO group III*). An echocardiogram performed 3 months earlier showed a normal left ventricular ejection fraction and a pulmonary artery systolic pressure of 59 mm Hg with a tricuspid regurgitant velocity of 3.5 m/sec and an estimated right atrial pressure of 10 mm Hg. Computed tomography of the chest showed a 1.5 × 1-cm right upper lobe nodule and a 1.4-cm right lower paratracheal lymph node. He had no other past medical history and was in excellent health except for mild exertional dyspnea (NYHA class II). The patient was referred for tissue diagnosis (Figure 16-1).
Case Resolution
Initial Evaluations
Physical Examination, Complementary Tests, and Functional Status Assessment
After the pulmonary nodule was discovered on chest radiograph, this patient underwent a chest computed tomography (CT). In addition to the history and physical examination, CT scan of the chest is recommended in patients suspected of having lung cancer who are eligible for treatment, because its potential benefits for staging and treatment planning outweigh the relatively low risk of radiation-induced damage.1 CT scan of the chest should be obtained before bronchoscopy because having a more accurate appreciation of the size and location of the tumor increases the diagnostic yield of bronchoscopy, which is low, however, for peripheral pulmonary nodules smaller than 2 cm. Yield is high for diagnosis of larger nodules and for staging of mediastinal lymphadenopathy when sonographic guidance (endobronchial ultrasound–transbronchial needle aspiration [EBUS-TBNA]) is used.
This patient showed evidence of ipsilateral lymph node involvement (right lower paratracheal; 4R), but contralateral lymph node involvement was not suspected on the basis of CT. Positron emission tomography (PET)-CT has greater sensitivity for detecting metastasis in the mediastinum compared with CT, but this procedure had not been performed before the patient was referred to our institution. Nor had other diagnostic studies been performed to search for extrathoracic disease. For example, for patients with clinical stage IB-IIIB being considered for curative treatment, cranial magnetic resonance imaging (MRI) is recommended by some authorities to detect brain metastases, even if clinical examination findings are negative.1 We elected to perform additional tests on our patient for complete staging after a diagnosis had been made. We chose to recommend bronchoscopy and EBUS-guided TBNA for both diagnosis and mediastinal staging.
Comorbidities
This patient likely had pulmonary hypertension secondary to chronic obstructive pulmonary disease (COPD) based on a Doppler echocardiographic study showing a pulmonary artery systolic pressure (PASP) of 59 mm Hg with a tricuspid regurgitant velocity (TRV) of 3.5 m/sec.2 In general, pulmonary hypertension is considered likely if the PASP is greater than 50 and the TRV is greater than 3.4; unlikely if the PASP is 36 or less and the TRV is 2.8 or less, and no other suggestive findings are reported; and possible with other combinations of findings. Doppler echocardiography, however, may be misleading in patients with suspected pulmonary hypertension, especially when an inadequate tricuspid regurgitant jet cannot be properly assessed owing to a poor echocardiographic window. This was demonstrated in an observational study† of 65 patients with various types of pulmonary hypertension.3 The pulmonary arterial pressure estimated by Doppler echocardiography was at least 10 mm Hg higher or lower than that obtained by right heart catheterization in 48% of patients.* Therefore, a low threshold for right heart catheterization is warranted when patients with suspected pulmonary hypertension are evaluated. However, perioperative insertion of pulmonary artery catheters is not routinely recommended as a strategy to reduce perioperative mortality or postoperative pulmonary complications, even in high-risk surgical patients.4
Procedural Strategies
Indications
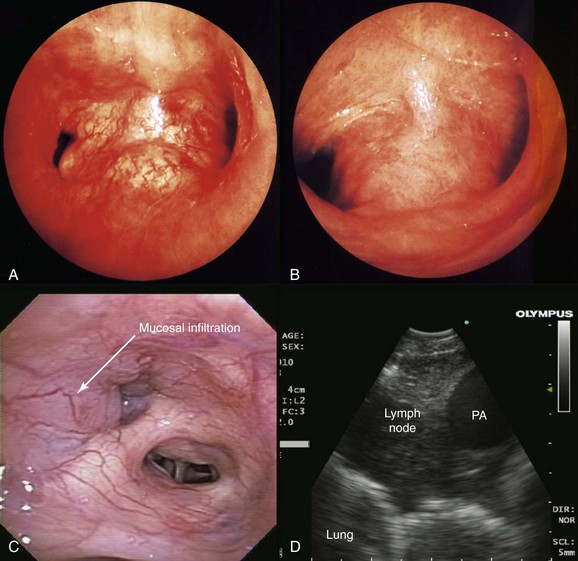
This patient likely had primary lung carcinoma. He needed a tissue diagnosis. We proposed bronchoscopy with EBUS-TBNA because the suspected N2 status (right lower paratracheal lymphadenopathy) had to be confirmed to decide on the need for further therapy: If N2 status were negative, therapy would be recommended for stage I-II non–small cell lung carcinoma (NSCLC) (with likely surgical resection once operability is determined); or for postoperative stage IIIA1* or IIIA2, according to strategies for stage IIIA. If, on the other hand, N2 status is known to be positive preoperatively, the patient would be assigned stage IIIA3 and would be treated according to the algorithm for stage IIIA. Careful white light bronchoscopic examination should be performed at the time of EBUS-TBNA because it may detect mucosal abnormalities, which may be malignant. Such findings would change the stage. For instance, in the setting of mediastinal lymphadenopathy, if the tracheal wall is involved, the tumor would be classified as T4 and therefore stage IIIB, but if no mucosal abnormalities are noted, the patient would have N2 disease, namely, stage IIIA (Figure 16-2); furthermore, if the needle passes through airway wall infiltrated with malignant cells before entering the lymph node target, the lymph node aspirate could become contaminated, and this could represent a false-positive aspirate. Thus we believe that abnormal airway mucosa at the puncture site should be biopsied (see Figure 16-2), and that bronchoscopists should do their best to avoid needle insertion through abnormal appearing tissue, if possible.†
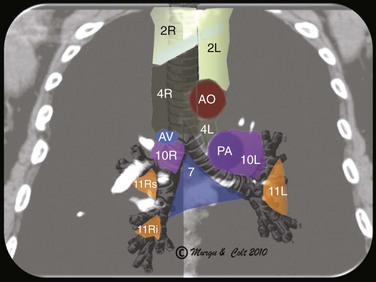
EBUS-TBNA can offer diagnosis and staging in one setting. With EBUS, one can potentially sample all stations adjacent to the trachea or bronchi (2, 4, 7, 10, 11) (Figure 16-3), but not level 5, 6, 8, and 9 lymph nodes.‡ Low-volume cytology specimens such as those obtained via EBUS-TBNA are considered adequate for molecular analysis, as with epidermal growth factor receptor (EGFR) and anaplastic lymphatic kinase (ALK).5,6 This is increasingly important because whole genome amplification enables multiple molecular analyses and may be used to expand starting deoxyribonucleic acid (i.e., DNA amplification) from low-volume lung biopsies, such as those obtained with EBUS-TBNA, for further analysis of advanced-stage NSCLC.7 It is noteworthy that even when the primary tumor (the pulmonary nodule) is biopsied and studied for gene alterations, molecular assessment of metastatic sites (e.g., lymph nodes) may change management decisions because of the possibility of genetic differences between primary tumor and metastatic sites caused by tumor heterogeneity.8,9 Mutations in genes involved in the EGFR/KRAS/BRAF pathway, for example, were shown to predict clinical response to EGFR-directed tyrosine kinase inhibitors (TKIs) in NSCLC patients. The presence of activating EGFR mutations and the absence of KRAS mutations have been shown to be favorable markers for response to EGFR-directed TKI therapy.9 One study, which compared the mutational status of EGFR, KRAS, and BRAF in primary tumors* (adenocarcinomas) with that in the corresponding lymph node metastases, found that mutations in primary tumors and lymph node metastases were identical in only 1 of 7 (14%) patients in case of EGFR mutation and in 11of 36 (31%) patients in case of KRAS mutation. Only one patient showed the same EGFR mutation in the primary tumor and corresponding lymph node metastasis. In this study, the remaining 6 patients had EGFR mutations identified in the primary tumor (3 patients) or in the lymph node metastasis (3 patients), but not in both. A different KRAS mutation in the primary tumor and the corresponding metastasis was seen in 1 patient, and 24 patients had KRAS mutations exclusively in primary (16 patients) or metastatic tumor (8 patients).9 These data suggest that the possibility of differences in the mutational status of EGFR, KRAS,† and BRAF‡ between primary tumors and corresponding lymph node metastases should be considered whenever these mutations are used for the selection of patients for EGFR-directed TKI therapy.9
Expected Results
The diagnostic rate of EBUS-TBNA for station 4R is 71% to 94%10,11 and, as has been mentioned, the likelihood of obtaining sufficient material for use in molecular analysis is high. The prevalence of genetic alterations seems to vary depending on the patient’s ethnicity; a meta-analysis of nine published studies showed that among those with adenocarcinoma, EGFR mutations were present in 48% of East Asian patients but in only 12% of those with other ethnicities.12 Relevant to our patient is that EGFR mutation is frequent among Koreans suffering from lung cancer. In one study, it was seen in 20 of 115 patients (17.4%).§13 Drugs that target mutant EGFR and ALK are now available, and it is possible that testing for prospective mutations could result in assigning a successful targeted therapy. For instance, erlotinib and gefitinib are already available to target mutant EGFR, and the ALK inhibitor crizotinib has demonstrated remarkable efficacy against ALK fusion–positive lung cancers.14
Other lung or nonlung primary tumors could be responsible for this patient’s presentation. For neuroendocrine tumors, correct classification of a tumor as small cell lung cancer (SCLC) or as large cell neuroendocrine carcinoma (LCNEC) is a particularly difficult problem because of the many overlapping features of these tumors.15 In the diagnosis of primary lung lesions, this distinction is important because of different treatment strategies. LCNEC is considered an NSCLC treated by surgical excision, whereas SCLC is usually treated with chemotherapy or chemoradiotherapy. Given the large numbers of background lymphocytes in EBUS-TBNA specimens, the distinction between lymphoid cells and a neuroendocrine tumor can be particularly difficult. If carcinoid is diagnosed, cytology specimens are often inadequate for a definitive diagnosis of typical or atypical morphology16—a crucial distinction in determining prognosis.
On a different note, the sensitivity and specificity of EBUS-TBNA for diagnosing mediastinal and hilar lymph node metastasis from nonpulmonary tumors (taken together) have been reported to be as high as 92.0% and 100%, respectively. Tumors encountered included colorectal, head and neck, ovarian, breast, esophageal, hepatocellular, prostate, renal, and germ cell cancers and malignant melanoma.17 For lymphoma, a small study evaluating patients with high pretest probability for lymphoma in a tertiary cancer center revealed an overall sensitivity of 90.9% and specificity of 100%.18 Larger, prospective studies showed that the sensitivity and the specificity of EBUS-TBNA for definitive diagnosis of lymphoma were 57% and 100%, respectively. Decreased diagnostic yield may be due in part to problems with the low-volume cytology specimens obtained via EBUS-TBNA. For example, baseline cellularity of the aspirates includes both lymphocytes and bronchial epithelial cells because specimens may be contaminated with bronchial epithelial cells captured when the needle traverses the bronchial wall; in addition, a variety of entities have overlapping cytomorphologic features, and a paucity of published literature pertains to cytomorphology of these tumors.15 For lymphoma, even though the diagnostic accuracy of EBUS-TBNA is less than that seen for other cancers, the procedure appears justified in patients with isolated mediastinal lymphadenopathy, given the significant proportion (76%) of patients with lymphoma who would thus avoid a surgical biopsy.19
Team Experience
Good communication with the cytopathologist is important to increase the yield of the procedure, because sample preparation, triage, and interpretation ultimately depend on the question being asked. The pathologist should be told whether the procedure is performed for suspected primary lung cancer, for staging purposes, or to rule out metastasis or second primaries. In patients with known or suspected lymphoma or infection, additional tests on samples may be warranted.* Failure to begin the procedure with an expected result in mind and failure of the pathologist to understand the question being asked contribute to suboptimal interpretation of cytologic findings.20
Diagnostic Alternatives
1. CT-guided percutaneous needle aspiration of the nodule: This technique has a high diagnostic rate (91%) but does not provide mediastinal staging and increases the risk for pneumothorax (5% to 60%).21
2. Esophageal ultrasound-guided fine-needle aspiration (EUS-FNA) alone: EUS alone is suitable for assessing lymph nodes in the posterior aspect of lymph node stations 4L, 5, and 7, and in the inferior mediastinum at stations 8 and 9. EUS alone has limited value for complete staging because right-sided nodes are usually inaccessible.
3. Mediastinoscopy: This approach is traditionally considered the gold standard for mediastinal staging. It is more invasive, and bronchoscopic airway inspection might still be required. Mediastinoscopy provides systematic exploration and biopsy under visual guidance of nodal stations 1, 2, 3, 4, and 7.
4. Combined EUS and EBUS followed by mediastinoscopy: Because EBUS alone provides no access to lymph node stations 5, 6, 8, and 9, a strategy combining endosonography (EUS and EBUS) and mediastinoscopy (if no nodal metastasis was detected at endosonography) resulted in greater sensitivity for mediastinal nodal metastases and fewer unnecessary thoracotomies (needed in only 1 of 7 patients) in comparison with mediastinoscopy alone.22 This study used thoracotomy with nodal dissection as the reference standard in both study groups. Although stage IIIA is likely in our patient with ipsilateral mediastinal lymphadenopathy, tissue confirmation was necessary because computed tomography is known to be inaccurate for staging purposes. In one study, up to 28% of patients with high clinical suspicion of nodal disease had mediastinal nodal metastases confirmed by mediastinoscopy despite negative EBUS-TBNA.23 Therefore, a negative EBUS-TBNA should be followed by mediastinoscopy in patients with suspected mediastinal nodal involvement based on CT or PET.
5. Thoracotomy with nodal dissection: In this patient with highly suspected mediastinal nodal disease, this technique is neither cost-effective nor therapeutically advantageous.
Risk-Benefit Analysis
No serious complications are reported in the published literature on EBUS-TBNA, but cough and bleeding at the puncture site are described infrequently, usually when procedures are performed under moderate (conscious) sedation.24 The benefits of performing this procedure were that diagnosis and staging could be done at the same time, expected yield was excellent, and specimens would be collected for genetic testing that potentially would be useful for selecting targeted therapy based on specific research protocols.
Informed Consent
After the risks, benefits, and alternatives were discussed, our patient chose to proceed with EBUS-TBNA. On further questioning, he stated he had no health care advance directives and had no intention of initiating one at that time. The importance of advance directives may not be equally appreciated by all ethnic groups. In one study, for example, a survey showed that no Korean respondents and few Hispanics had advance directives. Low rates of advance directive completion among nonwhites may reflect health care disparities, distrust of the health care system, different cultural perspectives regarding death and suffering, and differences in family dynamics and parent-child relationships.25
Techniques and Results
Anesthesia and Perioperative Care
The patient’s pulmonary hypertension could lead to perioperative complications. One study of 28 patients undergoing major or minor surgeries under general and regional anesthesia revealed a perioperative death rate of 7%.* Perioperative complications attributed to pulmonary hypertension occurred in 29% of patients, regardless of the underlying cause of the pulmonary hypertension. Most (92%) of the complications occurred in the first 48 hours following surgery, and risk factors for complications were greater for emergency and major surgery and with a long operative time (193 minutes vs. 112 minutes; P = .003).26 A larger study evaluated 145 surgical patients with pulmonary hypertension, excluding those in whom the condition was due to left heart disease.27 Complications included respiratory failure (n = 41), cardiac arrhythmia (n = 17), congestive heart failure (n = 16), renal insufficiency (n = 10), and sepsis (n = 10), and risk predictors included a history of pulmonary embolus, NYHA functional class of II or greater, intermediate- or high-risk surgery, and a duration of anesthesia greater than 3 hours.
Our patient underwent the procedure in the operating room, under general anesthesia, and was intubated with a No. 9 endotracheal tube (ETT). With this method, the EBUS scope is directed more centrally in the airway; this could make needle aspirations more difficult, especially if the nodes are laterally located. In such cases, the ETT is moved proximally in the upper trachea—a maneuver that may provide more space for manipulating the scope inside the lower trachea. Laryngeal mask airway (LMA) can also be used instead of ETT for this purpose. In addition, LMA permits evaluation of the upper paratracheal nodes, which may not be accessible if an ETT is used.†28 EBUS can also be performed under moderate sedation in the bronchoscopy suite. This may result in safety and cost savings compared with general anesthesia, but aspiration of smaller nodes is technically more difficult.29 Furthermore, at the time of this writing, most published studies of EBUS-TBNA showing high diagnostic rates were done with the patient under general anesthesia.
Technique and Instrumentation
We used a dedicated EBUS bronchoscope (BF-UC180 F, Olympus, Tokyo, Japan) with an integrated convex transducer at the tip of the scope, which scans parallel to the insertion direction of the bronchoscope, and a 22-gauge needle to perform TBNA (NA-201SX-4022, Olympus). After the lymph node had been penetrated, the internal stylet was used to push out the bronchial wall debris, which may clog the internal lumen, and then the stylet was removed. Negative pressure can be applied with a syringe and the needle moved back and forth inside the target 10 to 15 times over 30 to 60 seconds.30 In some instances, the bronchoscopist may choose to avoid suction, having obtained the specimen simply through needle insertion into the node.
Many times, the bronchoscopist notices the needle in the lesion, but aspirates are acellular, or only blood or bronchial cells are seen. From a “targeting” perspective, the node is properly accessed, but acquisition of lymph node material is suboptimal. The question that may have to be asked is not whether the needle is in the lesion, but whether the lesion is in the node.31 This means that penetration of the needle into the node does not guarantee the presence of lymph node tissue inside the needle. In this regard, the principles of any fine-needle aspiration technique include the following: (1) Ensure the cutting action of the needle by performing a fast, downstroke movement (see video on ExpertConsult.com) (Video IV.16.1![]() ); and (2) minimize the amount of blood in the sample by following a straight needle trajectory, for instance, if the node is sampled from different entry locations, the needle should be repositioned outside the target first, thus avoiding tissue tearing and minimizing suction within the target, especially for vascular nodes (i.e., renal cell carcinoma, melanoma). High negative pressure may rupture nodal capillaries (see video on ExpertConsult.com) (Video IV.16.2
); and (2) minimize the amount of blood in the sample by following a straight needle trajectory, for instance, if the node is sampled from different entry locations, the needle should be repositioned outside the target first, thus avoiding tissue tearing and minimizing suction within the target, especially for vascular nodes (i.e., renal cell carcinoma, melanoma). High negative pressure may rupture nodal capillaries (see video on ExpertConsult.com) (Video IV.16.2![]() ). Minimizing or eliminating suction altogether, while moving the needle fewer than a dozen times inside the node, reduces transit time and potentially decreases the chance for retrieving blood clot rather than a tissue sample.31 With a cytologist available for rapid on-site examination (ROSE) of the aspirates, TBNA is continued until adequate sampling is confirmed. Without ROSE, three aspirates per lymph node station or two aspirations with one core tissue specimen is recommended.32 Feedback from the cytopathologist, whether obtained at the time of ROSE or later after final results are available, is important to improve one’s technique. Operators can examine the quality of their stained smears to judge adequacy and quality of the aspirates.* In addition to technique, other reasons for false-negative aspirates have been identified, including partial tumor invasion within the lymph node. Complementary optical technologies such as spectroscopy or optical coherence tomography may have a role in the future by directly guiding needle aspiration toward abnormal zones within lymph nodes.33,34
). Minimizing or eliminating suction altogether, while moving the needle fewer than a dozen times inside the node, reduces transit time and potentially decreases the chance for retrieving blood clot rather than a tissue sample.31 With a cytologist available for rapid on-site examination (ROSE) of the aspirates, TBNA is continued until adequate sampling is confirmed. Without ROSE, three aspirates per lymph node station or two aspirations with one core tissue specimen is recommended.32 Feedback from the cytopathologist, whether obtained at the time of ROSE or later after final results are available, is important to improve one’s technique. Operators can examine the quality of their stained smears to judge adequacy and quality of the aspirates.* In addition to technique, other reasons for false-negative aspirates have been identified, including partial tumor invasion within the lymph node. Complementary optical technologies such as spectroscopy or optical coherence tomography may have a role in the future by directly guiding needle aspiration toward abnormal zones within lymph nodes.33,34
After we retrieved our specimen, the aspirated material was pushed onto a glass slide. We did not retrieve a core of tissue,* but if this had been obtained, it could have been placed on filter paper to absorb excess blood and then fixed with 10% neutral buffered formalin, so that a formalin-fixed paraffin-embedded sample would have been made and further stained with hematoxylin and eosin for histologic diagnosis.†30 The rest of the material was smeared onto glass slides for both air-dried smears and was immediately fixed with 95% ethanol smears. The air-dried smears were stained by Diff-Quick staining for ROSE or Giemsa stain solution. The ethanol-fixed smears were sent for Papanicolau staining. The contents of the EBUS-TBNA needle were washed into a tube containing saline and were used for molecular testing if warranted.
Anatomic Dangers and Other Risks
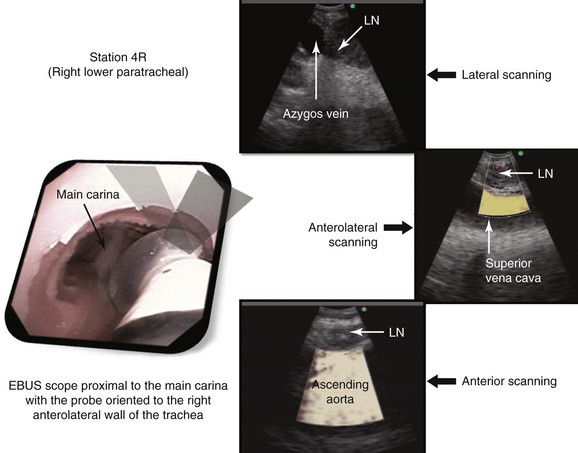
At station 4R, the major blood vessels include pulmonary artery, azygos vein, superior vena cava, and ascending aorta. The lung is also visualized (Figure 16-4). The risk of penetrating a major vessel may be reduced with Doppler mode imaging (see Figure 16-4). To date, no cases of major bleeding have been reported.35 It has been shown that dedicated EBUS-TBNA needles can release metal particles consisting of iron, titanium, nickel, and chromium, probably resulting from friction between the stylet and the needle. A potential risk is involved in injecting particles into nodes. The long-term consequences of lymph node contamination are currently unknown.36
Long-Term Management
Outcome Assessment
At the time of final interpretation, Papanicolaou- and Diff-Quick–stained smears, as well as hematoxylin and eosin (H&E)-stained sections of the formalin-fixed cell block, showed adenocarcinoma. The procedure was considered successful because a diagnosis was obtained by using a minimally invasive technique. Findings were shared with the patient’s daughter, who asked us to refrain from informing the patient of his diagnosis. She believed that learning he had cancer would cause him unnecessary anxiety and depression. Being asked to avoid truthfulness regarding diagnosis or prognosis often poses a significant ethical dilemma for health care providers, who must juggle with the principles of autonomy, beneficence, nonmaleficence, and justice in their daily practices.37 Truth telling respects the rights of patients and their families to receive accurate information about their illness. In fact, several studies demonstrate that most patients expect and desire truthfulness about their health. Nondisclosure can be viewed as a betrayal of trust, a rupture of the tacit agreement between patient and physician that both shall be forthright in their dialogue.
On the other hand, we were aware that certain ethnic groups prefer not to be directly informed of a life-threatening diagnosis, and that on many occasions, families might make medical decisions without disclosing the whole truth when one of its family members is ill. A long tradition of family-centered health care decisions38 has been noted, for example, in South America, where more than 60% of physicians reported informing families only in cases of a patient’s fatal prognosis.39 In Japan, Taiwan, and China, however, most patients with cancer were more likely than their families to believe that they should be informed of their diagnosis.40,41 In the culturally diverse United States, investigators compared persons of black and European descent versus those of Korean and Mexican American descent and found that Koreans and Mexican Americans were more likely to consider family members, rather than the patient alone, as holding decision-making power regarding life support.42 Even with acculturation, minorities may still view the decision-making process as a family-centered process.43 Among Asian cultures, family-based medical decisions are viewed as a function of filial devoutness, with an orientation toward the extended family as opposed to individual patient self-interest.43 Hence, illness is often considered a family affair rather than an individual event, and a sense of obligation can make it difficult for relatives to accept anything other than extraordinary measures of care.44
When treating patients from cultures with norms of nondisclosure, physicians might offer to provide diagnostic and treatment information to the patients themselves. Physicians can ask patients how they would like treatment decisions to be made, to help them determine the extent to which patients and family members wish to be involved in the decision-making process. By offering autonomy to patients,* cultural customs are respected, while rights to independent decision making are simultaneously acknowledged. A patient who refuses diagnostic information and prefers family- or physician-centered decision making has made a clear, voluntary choice. If a patient prefers that family members receive information, the physician should find out which family member(s) will be responsible for decision making. In our case, based on his request, the patient’s daughter was clearly identified as the primary decision maker.
Follow-up Tests and Procedures
We discussed with our pathologist and oncologist the rationale for sending specimens for genetic analysis. Advances in targeted therapies may lead to an era of personalized medicine. This is particularly important because lung cancer is proving to be a heterogeneous collection of diseases with highly diverse pathogeneses and molecular characteristics, even within a single histologic type such as adenocarcinoma.45 As of this writing, several biomarkers are thought to predict the likelihood of a patient’s response to a specific chemotherapeutic drug for NSCLC. Some oncologists apply testing and treatment algorithms when they encounter any new patient with advanced NSCLC,45 first, EGFR mutation analysis: If a mutation is present, the patient is a candidate for EGFR-TKI.46 If no response is noted, or if the mutation is not present, the tumor is assessed for EML4/ALK rearrangements and, if present, is treated with crizotinib.14 If no response or evidence of EML4/ALK fusion is noted, the tumor is assessed for ERCC1 and RRM1.* Patients with low levels are treated with a platinum plus gemcitabine combination.47 If no response is seen, levels of ERCC1† and TS‡ are assessed. Patients with low ERCC1 and TS levels are treated with a platinum plus pemetrexed combination. For those in whom these treatments are ineffective and for those who have high levels of ERCC1, default treatment with a taxane plus a nonplatinum drug is provided. For practical purposes, bronchoscopists may need to perform additional aspirates, ideally obtaining core biopsies so that specimens can be preserved for further molecular analysis if necessary. This is especially important today because almost 70% of patients with lung cancer are diagnosed on the basis of small biopsy or cytology specimens.48 In our patient, EGFR and EML/ALK4 mutations were negative. Testing for other biomarkers was deferred until a formal oncology consultation could be obtained.
Referrals
Targeted therapy is highly dependent on which pathway is activated for a particular tumor. In this regard, erlotinib targets EGFR signaling, sorafenib, ras/raf signaling, vandetanib, vascular endothelial growth factor (VEGF) and EGFR signaling, and bexarotene, the retinoid X receptor for signaling.49 The patient was referred to an oncologist, who actively practiced targeted therapy. This appointment was arranged to occur within 10 days after the procedure.
Quality Improvement
In this case, the EBUS-TBNA aspirate showed adenocarcinoma, confirming the diagnosis and staging the tumor as stage IIIA. The specimen was adequate for interpretation. Lymph node sampling is represented by abundant dispersed lymphocytes, lymphoid aggregates, anthracotic histiocytes, granulomatous aggregates, or tumor. The “adequate sampling” definition is controversial, prompting some experts to recommend using the term nondiagnostic only for those cases that truly have no evidence of lymph node sampling. Cases with sparse evidence of lymph node sampling should be reported as such, with the understanding that the chance of false-negative results may be increased.20
Discussion Points
1. Define the borders of station 4R and justify this definition.
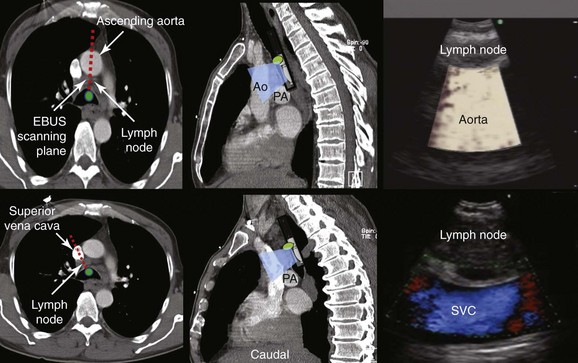
Station 4R includes right lower paratracheal nodes and pretracheal nodes extending to the left lateral border of the trachea. Lymphatic drainage in the superior mediastinum predominantly occurs to the right paratracheal area and extends past the midline of the trachea; therefore the boundary between lymph nodes of the right- and left-sided levels 2 and 4 has been reset to the left lateral wall of the trachea.50 When the scope is oriented anteriorly in the lower trachea or in the right mainstem bronchus at the level of the main carina, the node seen is still the right lower paratracheal node (station 4R). The Doppler-positive vessel behind it could represent the superior vena cava or the ascending aorta, depending on the actual orientation of the scope (Figure 16-5). Based on IASLC definitions, the upper border of station 4R is the intersection of the caudal margin of the innominate vein with the trachea, and the lower border is represented by the lower border of the azygos vein (see Figure 16-3).50
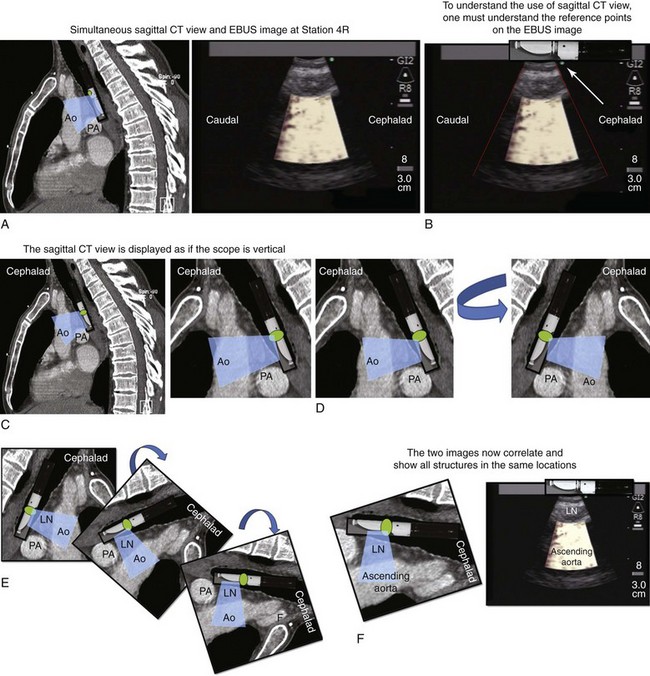
2. Describe how the sagittal view of a computed tomography scan is used to plan EBUS-TBNA at station 4R.
A sagittal (aka median) plane is perpendicular to the ground; this separates left from right. The midsagittal plane is the specific sagittal plane that is exactly in the middle of the body. To visualize station 4R in its anterior carinal position, the scope is positioned just proximal to the main carina and is oriented anteriorly toward the 12 o’clock position; therefore when 4R has a pretracheal component, the sagittal view is useful and the ascending aorta will be visualized just distal to the node; the superior vena cava (SVC) can be seen most times when the transducer is oriented slightly toward the right anterolateral wall (1 o’clock position), and the azygos vein, when oriented toward the right lateral wall (3 o’clock position) (see Figures 16-4 and 16-5). In this patient, the EBUS image at station 4R shows the pattern in Figure 16-6, when the scanning plane is anterior toward the ascending aorta (Doppler mode on). The scanning plane for the EBUS scope is the same as in the sagittal CT view (see Figure 16-6). However, although the sagittal CT view is projected as if the scope is vertical, the EBUS image is projected as if the scope is horizontal. Thus, to understand the reasons for use of the sagittal CT view, one must understand the reference points on the EBUS image (see Figure 16-6). The green dot on the monitor represents the point where the needle exits the scope and corresponds to the superior (cephalad) aspect of the body. This dot is by default toward the 1 o’clock position on the screen (see Figure 16-6). Reorientation of the sagittal CT image is necessary to bring the scope to a horizontal position and to bring the green dot cephalad (toward the 1 o’clock position on the screen) to match the EBUS image. For practical purposes, one can print out or save the sagittal CT image showing station 4R as a separate picture. This picture is flipped over like a page, then is rotated clockwise to “horizontalize” the scope and bring the green dot cephalad toward the 1 o’clock position (see Figure 16-6), so that the two images (EBUS and CT) now correlate and show the lymph node and the aorta in similar positions.
Expert Commentary
Malignant tumors often produce cellular aspirates, a manifestation of decreased cell cohesion. Establishing adequacy of the sample is not a problem when malignant cells are identified, because the specificity is close to 100%. However, in cases in which no malignant cells are readily identified (i.e., negative cases), classifying the sample as adequate is more challenging. The presence of abundant lymphocytes in an aspirate is a good indication that the needle was in a lymph node, and multiple passes usually ensure that the nodes were thoroughly sampled. However, the presence of a few lymphocytes, particularly in a background of mucus and bronchial epithelial cells, calls into question the adequacy of the sample and probably should not be classified as an indication of absence of malignancy.51
Cytopathologists rely on individual cell morphology for making a diagnosis, and they do not have the benefit of being able to assess relations between cell groups and tissues, as do their colleagues examining histopathologic material. Differentiation between some neoplasms based solely on isolated cytologic criteria can be difficult. The use of immunocytochemical ancillary techniques, however, greatly enhances the ability of cytopathology to close this gap.16
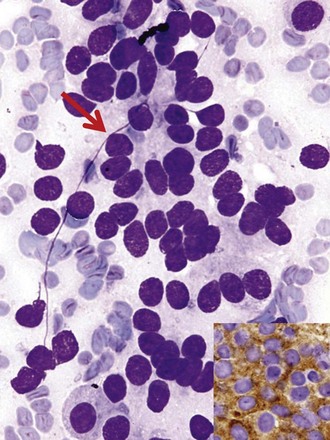
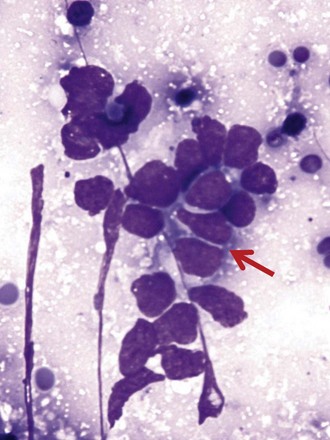
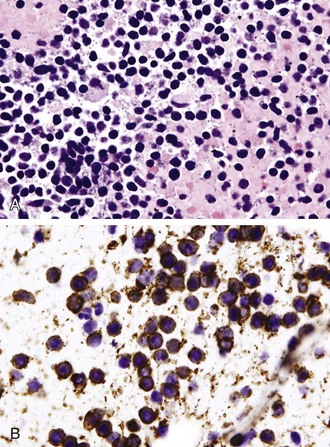
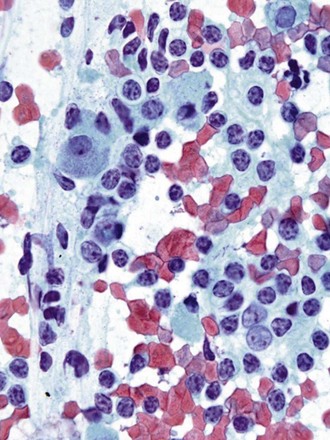
Differentiation between carcinoid tumor, small cell carcinoma, and lymphoma can be challenging cytologically. Carcinoid tumors produce cellular specimens with fairly uniform round to oval nuclei that have a characteristic “salt and pepper” chromatin clumping and occasionally an attempt at rosette formation (Figure 16-7). The granular cytoplasm stains positively for neuroendocrine markers such as chromogranin and synaptophysin. Differentiation between typical and atypical carcinoid tumors, however, is not always feasible on a cytologic basis alone, and requires additional sampling and correlation with clinical and imaging studies. Small cell carcinoma, another neuroendocrine tumor, is characterized by undifferentiated cells that are small, yet larger than lymphocytes (Figure 16-8). The cytoplasm is scant at best, unlike that of neuroendocrine large cell carcinoma. The nuclei show molding to accommodate neighboring nuclei, and streaks of nuclear material are often seen in the background as a result of smearing. Such crush artifact, although not diagnostic by itself, raises a red flag to the microscopist and is a reflection of the fragility of the nuclei. Malignant lymphoma is another tumor that enters into the differential diagnosis of small cell tumors. It can involve the lung as a primary lesion, or more often as part of systemic disease. The cytomorphology depends on the type of neoplasm. When an abundant infiltrate by lymphocytes is encountered in the aspirate, particularly if lymphocytes show atypia or the presence of nucleoli, a second aspirate dedicated to flow cytometric evaluation is recommended. If not, a cell block may be prepared and stained for various lymphocyte markers to establish monoclonality, and thus neoplasia (Figure 16-9). The sensitivity and specificity of cytologic diagnosis of non-Hodgkin lymphoma show variability among different studies; they are dependent on the availability of an adequate number of lymphocytes for flow cytometry analysis and dilution of the sample by a significant component of bronchial columnar cells and blood. Hodgkin lymphoma can be diagnosed if the clinical and cytologic characteristics are taken in consideration, but greater sampling is often necessary to subclassify the disease. Reactive lymph node aspirates demonstrate a polymorphous population of lymphocytes at various stages of maturation, unlike the monomorphous population encountered in aspirates from lymphomas that can be easily established by flow cytometric immunophenotyping. Identification of histiocytic cells with phagocytized material in their cytoplasm (tingible body macrophages) is also helpful in supporting a benign diagnosis. In cases where abundant histiocytes are encountered, a granulomatous reaction should be considered, and material should be reserved for microbiologic studies (Figure 16-10).
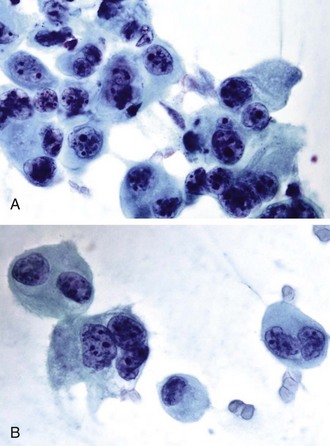
Neoplasms with large cells form a heterogeneous group that includes squamous cell carcinomas, adenocarcinomas, large cell carcinomas, metastatic carcinomas, and melanomas, among others. The cytologic characteristics of these tumors can establish the diagnosis in many cases, or at least can narrow the differential diagnosis. Immunohistochemical stains can further refine or confirm the diagnosis in cases where cytomorphologic features overlap. A detailed discussion of the specific morphologic characteristics of this heterogeneous group of tumors is beyond the scope of this commentary,* but a description of a few basic principles and problem areas is appropriate. Adenocarcinomas, for example, can present particular challenges in terms of their classification. The cytologic patterns of a tumor can be variable and often overlap with those of other tumor types. Decisions as to whether the tumor is primary or metastatic, the type of primary lesion, and the source of metastasis can be difficult to ascertain, particularly in the absence of a known primary. In general, malignant glandular cells tend to have abundant cytoplasm with evidence of polarization of their nuclei to one end. Bronchioloalveolar carcinomas (BACs), on histologic examination, have a characteristic pattern of spread along alveolar septa. Such a pattern, however, can be encountered with other adenocarcinomas, including metastasis.
Cytologic samples can be difficult to interpret because cells may be quite bland with minimal evidence of malignancy. Other BACs show abundant mucinous cells. Cytologic parameters for adenocarcinomas will have to be adapted to the classification of non–small cell carcinoma (NSCC) recently developed by the International Association for the Study of Lung Cancer and other American and European organizations. Data on patient survival support the validity of this new classification. In situ, minimally invasive and lepidic-predominant adenocarcinomas have excellent 5-year survival rates, and micropapillary-predominant and solid with mucin-predominant tumors are associated with poor survival. Papillary and acinar-predominant tumors have an intermediate survival rate.52 Giant cell tumors, as illustrated in Figure 16-11, are poorly differentiated neoplasms associated with aggressive behavior. Careful examination of the material in some of these tumors may reveal subtle clues of differentiation into a glandular or squamous cell line. By using immunocytochemical and molecular marker studies, as discussed later, our ability to further characterize these tumors may be enhanced, decreasing the number of tumors that cannot be further categorized. One has also to recognize that some neoplasms can show differentiation along two or more cell lines, such as squamous cell and/or small cell and/or adenocarcinoma.
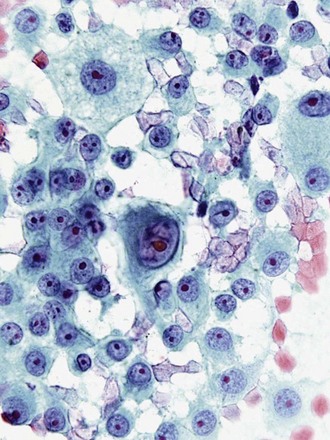
Last, metastatic neoplasms may present with poorly differentiated highly atypical cells, and some aspirates fail to elucidate the source of the primary tumor, unless a history of another primary has been reported. In some cases, cytologic criteria point to a specific tumor type. The presence of elongated cigar-shaped nuclei and a necrotic background often points to metastasis from the colon, rather than a primary lung adenocarcinoma. Prominent nucleoli, intranuclear cytoplasmic inclusions, and multinucleation point to a malignant melanoma, even in the absence of melanin pigment (Figure 16-12). In many cases, confirmation rests on positive staining with immunocytochemical markers, such as Melan-A and chromogranin.
* Detailed discussions of specific morphologic characteristics of these is tumors is available in A Colour Atlas of Endoscopic Diagnosis in Early Stage Lung Cancer by Kato H, Horai T (Mosby 1991); in Pulmonary Cytopathology by Erozan Y, Ramzy I (Springer 2009); and in online cytopathology and pathology resources (http://137.189.150.85/cytopathology/link.html).
1. Goeckenjan G, Sitter H, Thomas M, et al. Prevention, diagnosis, therapy, and follow-up of lung cancer: interdisciplinary guideline of the German Respiratory Society and the German Cancer Society. Pneumologie. 2011;65:39-59.
2. Task Force for Diagnosis and Treatment of Pulmonary Hypertension of European Society of Cardiology (ESC), European Respiratory Society (ERS), International Society of Heart and Lung Transplantation (ISHLT), et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2009;34:12-19.
3. Fisher MR, Forfia PR, Chamera E, et al. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med. 2009;179:615-621.
4. Sandham JD, Hull RD, Brant RF, et al. A randomized, controlled trial of the use of pulmonary-artery catheters in high-risk surgical patients. N Engl J Med. 2003;348:5.
5. Garcia-Olivé I, Monsó E, Andreo F, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for identifying EGFR mutations. Eur Respir J. 2010;35:391-395.
6. Sakairi Y, Nakajima T, Yasufuku K, et al. EML4-ALK fusion gene assessment using metastatic lymph node samples obtained by endobronchial ultrasound-guided transbronchial needle aspiration. Clin Cancer Res. 2010;16:4938-4945.
7. Lim EH, Zhang SL, Li JL, et al. Using whole genome amplification (WGA) of low-volume biopsies to assess the prognostic role of EGFR, KRAS, p53, and CMET mutations in advanced-stage non-small cell lung cancer (NSCLC). J Thorac Oncol. 2009;4:12-21.
8. Park S, Holmes-Tisch AJ, Cho EY, et al. Discordance of molecular biomarkers associated with epidermal growth factor receptor pathway between primary tumors and lymph node metastasis in non-small cell lung cancer. J Thorac Oncol. 2009;4:809-815.
9. Schmid K, Oehl N, Wrba F, et al. EGFR/KRAS/BRAF mutations in primary lung adenocarcinomas and corresponding locoregional lymph node metastases. Clin Cancer Res. 2009;15:4554-4560.
10. Herth F, Becker HD, Ernst A. Conventional vs endobronchial ultrasound-guided transbronchial needle aspiration: a randomized trial. Chest. 2004;125:322-325.
11. Herth FJ, Eberhardt R, Vilmann P, et al. Real-time endobronchial ultrasound guided transbronchial needle aspiration for sampling mediastinal lymph nodes. Thorax. 2006;61:795-798.
12. Shigematsu H, Gazdar AF. Somatic mutations of epidermal growth factor receptor signaling pathway in lung cancers. Int J Cancer. 2006;118:257-262.
13. Bae NC, Chae MH, Lee MH, et al. EGFR, ERBB2, and KRAS mutations in Korean non-small cell lung cancer patients. Cancer Genet Cytogenet. 2007;173:107-113.
14. Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693-1703.
15. Monaco SE, Schuchert MJ, Khalbuss WE. Diagnostic difficulties and pitfalls in rapid on-site evaluation of endobronchial ultrasound guided fine needle aspiration. Cyto J. 2010;7:9.
16. Erozan YS, Ramzy I. Primary epithelial malignancies. In: Rosenthal DL, editor. Pulmonary Cytopathology: Essential in Cytopathology Series. New York: Springer; 2008:146-153.
17. Nakajima T, Yasufuku K, Iyoda A, et al. The evaluation of lymph node metastasis by endobronchial ultrasound-guided transbronchial needle aspiration: crucial for selection of surgical candidates with metastatic lung tumors. J Thorac Cardiovasc Surg. 2007;134:1485-1490.
18. Kennedy MP, Jimenez CA, Bruzzi JF, et al. Endobronchial ultrasound-guided transbronchial needle aspiration in the diagnosis of lymphoma. Thorax. 2008;63:360-365.
19. Steinfort DP, Conron M, Tsui A, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for the evaluation of suspected lymphoma. J Thorac Oncol. 2010;5:804-809.
20. Stewart J. EBUS: a cytopathologist’s perspective, Conference syllabus. Interventional Pulmonology in Cancer Patients, 2011:175-183
21. Toloza EM, Harpole L, Detterbeck F, et al. Invasive staging of non-small cell lung cancer: a review of the current evidence. Chest. 2003;123:157S-166S.
22. Annema JT, van Meerbeeck JP, Rintoul RC, et al. Mediastinoscopy versus endosonography for mediastinal nodal staging of lung cancer: a randomized trial. JAMA. 2010;304:2245-2252.
23. Defranchi SA, Edell ES, Daniels CE, et al. Mediastinoscopy in patients with lung cancer and negative endobronchial ultrasound guided needle aspiration. Ann Thorac Surg. 2010;90:1753-1758.
24. Varela-Lema L, Fernandez-Villar A, Ruano-Ravina A. Effectiveness and safety of endobronchial ultrasound–transbronchial needle aspiration: a systematic review. Eur Respir J. 2009;33:1156-1164.
25. Blackhall LJ, Murphy ST, Frank G, et al. Ethnicity and attitudes toward patient autonomy. JAMA. 1995;274:820-825.
26. Price LC, Montani D, Jaïs X, et al. Noncardiothoracic nonobstetric surgery in mild-to-moderate pulmonary hypertension. Eur Respir J. 2010;35:1294-1302.
27. Ramakrishna G, Sprung J, Ravi BS, et al. Impact of pulmonary hypertension on the outcomes of noncardiac surgery: predictors of perioperative morbidity and mortality. J Am Coll Cardiol. 2005;45:1691-1699.
28. Douadi Y, Bentayeb H, Malinowski S, et al. Anaesthesia for bronchial echoendoscopy: experience with the laryngeal mask. Rev Mal Respir. 2010;27:37-41.
29. Sarkiss M, Kennedy M, Riedel B, et al. Anesthesia technique for endobronchial ultrasound-guided fine needle aspiration of mediastinal lymph node. J Cardiothorac Vasc Anesth. 2007;21:892-896.
30. Nakajima T, Yasufuku K. How I do it—optimal methodology for multidirectional analysis of endobronchial ultrasound-guided transbronchial needle aspiration samples. J Thorac Oncol. 2011;6:203-206.
31. Papanicolau Society of Cytopathology. Optimal FNA techniques. www.papsociety.org/fna.html. Accessed April 23, 2011
32. Lee HS, Lee GK, Lee HS, et al. Real-time endobronchial ultrasound-guided transbronchial needle aspiration in mediastinal staging of non-small cell lung cancer: how many aspirations per target lymph node station? Chest. 2008;134:368-374.
33. Kanick SC, van der Leest C, Aerts JG, et al. Integration of single-fiber reflectance spectroscopy into ultrasound-guided endoscopic lung cancer staging of mediastinal lymph nodes. J Biomed Opt. 2010;15:017004.
34. McLaughlin RA, Scolaro L, Robbins P, et al. Imaging of human lymph nodes using optical coherence tomography: potential for staging cancer. Cancer Res. 2010;70:2579-2584.
35. Yasufuku K, Chiyo M, Sekine Y, et al. Real-time endobronchial ultrasound-guided transbronchial needle aspiration of mediastinal and hilar lymph nodes. Chest. 2004;126:122-128.
36. Gounant V, Ninane V, Janson X, et al. Release of metal particles from needles used for transbronchial needle aspiration. Chest. 2011;139:138-143.
37. Brody H, Tomlinson T. Commentary. J Fam Pract. 1988;26:404-406.
38. Searight HR, Gafford J. Cultural diversity at the end of life: issues and guidelines for family physicians. Am Fam Physician. 2005;71:515-522.
39. de Souza Trindade E, de Azambuja LE, Andrade JP, et al. O médico frente ao diagnóstico e prognóstico do câncer avançado. Rev Assoc Med Bras. 2007;53:68-74.
40. Fujimori M, Parker PA, Akechi T, et al. Japanese cancer patients’ communication style preferences when receiving bad news. Psychooncology. 2007;16:617-625.
41. Jiang Y, Liu C, Li JY, et al. Different attitudes of Chinese patients and their families toward truth telling of different stages of cancer. Psychooncology. 2007;16:928-936.
42. Blackhall L, Murphy S, Frank G, et al. Ethnicity and attitudes toward patient autonomy. JAMA. 1995;274:820-825.
43. Kagawa-Singer M, Blackhall LJ. Negotiating cross-cultural issues at the end of life: “you got to go where he lives.”. JAMA. 2001;286:2993-3001.
44. Frank G, Blackhall LJ, Michel V, et al. A discourse of relationships in bioethics: patient autonomy and end-of-life decision making among elderly Korean Americans. Med Anthropol Q. 1998;12:403-423.
45. Gadgeel SM, Cote ML, Schwartz AG, et al. Parameters for individualizing systemic therapy in non-small cell lung cancer. Drug Resist Update. 2010;13:196-204.
46. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947-957.
47. Reynolds C, Obasaju C, Schell MJ, et al. Randomized phase III trial of gemcitabine-based chemotherapy with in situ RRM1 and ERCC1 protein levels for response prediction in non-small-cell lung cancer. J Clin Oncol. 2009;27:5808-5815.
48. Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244-285.
49. Printz C. BATTLE to personalize lung cancer treatment: novel clinical trial design and tissue gathering procedures drive biomarker discovery. Cancer. 2010;116:3307-3308.
50. Rusch VW, Asamura H, Watanabe H, et al. Members of IASLC Staging Committee. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol. 2009;4:568-577.
51. Baker JJ, Solanki PH, Schenk DA, et al. Transbronchial needle aspiration of the mediastinum: importance of lymphocytes as an indicator of specimen adequacy. Acta Cytol. 1990;34:517-523.
52. Russell PA, Wainer Z, Wright GM, et al. Does lung adenocarcinoma subtype predict patient survival? A clinicopathologic study based on the New International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society International Multidisciplinary Lung Adenocarcinoma Classification. J Thorac Oncol. 2011 Jun 2. [Epub ahead of print]
* This group includes pulmonary hypertension due to COPD, interstitial lung disease, other pulmonary diseases with a mixed restrictive and obstructive pattern, obstructive sleep apnea, alveolar hypoventilation disorders, and other causes of hypoxemia.
† A major limitation of the study was that catheterization and Doppler echocardiography were not performed simultaneously. Doppler echocardiography was performed within 1 hour of a clinically indicated right-heart catheterization.
* Although overestimations and underestimations occurred with similar frequency, the magnitude of the pressure underestimation was greater than that of overestimation (−30 ± 16 vs. +19 ± 11 mm Hg; P = .03).
* Stages IIIA1-4 are defined as follows: IIIA1, mediastinal lymph node metastases on postoperative histologic examination at one lymph node level; IIIA2, intraoperative findings of lymph node involvement at one level; IIIA3, involvement of one or more positions identified preoperatively by mediastinoscopy, needle biopsy, or PET. IIIA4, bulky disease (mediastinal lymph nodes >2-3 cm with extracapsular invasion, lymph node involvement of multiple N2 positions, and groups of multiple, positive, 1- to 2-cm lymph nodes).
† The inner stylet does not completely prevent contamination with bronchial wall cells (normal or abnormal).
‡ Although sometimes visualized via EBUS but not adjacent to the airway, these stations are not accessible using this technique. Stations 5 and 6 are approached by using anterior mediastinotomy (Chamberlain procedure), and stations 8 and 9 via endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) or thoracoscopy.
* KRAS and BRAF function downstream of EGFR in the signaling pathway, and activating mutations have been described as usually mutually exclusive in EGFR-mutated tumors.
† KRAS mutations are detected in 15% to 57% of patients with lung adenocarcinoma from the United States and Europe and are associated with poor response to EGFR-directed TKI therapy.
‡ BRAF, a serine/threonine kinase, is mutated in only 1% to 2% of lung cancer patients.
§ EGFR mutations were seen in adenocarcinoma and were more frequent among women and never-smokers. EGFR mutations in adenocarcinomas were not associated with pathologic stage in never-smokers, but were more frequent in pathologic stage II-IV than in stage I disease among current and previous smokers.
* For instance, low-grade follicular lymphoma is difficult to distinguish from normal lymph node by morphology alone, so flow cytometry should be performed as well.
* At the time of surgery, 75% of patients were in NYHA functional class I-II.
† This is because the EBUS scope is directed more centrally in the airway through the ETT, and coupling of the transducer and the airway wall may be impaired and biopsies may be more difficult than when the scope is passed directly through an LMA.
* EBUS-guided FNAs or a nondiagnostic or negative specimen can be very cellular with numerous reactive bronchial cells (if nondiagnostic) and numerous lymphocytes (if diagnostic). This high cellularity creates a large number of diagnostic difficulties and makes these cases difficult to screen at the time of ROSE. Thus, the focus is not so much on the quantitative features, but is more on the qualitative features and the need to determine whether a sufficient number of the correct cells are present to indicate sampling of the target.
* Part of the core tissue can be stored with the use of stabilizing solutions for extraction of RNA.
† This specimen can be used for in situ hybridization, and DNA/RNA can be extracted for genetic analysis.
* This can be done by asking the following question: “Some patients want to know everything about their medical condition, but others do not. What is your preference?”
* Regulatory subunit of ribonucleotide reductase.
† Excision repair cross-complementation group 1; high levels of this protein in NSCLC tumors predict longer survival in patients randomized to treatment without chemotherapy and a lower likelihood of response to platinum-based chemotherapy.
‡ Thymidylate synthase: Low expression of this protein in NSCLC predicts better outcome regardless of treatment and potentially better response to neoadjuvant chemotherapy with pemetrexed.