Chapter 19 EBUS-Guided TBNA for Isolated Subcarinal Lymphadenopathy (Station 7)
Case Description
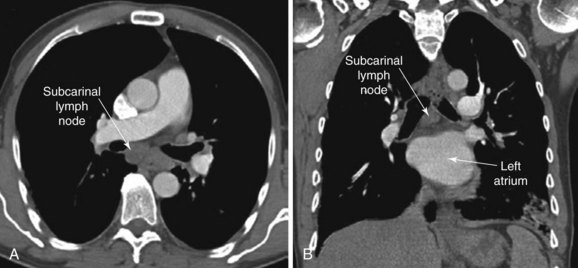
The patient is a 51-year-old male with a 10–pack-year history of smoking. His past medical history includes COPD (FEV1 60% predicted) controlled on tiotropium and albuterol inhalers and right toe amputation for melanoma 3 years earlier. A surveillance chest CT ordered by his oncologist showed a 2.5 × 2.7 cm subcarinal lymph node and no other abnormalities (Figure 19-1). The PET scan showed increased activity (SUV max 6) in the subcarinal node but no evidence of other thoracic or extrathoracic abnormalities. His physical examination was normal. He lives alone and is extremely concerned about a possible recurrence of melanoma. He has been referred for convex probe EBUS-guided TBNA.
Discussion Points
1. Describe how the coronal view of a computed tomography scan can be used to help plan the procedure in this patient.
2. Describe the yield of EBUS-guided TBNA versus conventional TBNA for sarcoidosis.
3. Describe the clinical implications of granulomatous inflammation detected on an EBUS-guided TBNA specimen.
4. List six sonographic criteria of morphology that can be used to describe mediastinal and hilar lymph nodes.
Case Resolution
Initial Evaluations
Physical Examination, Complementary Tests, and Functional Status Assessment
Chest computed tomography (CT) showed a large* 2.5 cm subcarinal lymph node with no associated pulmonary nodules, masses, or infiltrates. Although variations in normal node size are significant, depending on the location of the node, by convention, the upper limit of normal for mediastinal lymph nodes is considered to be 1 cm in the short axis, except in the subcarinal region, where an upper limit of 1.5 cm is generally used.1 Micrometastasis can be present, however, in the absence of lymph node enlargement in patients with known or suspected lung cancer or other malignancy. Conversely, enlarged lymph nodes may just be post inflammatory or hyperplastic, especially in a patient without cancer risk factors. Very large lymph nodes (short axis >2 cm) in the middle mediastinum, including the subcarinal region, often reflect metastatic primary lung carcinoma, metastatic extrapulmonary carcinoma,† lymphoma, tuberculosis, fungal disease (e.g., histoplasmosis, coccidioidomycosis), or sarcoidosis. A careful review of the chest CT is always warranted to avoid confusion with a bronchogenic cyst,‡ a dilated azygos vein, esophageal varices, or a hiatal hernia.
This patient does not have a high pretest probability for sarcoidosis or another granulomatous disorder. In sarcoidosis, lymph node enlargement is seen in more than 80% to 95% of cases but predominates in the right paratracheal, aorto-pulmonary window, and hilar regions. CT scans show enlarged subcarinal lymph nodes in approximately 65% of patients.2
Although enlargement of lymph nodes in a single station can be seen in patients with Hodgkin’s disease, this most often occurs in the anterior mediastinum. Non-Hodgkin’s lymphoma, on the other hand, may show involvement of only one node group in 40% of cases, most commonly involving the superior mediastinum. Another lymphoproliferative disorder, the hyaline vascular type of Castleman’s disease, is often asymptomatic, presents as a hilar or localized mediastinal mass in any mediastinal compartment, and could be responsible for this patient’s large subcarinal lymph node.2 The lack of multiple enlarged lymph nodes, constitutional symptoms, and normal laboratory markers makes rare lymphoproliferative disorders or leukemia less likely to explain this patient’s isolated subcarinal lymphadenopathy.
Comorbidities
Chronic obstructive pulmonary disease (COPD) is reportedly associated with an increased complication rate after bronchoscopy compared with that seen in patients with normal lung function. Concern for bronchospasm is increased, but premedication with inhaled short-acting agonists is not routinely recommended.3
Support System
This patient lived alone and had very few acquaintances. Given that our main differential diagnoses were cancer and sarcoidosis, we wondered whether he might eventually benefit from participation in support groups. For people with sarcoidosis, many of these groups are available online, and they may become necessary when physical and emotional problems associated with this disease arise. In self-help groups, patients might better cope with problems and concerns related to their disease and feelings of isolation.4 Similarly, for cancer, support groups focus on behavioral issues and symptoms, or on the expression of emotions. Most of these support programs are structured to ensure delivery of information and to provide emotional and social support, stress management strategies based on cognitive-behavioral approaches, and relaxation techniques. Group therapy helps patients gain emotional support from others with similar experiences and learn to use these experiences to reduce their fear of dying and uncertainty.5
Patient Preferences and Expectations
Our patient was anxious about his potential diagnosis and had many questions regarding his health. In such circumstances, effective communication skills improve a patient’s understanding of the condition and promote adherence to potential treatment regimens. Having good communication skills helps health care providers use time efficiently, avoid burnout, and enhance feelings of professional fulfillment. Blocking,* lecturing, depending on a routine, collusion,† coercion, and premature reassurance are usually ill advised. One well-recognized and effective communication skill is the “ask-tell-ask technique,” which is based on the notion that providing patient education requires knowing what the patient already knows and building on that knowledge. Of course, building a relationship with a patient requires that the physician listen to the patient, understand and empathize with his perspectives, show compassion, and respect the patient’s agenda, even if it is not quite in line at all times with the way the physician might want to do things.6
Procedural Strategies
Indications
In general, proposed indications for endobronchial ultrasound (EBUS)-guided transbronchial needle aspiration (TBNA) include nondiagnostic conventional TBNA, staging of the radiologically normal mediastinum in case of suspected or confirmed lung cancer, mediastinal restaging after induction chemotherapy, and, more commonly, diagnosis of mediastinal or hilar lymphadenopathy.7 In patients with a history of cancer, such as ours, the diagnosis of new mediastinal adenopathy suggests recurrence of malignancy; therefore this diagnosis needs to be excluded or confirmed. Because not all cases of PET-positive mediastinal adenopathy are due to cancer recurrence, lymph node sampling is usually warranted. In this patient with a newly discovered large subcarinal lymph node, a tissue diagnosis was going to be obtained using EBUS-guided TBNA with the patient under general anesthesia.
Expected Results
1. In patients with previous malignancy and PET-positive lymph nodes, EBUS-TBNA has a yield greater than 90%. In one study, 73 lymph nodes from 48 patients were sampled, with each patient undergoing mediastinoscopy or thoracoscopy immediately after needle aspiration for histologic confirmation. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of EBUS-TBNA were 97.4%, 100%, 100%, 87.5%, and 97.7%, respectively.8 In another study, similarly high sensitivity, specificity, diagnostic accuracy, and NPV of EBUS-TBNA for the diagnosis of mediastinal and hilar lymph node metastasis were found (92.0%, 100%, 95.3%, and 90%, respectively). Tumors encountered included colorectal, head and neck, ovarian, breast, esophageal, hepatocellular, prostate, renal, and germ cell cancers and melanoma.9
2. For primary lung carcinoma, a meta-analysis of 11 studies with 1299 patients found that overall, EBUS-guided TBNA (using both radial and convex probes) had a pooled sensitivity of 93% and a pooled specificity of 100%. The subgroup of patients selected on the basis of CT- or PET-positive results had higher pooled sensitivity (94%) than the subgroup of patients not selected on the basis of CT or PET findings (76%).10
3. The diagnosis of lymphoma may be controversial, although the use of flow cytometry, molecular biology techniques, and immunohistochemistry on cell block preparations may provide enough information for definitive diagnosis. Results from EBUS-TBNA were compared with a reference standard of pathologic tissue diagnosis of lymphoma or a composite of greater than 6 months of clinical follow-up with radiographic imaging. Nodes studied were larger than 5 mm and had a standardized uptake value (SUV) max higher than 4. Sensitivity was 90.9%, specificity 100%, PPV 100%, and NPV 92.9%.11 In another study, EBUS-TBNA had a sensitivity of 76%, but 20% of patients required surgical biopsy to completely characterize lymphoma subtypes, resulting in overall sensitivity of 57% and specificity of 100%.12
4. For fungal or mycobacterial infection, no studies have specifically addressed the yield of EBUS-TBNA. For patients with tuberculosis, however, in endemic areas, conventional TBNA has sensitivity of 83%, specificity of 100%, PPV of 100%, NPV of 38%, and accuracy of 85%.13 Future studies are necessary to determine whether EBUS-TBNA offers similar or better results.
5. For sarcoidosis, several studies have reported yields ranging from 82% to 95% for EBUS-TBNA.14–17
Team Experience
A single-institution study suggested that the learning curve for EBUS-TBNA for thoracic surgeons requires 10 procedures to reach the high yields reported in controlled clinical trials.18 In reality, however, the actual number of any given procedure performed does not account for the different rates at which people learn. With regard to EBUS-TBNA, one study assessed the learning curves of five independent operators by retrospectively applying cusum analysis* to the first 100 cases of each19; over a wide range of time, EBUS-TBNA competence was attained and the pooled sensitivity of EBUS-TBNA under conscious (moderate) sedation was 67.4%—lower than the high (>90%) rates reported in clinical trials.19
The usefulness of EBUS-TBNA resides in its clinical significance, accuracy, and reproducibility, which are usually shown to be excellent among pathologists experienced with these types of samples. Pathologists with little experience must climb what appears to be a steep learning curve.20
Diagnostic Alternatives
1. Conventional TBNA: For cancer, the yield of conventional TBNA for large subcarinal lymph nodes is similar to that of EBUS-TBNA, but EBUS guidance increases the yield of TBNA in all other stations.21 In the diagnosis of sarcoidosis, the overall diagnostic accuracy of TBNA cytology is as high as 86.2%.22
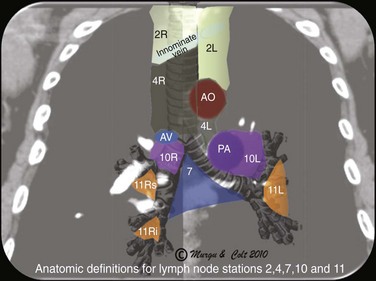
2. Endoscopic ultrasound (EUS)-FNA: EUS alone is suitable for assessing lymph nodes in the posterior aspect of lymph node stations 4L, 5 (when significantly enlarged), and 7, and in the inferior mediastinum at stations 8 and 9 (Figure 19-2); data from lung cancer studies show that EUS-FNA and EBUS-TBNA have similarly high yields for diagnosing cancer involving lymph node station 7 (subcarinal).23 The overall diagnostic accuracy and sensitivity of EUS-FNA in the diagnosis of sarcoidosis were found to be 94% and 100%, respectively.24 A disadvantage of EUS-FNA is its lesser ability to access different hilar lymph nodes and nodes situated anterior and to the right of the trachea. These nodes are in fact those most commonly involved in sarcoidosis.
3. Mediastinoscopy: Mediastinoscopy provides systematic exploration and biopsy under visual guidance of stations 1, 2, 3, 4, and 7 (see Figure 19-2); mediastinoscopy is more invasive and has higher complication rates than are seen with needle aspiration techniques. With regard to suspected lung cancer, it was shown that up to 28% of patients with a high clinical suspicion of nodal disease had mediastinal nodal metastases confirmed by mediastinoscopy despite negative EBUS-TBNA25; therefore a negative EBUS-TBNA should be followed by mediastinoscopy when cancer is suspected. For sarcoidosis, mediastinoscopy is used as the gold standard for histologic confirmation.26 For patients with isolated mediastinal lymphadenopathy, the sensitivity of mediastinoscopy is 96%, and before the introduction of dedicated EBUS-TBNA, some experts recommended it as a procedure of choice to diagnose lesions in the axial (middle) mediastinum.27
Cost-Effectiveness
It is debatable whether EBUS-TBNA is more cost-effective than mediastinoscopy for evaluation of mediastinal lymphadenopathy in non–small cell lung cancer.28 In patients with a benign disease such as sarcoidosis, this issue has not been studied in a systematic fashion. Mediastinoscopy is usually considered the gold standard approach for undiagnosed mediastinal adenopathy, including sarcoidosis,29 and before the introduction of EBUS in clinical practice, one cost-benefit analysis of mediastinoscopy for patients with suspected stage I sarcoidosis (asymptomatic bilateral hilar adenopathy) showed that benefits of mediastinoscopy would be minimal and likely would be offset by the procedure’s morbidity and mortality.30
Techniques and Results
Anesthesia and Perioperative Care
We performed the procedure with the patient under general anesthesia, but many operators perform it with the patient under moderate (conscious) sedation. Many studies reporting a high yield for the procedure were conducted with patients under general anesthesia.28
Instrumentation
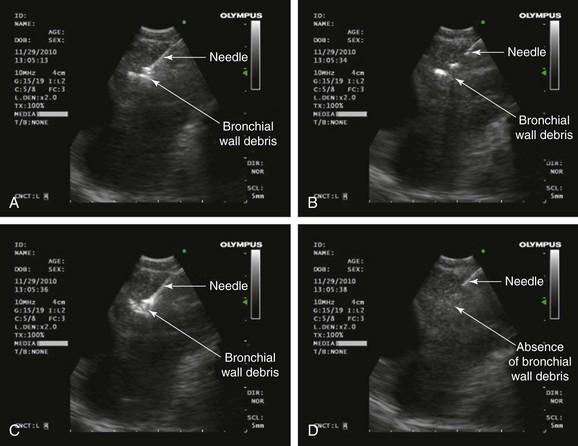
A dedicated EBUS bronchoscope, which offers direct real-time ultrasound imaging with a curved linear array transducer, was used. The frequency was set to 10 MHz. The associated ultrasound processor has adjustable gain and depth to optimize image quality, along with Doppler capabilities to distinguish vascular structures such as the heart and the inferior pulmonary veins; this is useful in sampling the subcarinal node. The 22 gauge acrogenic needle with an inner stylet allows for dislodgment of bronchial wall debris from the needle. This theoretically enhances the adequacy of the specimen (Figure 19-3). The needle guide system locks to the scope, and precise needle projection up to 4 cm is possible.
Anatomic Dangers and Other Risks
Concern has arisen regarding mediastinal abscess after EBUS-TBNA.31 In addition, pericarditis may occur in cases of inadvertent contamination of the pericardial space.32 With full-needle extension, the needle tip can be difficult to visualize, because small changes in the ultrasound angle can cause the needle tip to be out of plane with the ultrasound wave (see video on ExpertConsult.com) (Video IV.19.1![]() ). As a result, the pericardium (or vascular structures) may be violated but not visualized.32
). As a result, the pericardium (or vascular structures) may be violated but not visualized.32
Results and Procedure-Related Complications
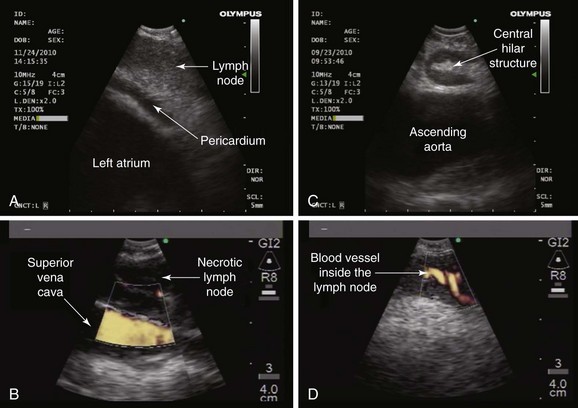
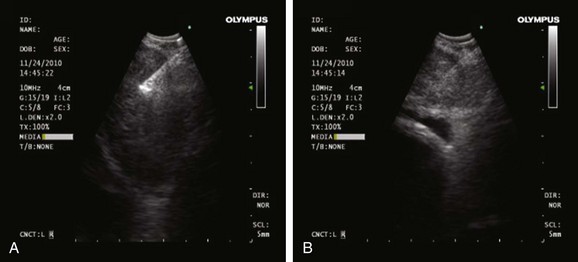
Under general anesthesia, the patient was intubated without difficulty using a 9.0 endotracheal tube. Bronchoscopic inspection showed normal airway mucosa and no endobronchial lesions. EBUS showed a subcarinal node greater than 1 cm in the short axis, of oval shape, homogeneous echogenicity, and indistinct margins; the node did not have a central necrosis sign (CNS),* nor did it have a central hilar structure (CHS)† (Figure 19-4). Studies show that sonographic features can be useful in evaluating lymph node metastasis in head and neck cancers, breast cancers, and thoracic malignancies.33,34 For example, operators may wish to avoid necrotic regions and obtain aspirates from the periphery of the node.35
In one study of 1061 lymph node stations from 487 patients with confirmed or suspected lung cancer, morphologic sonographic features of mediastinal and hilar lymph nodes reportedly helped predict the presence or absence of metastasis.36 The presence of CNS had the highest specificity (92.6%) and the highest hazard ratio (5.6) for prediction of metastatic lymph nodes. This evidence does not obviate the need for sampling the node, but the high negative predictive values could be useful in certain clinical scenarios. For instance, if EBUS-TBNA of a lymph node in a patient with suspected lung cancer provides an adequate cytologic specimen revealing only benign lymphocytes, then the lack of EBUS-related malignant lymph node characteristics could reinforce suspicions of true negativity.
Immediately after completing the first aspiration, while watching the needle deep within a lymph node (Figure 19-5, A), the operating room nurse interrupted the operator to ask whether she should record a video. After the operator answered her, a different image was apparent on the display monitor (Figure 19-5, B). It is likely that the operator rotated his wrist in such a way that the scanning plane of the ultrasound was changed while the needle remained in the same position (see video on ExpertConsult.com) (Video IV.19.1![]() ). This may not affect diagnostic yield but could result in accidental puncture of adjacent structures, because the tip of the needle is not seen.32
). This may not affect diagnostic yield but could result in accidental puncture of adjacent structures, because the tip of the needle is not seen.32
The first aspirate was prepared using a Diff-Quik stain, was read by the on-site cytologist, and showed scant lymphocytes and benign bronchial cells. An EBUS-TBNA cytology specimen is considered adequate or representative if frankly malignant cells, granulomas or lymphocytes, lymphoid tissue, or clusters of anthracotic pigment-laden macrophages are present.37 An EBUS-TBNA specimen is considered inadequate or nonrepresentative if no cellular components, scant lymphocytes (defined as <40 per high-power field [HPF]), and blood only, cartilage, or bronchial epithelial cells are noted.38 A quantitative cutoff value of at least 30% cellularity composed of lymphocytes has been arbitrarily proposed.39 In our case, aborting the procedure because lymphocytes were seen or assuming that the specimen was representative would have been premature.
The second aspirate was bloody but had been obtained from a different part of the subcarinal lymph node. The inferior pulmonary vein or left atrium could have been inadvertently penetrated. This was not the case, however, because the needle was seen in its entirety throughout the procedure. Blood vessels inside a lymph node are not uncommon and often appear as hypoechoic linear or circular structures that are Doppler positive. Their presence could explain bloody aspirates, even when the needle is well visualized inside the node (see Figure 19-4). The third and fourth aspirates were adequate and showed abundant lymphocytes and a suggestion of granulomatous inflammation. Specimens were sent for bacterial, fungal, and mycobacterial cultures. Cell block analysis was added to conventional cytologic evaluation because, in addition to increasing the yield for malignancy, it may result in a higher yield for granulomas in patients with suspected sarcoidosis.40 After the fourth aspirate, the procedure was terminated. No complications occurred, and the patient was discharged home after having been monitored in the postanesthesia care unit for 2 hours.
Long-Term Management
Outcome Assessment
Adequate specimens were obtained, but no specific diagnosis was made. However, the differential diagnosis was narrowed to infectious and noninfectious causes of granulomatous inflammation. If granulomatous inflammation is identified by EBUS-TBNA in a patient with suspected cancer recurrence, a reasonable clinical approach is to follow the patient radiographically without performing additional invasive testing, unless radiographic progression is noted.41 This is recommended because at least 5% of patients undergoing EBUS-TBNA in a tertiary cancer center were found to have a sarcoid-like lymphadenopathy mimicking cancer recurrence. Long-term follow-up imaging studies did not confirm tumor recurrence. The precise origin, natural history, and prognosis of this phenomenon are not yet known, but proposed mechanisms include immunologic dysfunction and a reaction due to previously administered chemotherapy.41
Follow-up Tests and Procedures
In view of the lack of evidence for infectious causes, the diagnosis of sarcoidosis or sarcoid-like reaction was made. No evidence of granulomatous inflammation was found at other sites to suggest sarcoidosis, nor were biochemical abnormalities such as hypercalcemia, hypercalciuria, hyperuricemia, elevated serum aminotransferase, alanine aminotransferase, or alkaline phosphatase present. The serum angiotensin-converting enzyme (SACE)* level was normal. Demonstration of granulomas remains an essential criterion for sarcoidosis, but because granulomatous inflammation can be seen in several conditions, it is necessary to exclude all possible causes and to correlate histology with other findings.42 To establish a diagnosis of sarcoidosis, for example, granulomas must be present in two or more organs and no agent known to cause a granulomatous response must be identified.43 This was not the case in our patient; therefore a diagnosis of sarcoid-like reaction was ultimately made. Because mediastinoscopy had been deferred, CT was scheduled 3 months later to re-evaluate the mediastinum; no change was noted. Continued surveillance scans during the next 12 months remained unchanged. We planned to monitor for stability for a total of 2 years.
Quality Improvement
No diagnosis was made on EBUS-TBNA, but we did not find evidence of primary lung tumor or recurrent melanoma. Attributing mediastinal lymphadenopathy to cancer recurrence without tissue confirmation can lead to unnecessary and toxic therapy44; therefore we certainly believed the procedure was warranted. We wondered, however, whether more aspirates should have been performed. For sarcoidosis, the yield of EBUS-TBNA exceeds 80% with five passes in one study and no further increase in yield, even after seven passes.15 For malignancy, however, the yield plateaus after a mean of only three aspirates.45 Therefore continuing the procedure “indefinitely” in the belief that eventually a diagnosis will be made is not warranted and probably increases the costs and risks for procedure-related complications or scope damage.
Discussion Points
1. Describe how the coronal view of a computed tomography scan can be used to help plan the procedure in this patient.
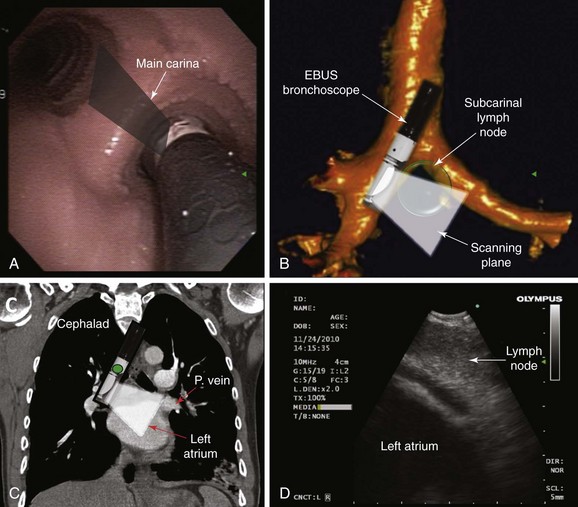
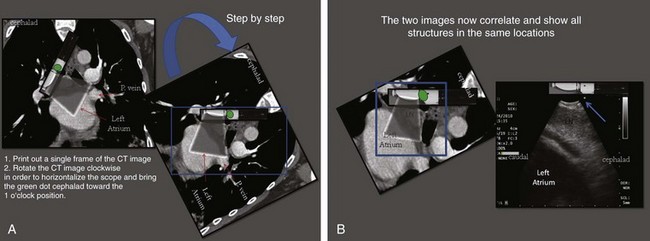
Based on the International Association for the Study of Lung Cancer (IASLC) lymph node map, the upper border of the subcarinal lymph node station is the carina of the trachea; the lower border is the upper border of the lower lobe bronchus on the left and the lower border of the bronchus intermedius on the right.46 This lymph node is located medial to the left or right mainstem bronchi, below the level of the main carina (see Figure 19-2). The EBUS scope should be placed in the right or left mainstem bronchus and the transducer turned medially to allow visualization of the subcarinal region. A coronal (aka frontal) plane is perpendicular to the ground and in humans separates the anterior from the posterior regions of the body (see Figure 19-1). The coronal CT view identifies the EBUS scanning plane and reveals the same structures but in different positions (Figure 19-6). To understand the EBUS image based on the coronal CT scan, however, several reference points should be recognized:
The coronal CT view is displayed as if the scope is nearly vertical (Figure 19-6, A). If one rotates the CT coronal image clockwise to horizontalize the scope and bring the green dot cephalad toward the 1 o’clock position, the two images (CT and EBUS) correlate and show all structures in the same locations (Figure 19-7). Because the green dot corresponds to the more cephalad, and therefore proximal, aspect of the body, the structure adjacent to the airway at approximately 1 o’clock is the subcarinal lymph node, and the anechoic structure at 7 o’clock is the left atrium (distal) (see Figure 19-7).
Several studies have shown that conventional TBNA (61% to 72%) with 19 gauge needles has an incremental diagnostic yield over other bronchoscopic techniques, including endobronchial biopsy (EBB) (45%) and transbronchial lung biopsy (TBLB) (40% to 52%), for stage I sarcoidosis. TBNA may actually have a higher yield than bronchioloalveolar lavage (BAL), EBB, or even TBLB alone.47,48 EBUS-TBNA was shown to significantly add to the yield of combined BAL and TBLB or EBB and TBLB for patients with suspicious stage I sarcoidosis.49,50 Both conventional and EBUS-TBNA procedures have a high yield.
3. Describe the clinical implications of granulomatous inflammation detected on EBUS-guided TBNA specimen.
4. List six sonographic criteria of morphology that can be used to describe mediastinal and hilar lymph nodes36:
Expert Commentary
Linear or convex probe endobronchial ultrasound-guided transbronchial needle aspiration (CP EBUS-TBNA) has revolutionized the evaluation of mediastinal lymph nodes since its introduction to clinical practice in 2004. Many studies have been published, but almost all of them have failed to meet rigorous proposed guidelines for the study of new diagnostic tests55—a problem also encountered when the published literature on cervical mediastinoscopy is reviewed. For example, to date, only two studies have directly compared cervical mediastinoscopy versus CP EBUS-TBNA alone56 or combined with transesophageal ultrasound.57
The mobility of mediastinal lymph nodes during the respiratory cycle has been blamed for the poor yield of conventional TBNA.58 The results of the only study that directly compared CP EBUS-TBNA versus cervical mediastinoscopy (diagnostic yield 98% vs. 78%; P = .007) suggest that CP EBUS-TBNA is superior when station 7 lymph nodes are evaluated.56 Conventional TBNA has not been compared directly with CP EBUS-TBNA but rather with radial probe EBUS,21 which does not allow real-time visualization of the needle as it enters the lymph node. In this study, conventional TBNA performed as well as radial probe EBUS-guided TBNA (diagnostic yields 72% vs. 80%; P = .3) in lymph nodes that measured 0.8 to 4.3 cm. CP EBUS-TBNA studies have consistently reported diagnostic yields above 90% in subcarinal lymph nodes. Therefore CP EBUS-TBNA could be recommended as the first diagnostic technique with which an isolated subcarinal lymphadenopathy can be evaluated, but if not readily available, conventional TBNA should be attempted.
In fact, the association between granulomatous inflammation and cancer has been recognized for over a century. Although this phenomenon has been reported more frequently in patients with testicular germ cell tumor, it presents with other cancer types as well, as described by our group,41 affecting patients treated with chemotherapy or surgical resection only. No clarity on the cause of the association has been obtained, but proposed explanations include an immunologic dysfunction shared by cancer and sarcoidosis, side effects of cancer therapy, and “antigenic shedding” from the malignant tumor, leading to granuloma formation. In our report, this phenomenon occurred in only 5.2% of patients studied, but we could expect a higher prevalence because before EBUS-TBNA became available in our institution, patients with a history of cancer and new onset of hilar or mediastinal lymphadenopathy might have often been treated for cancer recurrence without histologic confirmation. We recommend that with radiographic findings suggestive of cancer recurrence, such as enlarged or FDG-avid mediastinal lymph nodes, patients should undergo histologic confirmation of the recurrence to avoid unnecessary and toxic therapies.
1. Glazer GM, Gross BH, Quint LE, et al. Normal mediastinal lymph nodes: number and size according to American Thoracic Society mapping. AJR Am J Roentgenol. 1985;144:261-265.
2. Webb R. The mediastinum: mediastinal masses. In: Webb Richard, Higgins Charles. Thoracic Imaging. Philadelphia: Lippincott Williams & Wilkins; 2005:212-270.
3. Stolz D, Pollak V, Chhajed PN, et al. A randomized, placebo-controlled trial of bronchodilators for bronchoscopy in patients with COPD. Chest. 2007;131:765-772.
4. Swayze S. Helping them cope. J Psychosoc Nurs Ment Health Serv. 1991;29:35-37.
5. Weis J. Support groups for cancer patients. Support Care Cancer. 2003;11:763-768.
6. Back AL, Arnold RM, Baile WF, et al. Approaching difficult communication tasks in oncology. CA Cancer J Clin. 2005;55:164-177.
7. Yasufuku K, Nakajima T, Chiyo M, et al. Endobronchial ultrasonography: current status and future directions. J Thorac Oncol. 2007;2:970-979.
8. Nosotti M, Tosi D, Palleschi A, et al. Transbronchial needle aspiration under direct endobronchial ultrasound guidance of PET-positive isolated mediastinal adenopathy in patients with previous malignancy. Surg Endosc. 2009;23:1356-1359.
9. Nakajima T, Yasufuku K, Iyoda A, et al. The evaluation of lymph node metastasis by endobronchial ultrasound-guided transbronchial needle aspiration: crucial for selection of surgical candidates with metastatic lung tumors. J Thorac Cardiovasc Surg. 2007;134:1485-1490.
10. Gu P, Zhao Y, Jiang L, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for staging of lung cancer: a systematic review and meta-analysis. Eur J Cancer. 2009;45:1389-1396.
11. Kennedy MP, Jimenez CA, Bruzzi JF, et al. Endobronchial ultrasound-guided transbronchial needle aspiration in the diagnosis of lymphoma. Thorax. 2008;63:360-365.
12. Steinfort DP, Conron M, Tsui A, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for the evaluation of suspected lymphoma. J Thorac Oncol. 2010;5:804-809.
13. Bilaçeroğlu S, Günel O, Eris N, et al. Transbronchial needle aspiration in diagnosing intrathoracic tuberculous lymphadenitis. Chest. 2004;126:259-267.
14. Oki M, Saka H, Kitagawa C, et al. Real-time endobronchial ultrasound-guided transbronchial needle aspiration is useful for diagnosing sarcoidosis. Respirology. 2007;12:863-868.
15. Garwood S, Judson MA, Silvestri G, et al. Endobronchial ultrasound for the diagnosis of pulmonary sarcoidosis. Chest. 2007;132:1298-1304.
16. Wong M, Yasufuku K, Nakajima T, et al. Endobronchial ultrasound: new insight for the diagnosis of sarcoidosis. Eur Respir J. 2007;29:1182-1186.
17. Yasufuku K, Chiyo M, Sekine Y, et al. Real-time endobronchial ultrasound-guided transbronchial needle aspiration of mediastinal and hilar lymph nodes. Chest. 2004;126:122-128.
18. Groth SS, Whitson BA, D’Cunha J, et al. Endobronchial ultrasound-guided fine-needle aspiration of mediastinal lymph nodes: a single institution’s early learning curve. Ann Thorac Surg. 2008;86:1104-1109. discussion 1109-1110
19. Kemp SV, El Batrawy SH, Harrison RN, et al. Learning curves for endobronchial ultrasound using Cusum analysis. Thorax. 2010;65:534-538.
20. Skov BG, Baandrup U, Jakobsen GK, et al. Cytopathologic diagnoses of fine-needle aspirations from endoscopic ultrasound of the mediastinum: reproducibility of the diagnoses and representativeness of aspirates from lymph nodes. Cancer. 2007;111:234-241.
21. Herth F, Becker H, Ernst A. Conventional versus ultrasound-guided transbronchial needle aspiration: a randomized trial. Chest. 2004;125:322-325.
22. Smojver-Jezek S, Peros-Golubicic T, Tekavec-Trkanjec T, et al. Transbronchial fine needle aspiration cytology in the diagnosis of mediastinal/hilar sarcoidosis. Cytopathology. 2007;18:3-7.
23. Herth FJ, Lunn W, Eberhardt R, et al. Transbronchial versus transesophageal ultrasound-guided aspiration of enlarged mediastinal lymph nodes. Am J Respir Crit Care Med. 2005;171:1164-1167.
24. Fritscher-Ravens A, Sriram PV, Topalidis T, et al. Diagnosing sarcoidosis using endosonography-guided fine-needle aspiration. Chest. 2000;118:928-935.
25. Defranchi SA, Edell ES, Daniels CE, et al. Mediastinoscopy in patients with lung cancer and negative endobronchial ultrasound guided needle aspiration. Ann Thorac Surg. 2010;90:1753-1758.
26. Pakhale SS, Unruh H, Tan L, Sharma S. Has mediastinoscopy still a role in suspected stage I sarcoidosis? Sarcoidosis Vasc Diffuse Lung Dis. 2006;23:66-69.
27. Porte H, Roumilhac D, Eraldi L, et al. The role of mediastinoscopy in the diagnosis of mediastinal lymphadenopathy. Eur J Cardiothorac Surg. 1998;13:196-199.
28. Shrager JB. Mediastinoscopy: still the gold standard. Ann Thorac Surg. 2010;89:S2084-S2089.
29. Hunninghake GW, Costabel U, Ando M, et al. ATS/ERS/WASOG statement on sarcoidosis: American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16:149-173.
30. Reich JM, Brouns MC, O’Connor EA, et al. Mediastinoscopy in patients with presumptive stage I sarcoidosis: a risk/benefit, cost/benefit analysis. Chest. 1998;113:147-153.
31. Moffatt-Bruce SD, Ross PJr. Mediastinal abscess after endobronchial ultrasound with transbronchial needle aspiration: a case report. J Cardiothorac Surg. 2010;5:33.
32. Haas AR. Infectious complications from full extension endobronchial ultrasound transbronchial needle aspiration. Eur Respir J. 2009;33:935-938.
33. Lee N, Inoue K, Yamamoto R, et al. Patterns of internal echoes in lymph nodes in the diagnosis of lung cancer metastasis. World J Surg. 1992;16:986-994.
34. Bhutani MS, Hawes RH, Hoffman BJ. A comparison of the accuracy of echo features during endoscopic ultrasound (EUS) and EUS-guided fine-needle aspiration for diagnosis of malignant lymph node invasion. Gastrointest Endosc. 1997;45:474-479.
35. Fujiwara T, Yasufuku K, Nakajima T, et al. The utility of sonographic features during endobronchial ultrasound-guided transbronchial needle aspiration for lymph node staging in patients with lung cancer: a standard endobronchial ultrasound image classification system. Chest. 2010;138:641-647.
36. Wallace M, Hoffman B. Needle device systems for interventional endoscopic ultrasound and general technique of endoscopic ultrasound-guided fine-needle aspiration. Tech Gastrointest Endosc. 2000;2:136-141.
37. Alsharif M, Andrade RS, Groth SS, et al. Endobronchial ultrasound-guided transbronchial fine-needle aspiration: the University of Minnesota experience, with emphasis on usefulness, adequacy assessment, and diagnostic difficulties. Am J Clin Pathol. 2008;130:434-443.
38. Lee HS, Lee GK, Lee HS, et al. Real-time endobronchial ultrasound-guided transbronchial needle aspiration in mediastinal staging of non-small cell lung cancer: how many aspirations per target lymph node station? Chest. 2008;134:368-374.
39. Trisolini R, Lazzari Agli L, Patelli M. Conventional vs endobronchial ultrasound-guided transbronchial needle aspiration of the mediastinum. Chest. 2004;126:1005-1006.
40. von Bartheld MB, Veselic Charvat M, Rabe KF, et al. Endoscopic ultrasound-guided fine-needle aspiration for the diagnosis of sarcoidosis. Endoscopy. 2010;42:213-217.
41. Kennedy MP, Jimenez CA, Mhatre AD, et al. Clinical implications of granulomatous inflammation detected by endobronchial ultrasound transbronchial needle aspiration in patients with suspected cancer recurrence in the mediastinum. J Cardiothorac Surg. 2008;3:8.
42. Mehrotra R, Dhingra V. Cytological diagnosis of sarcoidosis revisited: a state of the art review. Diagn Cytopathol. 2010;39:541-548.
43. Barnett BP, Sheth S, Ali SZ. Cytopathologic analysis of paratracheal masses: a study of 737 cases with clinicoradiologic correlation. Acta Cytol. 2009;5:672-678.
44. Kok TC, Haasjes JG, Splinter TA, et al. Sarcoid-like lymphadenopathy mimicking metastatic testicular cancer. Cancer. 1991;68:1845-1847.
45. Lee HS, Lee GK, Lee HS, et al. Real-time endobronchial ultrasound-guided transbronchial needle aspiration in mediastinal staging of non-small cell lung cancer: how many aspirations per target lymph node station? Chest. 2008;134:368-374.
46. Rusch VW, Asamura H, Watanabe H, et al. Members of IASLC Staging Committee. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol. 2009;4:568-577.
47. Bilaceroglu S, Perim K, Gunel O, et al. Combining transbronchial aspiration with endobronchial and transbronchial biopsy in sarcoidosis. Monaldi Arch Chest Dis. 1999;54:217-223.
48. Trisolini R, Agli LL, Cancellieri A, et al. The value of flexible transbronchial needle aspiration in the diagnosis of stage I sarcoidosis. Chest. 2003;124:2126-2130.
49. Nakajima T, Yasufuku K, Kurosu K, et al. The role of EBUS-TBNA for the diagnosis of sarcoidosis—comparisons with other bronchoscopic diagnostic modalities. Respir Med. 2009;103:1796-1800.
50. Navani N, Booth HL, Kocjan G, et al. Combination of endobronchial ultrasound-guided transbronchial needle aspiration with standard bronchoscopic techniques for the diagnosis of stage I and stage II pulmonary sarcoidosis. Respirology. 2011;16:467-472.
51. Pisircriler R, Atay Z, Lang W. Cytological diagnosis of intrathoracic epithelioid cellular inflammatory process. Pneumologie. 1990;44:767-770.
52. Tremblay A, Stather DR, Maceachern P, et al. A randomized controlled trial of standard vs endobronchial ultrasonography-guided transbronchial needle aspiration in patients with suspected sarcoidosis. Chest. 2009;136:340-346.
53. Laurberg P. Sarcoid reactions in pulmonary neoplasms. Scand J Respir Dis. 1975;56:20-27.
54. Steinfort DP, Irving LB. Sarcoidal reactions in regional lymph nodes of patients with non-small cell lung cancer: incidence and implications for minimally invasive staging with endobronchial ultrasound. Lung Cancer. 2009;66:305-308.
55. Schunemann HJ, Oxman AD, Brozek J, et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ. 2008;336:1106-1110.
56. Ernst A, Anantham D, Eberhardt R, et al. Diagnosis of mediastinal adenopathy-real-time endobronchial ultrasound guided needle aspiration versus mediastinoscopy. J Thorac Oncol. 2008;3:577-582.
57. Annema JT, van Meerbeeck JP, Rintoul RC, et al. Mediastinoscopy vs endosonography for mediastinal nodal staging of lung cancer: a randomized trial. JAMA. 2010;304:2245-2252.
58. Piet AH, Lagerwaard FJ, Kunst PW, et al. Can mediastinal nodal mobility explain the low yield rates for transbronchial needle aspiration without real-time imaging? Chest. 2007;131:1783-1787.
* The short axis or least diameter in cross-section should be used in measuring lymph node size; this value more closely reflects the actual node diameter when nodes are obliquely oriented relative to the scan plane and shows less variation among normal subjects than the long axis.
† Metastases to mediastinal or hilar lymph nodes from extrathoracic tumors usually occur from carcinomas of the head and neck, genitourinary tract, and breast, as well as melanoma.
‡ Round, smooth, sharply defined; 50% are subcarinal.
* Blocking occurs when a patient raises a concern, but the physician either fails to respond or redirects the conversation.
† Collusion occurs when patients hesitate to bring up difficult topics and their physicians do not ask them specifically about them.
* Cusum analysis is a method of continuously assessing the performance of an individual or process against a predetermined standard to detect adverse trends and to allow for early intervention (e.g., retraining).
* CNS is a hypoechoic area within the lymph node without blood flow.
† CHS is defined as a linear, flat, hyperechoic area in the center of the lymph node.
* Elevated serum angiotensin-converting enzyme levels are seen in fact in only 50% to 60% of patients with sarcoidosis. Although these levels may reflect the total granuloma burden, they do not reliably correlate with disease activity nor with its prognosis.
* Typical CNS is described as one low echoic area within the lymph node, but sometimes it may occupy most of the lymph node.