CHAPTER 48 Ebstein Anomaly
The incidence of congenital heart disease ranges between 2.2 and 8.8/1000 live births.1 Cyanotic lesions account for a low percentage of these defects, but result in a significant proportion of congenital heart morbidity and mortality, particularly if left untreated. Characterization of the pulmonary blood flow (PBF) and cardiac chamber morphology remains a fundamental step in evaluating suspected cyanotic congenital heart disease. This chapter will review cyanotic congenital heart disease with cardiomegaly and decreased PBF, specifically focusing on Ebstein anomaly.
EVALUATING THE CYANOTIC PATIENT
MRI, MRA, and CTA are alternative noninvasive modalities to catheterization and may provide comprehensive evaluation of cardiac and pulmonary morphology and function, which is key to diagnosing the cyanotic patient with decreased pulmonary vascularity (Figs. 48-1 and 48-2). Both can generate cardiac chamber volume and qualitative and quantitative functional data. Image postprocessing can readily be facilitated for data sets from both modalities.
EBSTEIN ANOMALY
Ebstein anomaly is a rare cardiac disorder, accounting for 0.04% to 0.93% of congenital heart malformations.1–4 The distinguishing pathology is dysplastic tricuspid valve leaflets, which extend into and attach to the right ventricle, rather than the annulus at the atrioventricular junction, leading to tricuspid valve incompetence. Usually, the septal and posterior leaflets are involved, with insertion at the margin of the inlet and trabecular right ventricular zones, either directly or by way of anomalous chordae tendinae and papillary muscles. The degree of septal and posterior leaflet displacement and abnormal morphology has wide variability.
In distinction to the septal and posterior leaflets, the anterior leaflet typically has dysplastic morphology, but maintains normal attachments. It is enlarged, with a sail appearance and fibrous strands, which may be muscularized. Rarely, the anterior leaflet may have abnormal displacement along the right ventricular anterior free wall, leading to tricuspid stenosis. In 10% of cases, the anterior leaflet may have downward displacement, obstructing the ventricular inlet, leading to an obstructing, imperforate tricuspid valve.5,6
Downward leaflet displacement divides the right ventricle into a basilar, atrialized inlet portion and a small functional true right ventricle. Both portions are typically dilated, with a loss of myocytes, leading to decreased contractility.6 Less commonly, only the atrialized portion is dilated. The native functional right ventricle has reduced right ventricular (RV) filling capacity and cardiac output. RV preload volume is further reduced by virtue of the atrialized portion functioning as a false aneurysm such that as the native right atrium contracts, the abnormal segment above the functional right ventricle expands, collecting blood. In addition to these factors, tricuspid insufficiency and possible anterior leaflet stenosis or obstruction decrease forward flow, contributing to the diminished right ventricle filling volumes.
Hemodynamically, the decreased diastolic filling and myocardial contractility lead to diminished PBF, elevated right atrial and systemic venous pressures and, ultimately, right-sided failure. With elevated right atrial pressures, right-to-left shunting will occur across a patent foramen ovale (PFO) or secundum atrial septal defect (ASD), leading to cyanosis.5,7 Increased pulmonary vascular resistance in the early neonatal period increases afterload, further exacerbating cyanosis in these patients.8 As the intrapulmonary vascular resistance falls, cyanosis may improve.
Electrical conduction pathways may be abnormal with regard to the location and morphology of native pathways and the presence of accessory conduction pathways (Wolf-Parkinson-White; WPW). The conduction pathway abnormalities lead to dysrhythmias; the most common are atrial tachycardias and supraventricular and ventricular arrhythmias.9,10
In most cases of Ebstein anomaly, there is visceral situs solitus. The ASD or PFO has been confirmed by select angiography or autopsy to be present in at least 33% to 56% of patients.9–11 Additional cardiac anomalies may be present in at least 22% of cases. Among these anomalies, common lesions include pulmonary stenosis (35%), ventricular septal defect (VSD; 21%), patent ductus arteriosus (PDA) (20%), and pulmonary stenosis with VSD (9%).
Clinical Manifestations
Ebstein anomaly shows almost equal distribution between males and females.11 Depending on the degree of heart failure and shunting, and the occurrence of arrhythmias, patients may present as a neonate, infant, child, adolescent, or adult. Infants born with Ebstein anomaly are most commonly full term, with a weight between 2 and 4 kg.12,13
In addition to cyanosis, presenting symptoms may include dyspnea, palpitations, fatigue, epigastric or chest pain, and congestive heart failure. The neonate and infant may present with feeding intolerance, whereas children and older patients may use squatting to alleviate symptoms. Physical examination may be remarkable for a systolic and less commonly a diastolic murmur, systolic thrill, digital clubbing, and precordial deformity.10
Most patients with Ebstein anomaly have an abnormal electrocardiogram (ECG). Specific findings include an elevated P wave (indicative of right atrial enlargement), right bundle branch block, diminished right precordial lead voltage or dominant left ventricular voltage (indicative of a small right ventricle), notched QRS complex (secondary to delayed activation in the atrialized right ventricle), prolonged PR interval, and WPW (type B) pathway.10
Diagnostic Imaging
The degree of cardiomegaly on chest radiography is moderate to massive, with dominance of the right atrium (Fig. 48-3). This reflects the degree of elevated RV pressure and tricuspid insufficiency.10 Cardiac size decreases with age, so that the mean cardiothoracic ratio is 0.73 as a neonate, 0.57 during infancy and childhood, and 0.55 during adolescence and adulthood.11 Pulmonary vascularity may be normal to decreased, and directly correlates with the degree of valvular deformity, right-to-left shunting, and diminished right ventricular cardiac output,
Echocardiography, MRI, MRA, and CTA can all readily diagnose Ebstein anomaly. In a four-chamber projection, apical leaflet displacement into the right ventricle is identified by recognizing that it extends beyond the atrioventricular level of the mitral valve insertion (Fig. 48-4). Atrialized and native portions of the right ventricle are defined based on the insertion level. The size of the enlarged atrium, atrialized right ventricle, and small functional native right ventricle are characterized qualitatively and quantitatively. An atrial septal defect with right-to-left interatrial septal bowing is recognized with a short-axis or four-chamber projection.
Treatment
Conservative management is appropriate for patients who are asymptomatic, have no right-to-left shunting, and have only mild cardiomegaly.14 Patients should be given prophylaxis for endocarditis, and patients who experience cardiac failure should be treated with diuretics and digoxin.
Arrhythmias can be treated with antiarrhythmic medications or with catheter ablation or surgery. Catheter ablation in patients with Ebstein anomaly has a lower rate of success than in patients with morphologically normal hearts.15 Permanent pacemaker placement may be required in the setting of atrioventricular block or sinus node dysfunction.16
Surgery
Indications for surgery include symptoms such as paradoxic emboli, progressive increase in size of the right heart, compromise of systolic function, severe heart failure, or onset of tachyarrhythmias.14 Biventricular reconstruction is appropriate for most patients. When a patient has severe biventricular dysfunction, heart transplantation should be considered.
1 Samánek M, Vorísková M. Congenital heart disease among 815,569 children born between 1980 and 1990 and their 15-year survival: a prospective Bohemia survival study. Pediatr Cardiol. 1999;20:411-417.
2 Campbell M. Incidence of cardiac malformations at birth and later, and neonatal mortality. Br Heart J. 1973;35:189-200.
3 Goetzová J, Benesová D. Congenital heart diseases at autopsy of still-born and deceased children in the Central Bohemian region. Cor Vasa. 1981;23:8-13.
4 Samánek M, Slavik Z, Zborilová B, et al. Prevalence, treatment, and outcome of heart disease in live-born children: a prospective analysis of 91,823 live-born children. Pediatr Cardiol. 1989;10:205-211.
5 Zuberbuhler JR, Allwork SP, Anderson RH. The spectrum of Ebstein’s anomaly of the tricuspid valve. J Thorac Cardiovasc Surg. 1979;77:202-211.
6 Anderson KR, Zuberbuhler JR, Anderson RH, et al. Morphologic spectrum of Ebstein’s anomaly of the heart: a review. Mayo Clin Proc. 1979;54:174-180.
7 Genton E, Blount SGJr. The spectrum of Ebstein’s anomaly. Am Heart J. 1967;73:395-425.
8 Jaquiss RD, Imamura M. Management of Ebstein’s anomaly and pure tricuspid insufficiency in the neonate. Semin Thorac Cardiovasc Surg. 2007;19:258-263.
9 Watson H. Natural history of Ebstein’s anomaly of tricuspid valve in childhood and adolescence: An international co-operative study of 505 cases. Br Heart J. 1974;36:417-427.
10 Bialostozky D, Horwitz S, Espino-Vela J. Ebstein’s malformation of the tricuspid valve. A review of 65 cases. Am J Cardiol. 1972;29:826-836.
11 Radford DJ, Graff RF, Neilson GH. Diagnosis and natural history of Ebstein’s anomaly. Br Heart J. 1985;54:517-522.
12 Shinkawa T, Polimenakos AC, Gomez-Fifer CA, et al. Management and long-term outcome of neonatal Ebstein anomaly. J Thorac Cardiovasc Surg. 2010;139:354-358.
13 Knott-Craig CJ, Overholt ED, Ward KE, et al. Neonatal repair of Ebstein’s anomaly: indications, surgical technique, and medium-term follow-up. Ann Thorac Surg. 2000;69:1505-1510.
14 Attenhofer Jost CH, Connolly HM, Dearani JA, et al. Congenital heart disease for the adult cardiologist: Ebstein’s anomaly. Circulation. 2007;115:277-285.
15 Hebe J. Ebstein’s anomaly in adults: arrhythmias: diagnosis and therapeutic approach. Thorac Cardiovasc Surg. 2000;48:214-219.
16 Allen MR, Hayes DL, Warnes CA, Danielson GK. Permanent pacing in Ebstein’s anomaly. Pacing Clin Electrophysiol. 1997;20:1243-1246.

 FIGURE 48-1
FIGURE 48-1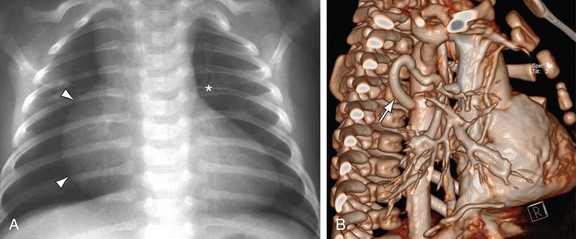
 FIGURE 48-2
FIGURE 48-2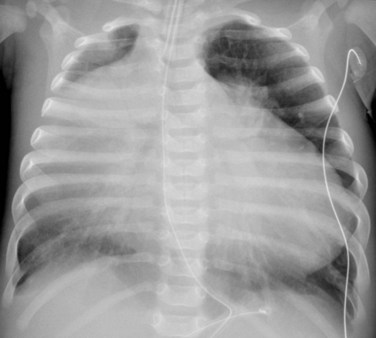
 FIGURE 48-3
FIGURE 48-3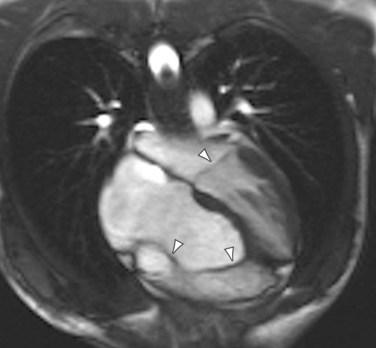
 FIGURE 48-4
FIGURE 48-4