Early Assessment and Management of Trauma
Trauma is the leading cause of morbidity and mortality in children from ages 1 to 14 years. It results in more disability and death than all other childhood diseases combined.1 More than 10,000 pediatric patients die from trauma in the USA each year.1 Approximately 10% of pediatric hospitalizations,2 15% of pediatric intensive care unit (PICU) admissions,3 25% of pediatric emergency department visits,4 and 50% or more of pediatric ambulance runs are due to trauma.5 Moreover, it also represents nearly 20% of hospitalizations for serious injury among all age groups combined.6
Trauma Epidemiology
Several injury severity scales exist in practice and in the literature. The large number of injury severity scales arises from the markedly different perspectives used in the application of the scales. The Abbreviated Injury Scale (AIS), primarily an anatomical measure of injury severity, was the first widely implemented scale used in practice. Criticism of the AIS included the inability to take into account multiple injuries to the same body region and the poor correlation of the AIS with severity and survival. The Injury Severity Score (ISS), New Injury Severity Score (NISS) and Pediatric Trauma Score (PTS) are just some examples of scoring systems developed to overcome the issues described. Despite the controversies in these scales, it is commonly accepted that injuries whose severity are a threat to life correspond to an ISS of 10 or higher, or a PTS of 8 or lower.2
The incidence of serious traumatic injury is approximately 420/100,000.7 Although the hospital-based fatality rate is 2.4/100,000, the population-based mortality rate is 11.8/100,000, indicating that 78% of lethally injured children die before hospital admission and demonstrating the need for effective injury prevention and prehospital care.8
Blunt injuries outnumber penetrating injuries in children by a ratio of 12 : 1, a ratio that has decreased somewhat in recent years. While blunt injuries are more common, penetrating injuries are more lethal. However, despite the decline in penetrating injuries, firearm related deaths continue as one of the top three causes of death in the American youth. Most blunt trauma in childhood is sustained unintentionally, but between 5–10% of serious injuries are due to intentional physical assault (of which half are due to physical abuse).9 Still, the leading cause of death in children is the motor vehicle, responsible for approximately 75% of all childhood deaths, which are evenly split between those due to pedestrian trauma and those resulting from occupant injuries (Table 14-1).
TABLE 14-1
Incidence and Mortality from the Major Categories of Pediatric Trauma
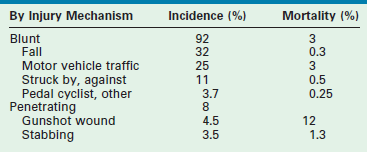
Data from the American College of Surgeons, National Trauma Data Bank 2012.
Injury Risks
The lack of adequate supervision of children during play involving possible injury hazards is recognized as a major risk factor for unintentional injury in pediatric patients. However, drug and alcohol use, obesity, poverty, and race also influence injury frequency. Toxicology screens are reportedly positive in 10–40% of injured adolescents, while obese children and adolescents appear to have more complications and require longer stays in the intensive care unit.10–13 Socioeconomic status has also been associated with increased hospitalization and mortality following major trauma, owing to a higher frequency and more lethal mechanisms of injury, rather than injury severity.14 Race and ethnicity affect injury risk independent of socioeconomic status, particularly among African-American children, whose rate of death from preventable injuries, head injuries, and child abuse is three to six times higher than that of white children.15–18 Improper use of restraints may contribute to the increased fatality rates observed in African-American children, who are half as likely to be restrained as white children when involved in motor vehicle crashes (MVCs) and one-third as likely to be placed in car seats during MVCs.19
Analysis of the Crash Injury Research Engineering Network (CIREN) database has recently yielded valuable information about the pattern of childhood injuries after MVCs: (1) child victims in frontal crashes are more likely to suffer severe spine and musculoskeletal injuries; (2) those in lateral crashes are more likely to suffer head and chest injuries; (3) those in front seats sustain more injuries to the chest, abdomen, pelvis, and axial skeleton than those in the rear seats; (4) seat belts are especially protective against pelvic and musculoskeletal injuries; (5) children involved in high-severity, lateral-impact crashes typically sustain injuries characterized by higher ISS and lower Glasgow Coma Scale (GCS) scores.20,21 Restraint devices have also been subjected to careful analysis in recent years: (1) they do not appear to protect young victims of MVCs as well as older victims; (2) car seats may not significantly affect injury outcome; (3) improper application may predispose to abdominal injuries, even in low-severity crashes; (4) the presence of abdominal wall bruising in restrained children, although not commonly observed, is frequently indicative of intra-abdominal injury.22–27
Injury Outcomes
In recent years, much effort has been devoted to outcomes research in pediatric trauma with the hope that benchmarking of treatment results may lead to better care for injured children. Both historical studies and contemporary investigations indicate that children survive more frequently and recover more fully in hospitals that specialize in pediatric trauma than in other hospitals.28–45 No less important than survival outcome is functional outcome, for which numerous studies now indicate improved outcomes in hospitals that specialize in pediatric trauma care.32–46 However, these studies also suggest that whereas children may recover from injury more quickly than adults, physical function may not fully normalize. Even so, self-perceived long-term quality of life among seriously injured children may not be adversely affected, justifying an aggressive approach to their resuscitation.47
Perhaps the most important recent development for outcomes research in pediatric trauma has been the expansion of the National Trauma Data BankTM (NTDBTM) of the American College of Surgeons (ACS) to include children. Initially designed as a simple case repository, efforts continue to analyze submitted cases to provide population estimates of severe pediatric injury and develop quality benchmarks for pediatric trauma care. Preliminary data suggest that these benchmarks perform as well as existing measures.48
Injury Prevention
Injuries are not accidents, but rather predictable events that respond to harm-reduction strategies similar to those applied for other diseases. The Haddon Factor Phase Matrix neatly depicts these in graphic form (Fig. 14-1).49 Strategies to lessen the burden of injury are applied to the host, agent, and environment before, during, and after the traumatic event using enforcement, engineering, education, and economics as techniques to limit the adverse impact of each factor.
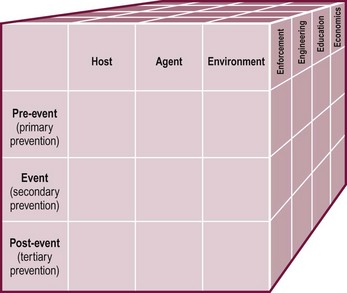
FIGURE 14-1 The Haddon Factor Phase Matrix, as modified and refined to include a third strategic dimension, integrates all phases of injury control into a single system. (Adapted from Haddon W. Advances in the epidemiology of injuries as a basis for public policy. Public Health Rep 1980;95:411–21; and Runyan CW. Using the Haddon Matrix: Introducing the third dimension. Inj Prev 1998;4:302–7.)
Effective injury-prevention programs are community-based and require extensive collaboration with civic leaders, governmental agencies, community-based organizations, and neighborhood coalitions. Programs such as the National Safe Kids Campaign (http://www.safekids.org) and the Injury Free Coalition for Kids (http://www.injuryfree.org) have proven highly successful in reducing the burden of childhood injury in many communities.
Injury Patterns
Injury mechanism is the main predictor of injury pattern. The body regions most frequently injured in major childhood trauma are the lower extremities, head and neck, and abdomen. In minor childhood injury, soft tissue and upper extremity injuries predominate. Motor vehicle versus pedestrian trauma results in the Waddell triad of injuries to the head, torso, and lower extremity (pelvis, femur, or tibia; Fig. 14-2). Motor vehicle accidents may cause head, face, and neck injuries in unrestrained passengers. Cervical spine injuries, bowel disruption or hematoma, and Chance fractures occur in restrained passengers (Fig. 14-3). Bicycle trauma results in head injury in the unhelmeted riders as well as upper extremity and upper abdominal injuries, the latter the result of contact with the handlebar (Fig. 14-4 and Table 14-2). Direct impact from a bicycle handlebar remains highly predictive of the need for operation.27
TABLE 14-2
Common Injury Mechanisms and Corresponding Injury Patterns in Childhood Trauma
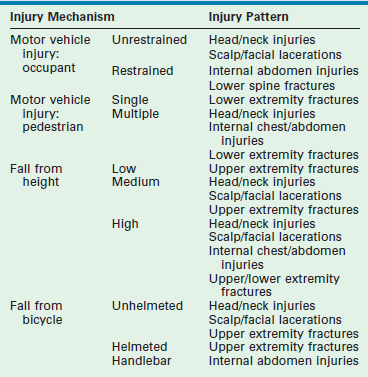
From American College of Surgeons Committee on Trauma. Advanced Trauma Life Support® ATLS® Student Course Manual. 9th ed. Chicago: American College of Surgeons; 2012.
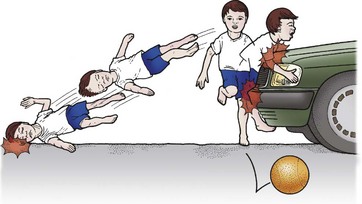
FIGURE 14-2 The Waddell triad of injuries to head, torso, and lower extremity is depicted. (From Foltin G, Tunik M, Cooper A, et al, editors. Teaching Resource for Instructors of Prehospital Pediatrics. New York: Center for Pediatric Emergency Medicine; 1998. Accessed at http://www.cpem.org.)
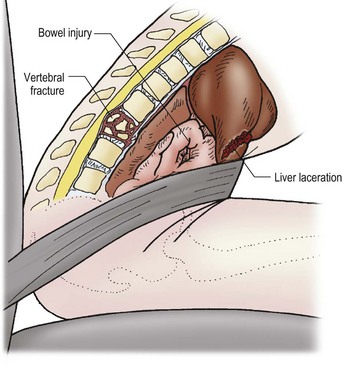
FIGURE 14-3 The mechanism for the development of intestinal and vertebral injuries from lap belts is shown. (From Foltin G, Tunik M, Cooper A, et al, editors. Teaching Resource for Instructors of Prehospital Pediatrics. New York: Center for Pediatric Emergency Medicine; 1998. Accessed at http://www.cpem.org.)
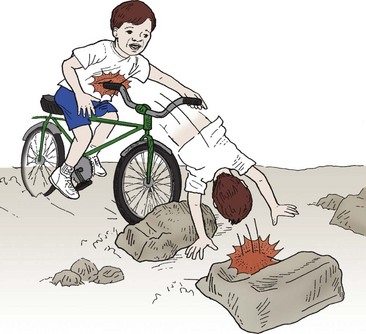
FIGURE 14-4 Children riding bicycles can sustain blunt abdominal trauma after contact with handlebars or head trauma from falling off the bicycle. (From Foltin G, Tunik M, Cooper A, et al, editors. Teaching Resource for Instructors of Prehospital Pediatrics. New York: Center for Pediatric Emergency Medicine; 1998.)
Head
Head injuries are potentially more dangerous in children than in adults for several reasons. First, developing neural tissue is delicate and the softer bones of the pediatric skull allow impact forces to be transmitted directly to the underlying brain, especially at points of bony contact. Second, intracranial bleeding in infants in whom the fontanelles and sutures remain open may, on rare occasions, be severe enough to cause hypotensive shock. Third, the proportionately larger size of the head, when coupled with the injury mechanisms commonly observed in children, generally leads to head trauma with a loss of consciousness. As a consequence, the voluntary muscles of the neck lose their tone which can lead to soft tissue obstruction in the upper airway and hypoxia. Hypoxia exacerbates and potentiates the initial traumatic injury to the brain (secondary insults). See Chapter 17 for more information about head injuries.
Neck
Cervical spine injury is a relatively uncommon event in pediatric trauma. It affects approximately 1.5% of all seriously injured children, and occurs at a rate of 1.8/100,000 population, in contrast to closed-head injury, which occurs at a rate of 185/100,000 population.50–52 The pediatric surgeon should also be aware of normal variants of cervical spine anatomy. The greater elasticity of the interspinous ligaments and the more horizontal apposition of the cervical vertebrae also give rise to a normal anatomic variant known as pseudosubluxation, which affects up to 40% of children younger than age 7 years. The most common finding is a short (2–3 mm) anterior displacement of C2 on C3, although anterior displacement of C3 on C4 also may occur. This pseudosubluxation is accentuated when the pediatric patient is placed in a supine position, which forces the cervical spine of the young child into mild flexion because of the forward displacement of the head by the more prominent occiput. The greater elasticity of the interspinous ligaments also is responsible for the increased distance between the dens and the anterior arch of C1 that is found in up to 20% of children.
When an injury to the cervical spine does occur, it frequently occurs at C2, C1, and the occipitoatlantal junction. The injuries are above the nerve roots that give rise to diaphragmatic innervation (C4) and predispose the afflicted child to respiratory arrest as well as paralysis. The increased angular momentum produced by movement of the proportionately larger head, the greater elasticity of the interspinous ligaments, and the more horizontal apposition of the cervical vertebrae are responsible for this spectrum of injuries. Subluxation without dislocation may cause spinal cord injury without radiographic abnormalities (SCIWORA). SCIWORA accounts for up to 20% of pediatric spinal cord injuries as well as a number of prehospital deaths that were previously attributed to head trauma.53–55
Chest
Serious intrathoracic injuries occur in 6% of pediatric blunt trauma victims.56 Although thoracostomy is required in about 50%, thoracotomy is seldom needed. Lung injuries, pneumothorax and hemothorax, and rib and sternal fractures occur most frequently (Table 14-3). Injuries to the heart, diaphragm, great vessels, bronchi, and esophagus occur less frequently, but have higher mortality rates associated with them. Because blunt trauma is nearly ten times more deadly when associated with major intrathoracic injury, thoracic injury serves as a marker of injury severity, even though it is the proximate cause of death in less than 1% of all pediatric blunt trauma.57
TABLE 14-3
Incidence and Mortality of Injuries to Thoracic and Abdominal Organs
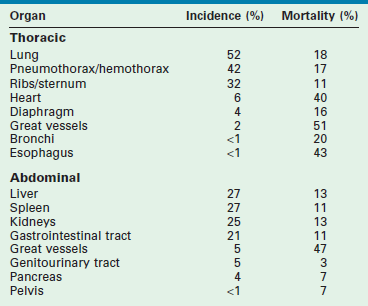
From Cooper A, Barlow B, DiScala C, et al. Mortality and truncal injury: The pediatric perspective. J Pediatric Surg 1994;29:33–8.
Abdomen
Serious intra-abdominal injuries occur in 8% of pediatric blunt trauma victims and are caused by crushing the solid upper abdominal viscera against the vertebral column, sudden compression and bursting of the hollow upper abdominal viscera against the vertebral column, or shearing of the posterior attachments, including the vascular supply, of the upper abdominal viscera after rapid deceleration (see Table 14-3).56,58 Injuries to the liver (27%), spleen (27%), kidneys (25%), and gastrointestinal tract (21%) occur most frequently and account for most of the deaths from intra-abdominal injury. Injuries to the great vessels (5%), genitourinary tract (5%), pancreas (4%), and pelvis (<1%) occur less frequently and account for few of the deaths that result from intra-abdominal injury. Most solid visceral injuries are successfully managed nonoperatively, especially those involving the kidneys (98%), the spleen (95%), and the liver (90%).59–61
Skeleton
Although they are the leading cause of disability, fractures are rarely an immediate cause of death from blunt trauma. They are reported to occur in 26% of serious blunt-injury cases and constitute the principal anatomic diagnosis in 22%.9 Upper extremity fractures outnumber lower extremity fractures by 7 : 1, although, in serious blunt trauma, this ratio is 2 : 3. The most common long bone fractures sustained during pedestrian/MVCs in children are fractures of the femur and tibia. Falls are typically associated with both upper and lower extremity fractures if the fall height is significant (from the window of a high-rise dwelling or the top of a bunk bed, but not from falls from standard beds or down stairs).62–65 Because isolated long bone and stable pelvic fractures are infrequently associated with significant hemorrhage, a diligent search must be made for another source of bleeding if signs of shock are observed.66,67 Unstable pelvic fractures are an uncommon feature of childhood injury, but unilateral (type III) or bilateral (type IV) anterior and posterior disruptions are those most often associated with major hemorrhage and must be recognized early and treated.68
Prehospital Care
Basic life support for the pediatric trauma patient consists of oxygen administration, airway adjuncts, bleeding control, spine stabilization, and temperature maintenance. Assisted ventilation and fracture immobilization should be provided as needed. Spinal immobilization requires both neutral positioning (which cannot be achieved without placing an approximate 2.5 cm layer of padding beneath the torso from shoulders to hips) and careful strapping (because forced vital capacity may be decreased by up to 60%).69,70 One study suggested that cervical spine immobilization can be safely avoided in most pediatric trauma patients with minor injuries, but caution was urged in view of the known risks of SCIWORA and atlanto-axial instability.71 Advanced life support of the pediatric trauma patient theoretically adds endotracheal intubation and volume resuscitation to this armamentarium, but neither intervention has been shown to improve outcome.72–76
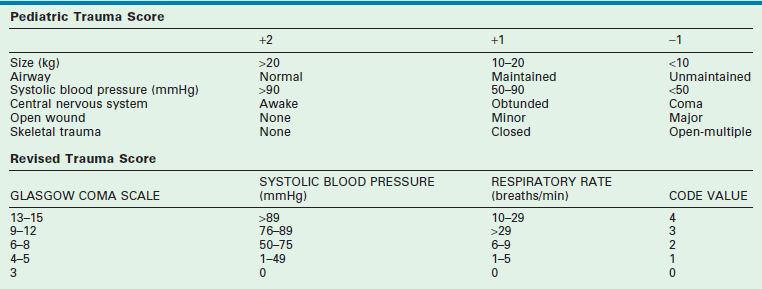
Field triage of pediatric trauma patients to pediatric trauma centers is now well established. Regional protocols should direct ambulance transports to such centers where available. The use of scoring systems to assist in predicting the need for specialty pediatric trauma care, such as the PTS and the Revised Trauma Score (RTS), may reliably predict the need for specialty pediatric trauma care but neither is optimally sensitive nor specific (Table 14-4).77,78 The most sensitive and specific indicators for the need for specialty pediatric trauma care are: a score of 1 in best motor response in the calculation of the GCS or a selection of ‘unresponsive/unconscious’ in the calculation of an AVPU Score.79 Good results also have been achieved by using checklists to identify anatomic, physiologic, and mechanistic criteria (Box 14-1) rather than calculated scores. These checklists are currently advocated by the ACS Committee on Trauma, based upon the advice of an expert panel convened in 2011 by the United States Centers for Disease Control and Prevention (CDC).80
Emergency Care
Primary Survey
Early management of childhood trauma begins in the field and continues in the emergency department.81,82 A primary survey of the airway, breathing, circulation (see Box 14-1) and neurologic disabilities (Box 14-2) should be completed to identify and correct deficits that pose an immediate threat to life. The primary survey continues with complete exposure of the patient to ensure that no injuries are missed, taking care to avoid hypothermia. The placement of therapeutic adjuncts, such as a urinary and gastric catheter (unless contraindicated), is also completed during this initial survey. Diagnostic adjuncts, such as pulse oximetry, radiographs, and focused assessment by sonography in trauma (FAST), facilitate the early recognition and treatment of immediate threats to vital functions (Box 14-3). The complete ‘trauma series’ of radiographs obtained as an adjunct to the primary survey in adults may not always be necessary in children, since the lateral cervical spine radiograph will not detect SCIWORA, and the screening pelvic radiograph seldom identifies a pelvic fracture. If a pelvic fracture is suspected on physical examination, a computed tomography (CT) scan should be obtained.83,84
Resuscitation
The first step in management of the circulation is control of bleeding. Direct pressure using sterile dressings is applied to all actively bleeding external wounds. Blind clamping is avoided, owing to the potential risk of injury to neurovascular bundles. Military experience suggests that commercial arterial tourniquets, and topical hemostatic agents such as chitosan granules or powder, zeolite granules, and kaolin clay, are effective in stopping major arterial hemorrhage, and massive arteriolar, venular, and capillary oozing from large open wounds. Recent data suggests equivalent effectiveness for tourniquets in children.85 However, because no reports of topical hemostatic agent use in children have been published to date, no recommendation can be made regarding its applicability in civilian pediatric trauma.
The child with significant trauma will require volume resuscitation if signs of hypovolemic shock are present. Intraosseous access should be used if conventional intravenous access with peripheral large bore catheters is not rapidly obtainable. Central venous catheter insertion, except in cases when venous access cannot otherwise readily be obtained, is not warranted. Simple hypovolemia usually responds to 20–40 mL/kg of warmed lactated Ringer’s solution. However, frank hypotension (defined clinically by a systolic blood pressure less than 70 mmHg plus twice the age in years) typically requires 40–60 mL/kg of warmed lactated Ringer’s solution followed by 10–20 mL/kg of warmed packed red blood cells. To avoid the greater mortality associated with coagulopathy and shock on hospital admission, a 1 : 1 : 1 or 2 : 1 : 1 ratio with fresh frozen plasma and platelet concentrates should be instituted when massive uncontrolled hemorrhage is present and a massive transfusion protocol is invoked.86–88 Urinary output should be measured in all seriously injured children as an indication of tissue perfusion. The minimum urinary output that indicates adequate renal perfusion is 2 mL/kg/h in infants, 1 mL/kg/h in children, and 0.5 mL/kg/h in adolescents.
Due to the ability of a child’s blood vessels to compensate vigorously for hypovolemia by intense vasoconstriction, systolic hypotension is a late sign of shock and may not develop until 30–35% of circulating blood volume is lost.89 Thus, any child who cannot be stabilized after infusion of 40–60 mL/kg of lactated Ringer’s solution and 10–20 mL/kg of packed red blood cells likely has internal bleeding and needs an operation. If a child initially is in shock, has no signs of intrathoracic, intra-abdominal, or intrapelvic bleeding, but fails to improve despite seemingly adequate volume resuscitation, other forms of shock (obstructive, cardiogenic, neurogenic) should be considered. Most children in hypotensive shock are victims of unrecognized hemorrhage that can be reversed only if promptly recognized and appropriately treated by means of rapid blood transfusion and immediate intervention, particularly if major intrathoracic or intra-abdominal vessels are injured.90–93
Secondary Survey
Selective laboratory evaluation is an integral part of the secondary survey, although routine trauma laboratory panels are of limited utility owing to their relatively low sensitivity and specificity.94–96 Arterial blood gases are important in determining the adequacy of ventilation (PsCO2), oxygenation (PsO2), and perfusion (base deficit).97,98 However, the critically important determinant of blood oxygen content is the blood hemoglobin concentration. Serial hemoglobin values better reflect the extent of blood loss than does the initial value. Elevations in serum levels of transaminases or amylase and lipase suggest injury to the liver or pancreas, but the infrequency of pancreatic injury makes the latter cost ineffective versus the former.99,100 Urine that is grossly bloody or is positive for blood by dipstick or microscopy (>50 red blood cells per high-power field) suggests kidney trauma.101
Selective radiologic evaluation is another important part of the secondary survey. CT of the head (without contrast) and abdomen (intravenous and oral) should be obtained as indicated. However, CT should be employed only when the short-term benefit of accurate diagnosis is felt to outweigh the long-term risk of late malignancy, particularly for body regions such as the cervical spine and the thorax for which conventional imaging is adequate. When utilized, the CT should be performed using radiation doses ‘as low as reasonably achievable’ (ALARA), consistent with the ‘image gently®’ protocols advocated by The Alliance for Radiation Safety in Pediatric Imaging.102–108
CT of the head should be performed whenever loss of consciousness has occurred, or if the GCS score is <15.109 It can be safely avoided in children <2 years of age with: (1) normal mental status; (2) no scalp hematoma except frontal; (3) no loss of consciousness or loss of consciousness for less than five seconds; (4) nonsevere injury mechanism; (5) no palpable skull fracture; and (6) normal activity according to parents. Recommendations for not obtaining a head CT in children ≥2 years of age and older include: (1) normal mental status; (2) no loss of consciousness; (3) no vomiting; (4) nonsevere injury mechanism; (5) no signs of basilar skull fracture; and (6) no severe headache.110 CT of the chest adds little to what is already known from the chest radiograph obtained during the primary survey, since the incidental pulmonary contusions identified by CT of the chest do not correlate with increased fatality.111,112 CT of the cervical spine may facilitate earlier identification of vertebral injury, but does so at the cost of increased radiation dose.113 CT of the abdomen should be obtained: (1) in intubated patients; (2) with signs of internal bleeding (abdominal tenderness, distention, bruising, or gross hematuria), a history of hypotensive shock (which has responded to volume resuscitation), or a hematocrit <30%; (3) if a femur fracture is evident; (4) if serum transaminase levels are elevated; (5) if significant microscopic hematuria is present, or (6) if the mechanism of injury is deemed significant.114,115
Sonography serves an adjunctive role in the imaging of pediatric trauma. FAST itself is most useful in detecting intra-abdominal blood, but is not sufficiently reliable to exclude blunt abdominal injury, although it does have the advantage that such injuries can be detected by repeated examination.116–123 Therefore, like its historical predecessor diagnostic peritoneal lavage, FAST adds relatively little to the management of pediatric abdominal trauma, since unstable patients with presumed intra-abdominal injuries need immediate operation, while stable patients are managed nonoperatively without regard to the presence of intra-abdominal blood.124–128 However, diagnostic sonography has been successfully used in screening for intra-abdominal injuries when abdominal CT is unavailable or contraindicated.129
Special Considerations in Trauma Care
Definitive management of childhood trauma also depends on the type, extent, and severity of the injuries sustained. Any child requiring resuscitation should be admitted to the hospital under the care of a surgeon experienced in the management of childhood injuries. Further information and details regarding the management and treatment of traumatic injuries in children may found throughout this textbook including: vascular access (Chapter 8), burns (Chapter 13), thoracic trauma (Chapter 15), abdominal trauma (Chapter 16), traumatic brain injury (Chapter 17), and orthopedic and spinal trauma (Chapter 18).
Physical Support
The care of children with major traumatic injury also involves assessment and treatment of somatic pain. Two pain scales have now been validated.130 In patients who are not eating, nutritional support is recommended.131 In children who have sustained injuries resulting in hematomas, low-grade fever may develop as these are resorbed. However, high spiking fevers should prompt investigation for a source such as infected hematomas, effusions, or pelvic osteomyelitis. Children with chest tubes or long-term indwelling urinary catheters are at risk for systemic infection and should receive prophylactic or suppressive antibiotics as long as the tube or catheter is required.
Emotional Support
Efforts must be made to attend to the emotional needs of the child and family, especially for those families facing the death of a child or a sibling.132 In addition to the loss of control over their child’s destiny, parents of seriously injured children also may feel enormous guilt, whether or not these feelings are warranted. The pediatric surgeon should attempt to create as normal an environment as possible for the child and allow the parents to participate meaningfully in postinjury care, as acute stress disorder symptoms in children and parents are common after injury.133 In so doing, treatment interventions will be facilitated as the child perceives that parents and staff are working together to ensure an optimal recovery, with the added benefit that long-term psychological effects such as posttraumatic stress disorder may be averted.134 Even so, depression is increasingly recognized as a serious complication in adolescents after major trauma. Risk factors for depression include high injury severity, other family members injured, low socioeconomic status, and suicidal ideation or attempt prior to the current traumatic event.135
Nonaccidental Trauma
Nonaccidental trauma (NAT) is the underlying cause of 3–5% of significant traumatic injuries in childhood, and is a major cause of morbidity and mortality among children referred to pediatric trauma centers.9,136 Although a detailed review of the mechanisms, patterns, presentations, and findings of NAT is beyond the scope of this chapter, NAT should be suspected when: (1) the injury remains unexplained; (2) a lengthy delay occurs in obtaining treatment; (3) the history is vague or otherwise incompatible with the observed physical findings; (4) the caretaker blames siblings or playmates or other third parties, or protects other adults rather than the child; (5) cutaneous bruises or skeletal fractures are found in multiple stages of healing or in unusual locations; (6) skeletal fractures are found in the diaphyses of long bones in infants or children too young to walk; (7) scald or contact burns are found in unusual locations or patterns; or (8) unconsciousness is said to have occurred in association with a low fall.137,138 As with unintentional trauma, traumatic brain injury is the leading cause of death in NAT. The term ‘shaken baby syndrome’, characterized by the classic triad of altered mental status, bilateral subdural hematomas, and retinal hemorrhages, has largely been supplanted by the more inclusive term ‘abusive head trauma’.139,140 Although the initial assessment and medical treatment of physical injuries is no different from that for any other mechanism of injury, the sociomedicolegal management of suspected cases of NAT requires a special approach. The crucial role played by the pediatric trauma service and the pediatric trauma registry in early recognition and adequate documentation of potentially abusive injuries is paramount.141,142 Reports of suspected NAT must be filed with local child protective services in every North American state, province, and territory, as well as in most developed nations worldwide. Still, it must be emphasized that confrontation and accusation hinder treatment and rehabilitation, and have no place in the management of the potentially abused child, regardless of the nature of the injury, or the identity of the perpetrators.
Penetrating Injuries
All penetrating wounds are contaminated and must be treated as infected. Accessible missile fragments should be removed (once swelling has subsided) to prevent the development of lead poisoning (especially those in contact with bone or joint fluid).143 Thoracotomy is usually not required except for massive hemothorax (20 mL/kg) or ongoing hemorrhage (2–4 mL/kg/h) from the chest tube, persistent massive air leak, or food or salivary drainage from the chest tube. Laparotomy is nearly always required for gunshot wounds as well as stab wounds associated with hemorrhagic shock, peritonitis, or evisceration, although nonoperative management may be employed in carefully selected cases.144 Thoracoabdominal injury should be suspected whenever the torso is penetrated between the nipple line and the costal margin. A diaphragmatic injury should be suspected if peritoneal irritation develops after thoracic penetration, food or chyle is recovered from the chest tube, or if injury-trajectory or imaging studies suggest the possibility of diaphragmatic penetration. Tube thoracostomy, followed by laparotomy for repair of the diaphragm and/or damaged organs, is mandated with such signs, although laparoscopy is being used with increasing frequency.145–148
Systems Issues
Pediatric patients, at significant risk for death from multiple and severe injuries, are best served by a fully inclusive trauma system that incorporates all appropriate health care facilities and personnel to the level of their resources and capabilities.149,150 Unfortunately, access to specialty pediatric trauma care, including pediatric intensive care, remains highly variable.151,152 Moreover, collaboration with local public health agencies (in programs for injury prevention and control), as well as local public health, public safety, and emergency-management agencies (in regional disaster-planning efforts) is necessary.149,150 Although the regional trauma center is at the hub of the system, area trauma centers may be needed in localities distant from the regional trauma center. All trauma centers, whether adult or pediatric, must be capable of the initial management of the injured child or infant. This requires the immediate availability of a resuscitation team trained and credentialed for the management of pediatric trauma, for which structured review and simulation training have been shown to improve team performance, while family presence during resuscitation rarely hinders it.153–158 All other hospitals in the region should participate as they are able, and must be fully capable of initial resuscitation, stabilization, and transfer of pediatric trauma patients. Finally, a regional trauma advisory committee should include pediatric representation that has the authority to develop and implement guidelines for triage of pediatric trauma within the system to verified pediatric-capable trauma centers.159,160 Mature systems should expect that seriously injured pediatric patients will be primarily transported to pediatric trauma centers.161
Transport Issues
Pediatric victims of multisystem trauma should undergo direct primary transport from the injury scene to a pediatric-capable trauma center.28–47,82,150 If this is not possible, additional secondary transport from the initial receiving hospital to the pediatric trauma center is needed. Transport providers must be capable of critical pediatric assessment and monitoring, and skilled in the techniques of endotracheal intubation and vascular access, as well as drug and fluid administration in children.162,163 Specialized pediatric transport teams, staffed by physicians and nurses with advanced training in pediatric trauma and critical care treatment and transport, should be used whenever possible, as complications related to endotracheal intubation and vascular access are the leading causes of adverse events during transport, which occur twice as often as in the PICU and ten times more often without a specialized team.164,165
Hospital Preparedness
Regional pediatric trauma centers should be located in trauma hospitals with comprehensive pediatric services (a full-service general, university, or children’s hospital) that demonstrate an institutional commitment to pediatric trauma care, including child abuse.28–47,150 Adult trauma centers can achieve results comparable to those of pediatric trauma centers if pediatric subspecialty and nursing support (pediatric emergency, acute care, and critical care medicine) are available.38–47,150 Finally, an organized pediatric trauma service must be available within the regional pediatric trauma center that, in addition to exemplary patient care, provides education and research in pediatric trauma, and leadership in pediatric trauma system coordination.
References
1. National Center for Injury Prevention and Control. CDC Injury Fact Book. Atlanta: Centers for Disease Control and Prevention; 2006.
2. Buie, VC, Owings, MF, DeFrances, CJ, et al. National Hospital Discharge Survey: 2006 Summary. National Center for Health Statistics. Vital Health Stat. 13(168), 2010.
3. Klem, SA, Pollack, MM, Glass, NL, et al. Resource use, efficiency, and outcome prediction in pediatric intensive care of trauma patients. J Trauma. 1990; 30:32–36.
4. Krauss, BS, Harakal, T, Fleisher, GR. The spectrum and frequency of illness presenting to a pediatric emergency department. Pediatr Emerg Care. 1991; 7:67–71.
5. Tsai, A, Kallsen, G. Epidemiology of pediatric prehospital care. Ann Emerg Med. 1987; 16:284–292.
6. Hale, GC, Caudill, SA, Hicks-Waller, CM, et al. The New York State Trauma System: A Special Report on Pediatric Trauma. Albany, NY: New York State Department of Health; 2002.
7. Tepas, JJ, Ramenofsky, ML, Mollitt, DL, et al. The pediatric trauma score as a predictor of injury severity: An objective assessment. J Trauma. 1988; 28:425–429.
8. Cooper, A, Barlow, B, Davidson, L, et al. Epidemiology of pediatric trauma: Importance of population-based statistics. J Pediatr Surg. 1992; 27:149–154.
9. DiScala, C. National Pediatric Trauma Registry Annual Report. Boston: Tufts University Rehabilitation and Childhood Trauma Research and Training Center; 2002.
10. Ehrlich, PF, Brown, JK, Drogonowski, R. Characterization of the drug-positive adolescent trauma population: Should we, do we, and does it make a difference if we test? J Pediatr Surg. 2006; 41:927–930.
11. Draus, JM, Santos, AP, Franklin, GA, et al. Drug and alcohol use among adolescent blunt trauma patients: Dying to get high? J Pediatr Surg. 2007; 43:208–211.
12. Ehrlich, PF, Drogonowski, A, Swisher-McClure, S, et al. The importance of a preclinical trial: A selected intervention program for pediatric trauma centers. J Trauma. 2008; 65:189–195.
13. Brown, CVR, Nevill, AL, Salim, A, et al. The impact of obesity on severely injured children and adolescents. J Pediatr Surg. 2006; 41:88–91.
14. Marcin, JP, Schembri, MS, Jingsong, H, et al. A population-based analysis of socioeconomic status and insurance status and their relationship with pediatric trauma hospitalization and mortality rates. Am J Publ Health. 2003; 93:461–466.
15. Falcone, RA, Brown, RL, Garcia, VF. The epidemiology of infant injuries and alarming health disparities. J Pediatr Surg. 2007; 42:172–177.
16. Haider, AH, Efron, DT, Haut, ER, et al. Black children experience worse clinical and functional outcomes after traumatic brain injury: An analysis of the National Pediatric Trauma Registry. J Trauma. 2007; 62:1259–1263.
17. Falcone, RA, Martin, F, Brown, RL, et al. Despite overall low pediatric head injury mortality, disparities exist between races. J Pediatr Surg. 2008; 43:1858–1864.
18. Falcone, RA, Brown, RL, Garcia, VF. Disparities in child abuse mortality are not explained by injury severity. J Pediatr Surg. 2007; 42:1031–1037.
19. Rangel, SJ, Martin, CA, Brown, RL, et al. Alarming trends in the improper use of motor vehicle restraints in children: Implications for public policy and the development of race-based strategies for improving compliance. J Pediatr Surg. 2008; 43:200–207.
20. Brown, JK, Ying, Y, Wang, S, et al. Patterns of severe injury in pediatric car crash victims: Crash Injury Research Engineering Network database. J Pediatr Surg. 2006; 41:362–367.
21. Ehrlich, PF, Brown, JK, Sochor, MR, et al. Factors influencing pediatric Injury Severity Score and Glasgow Coma Scale in pediatric automobile crashes: Results from the Crash Injury Research Engineering Network. J Pediatr Surg. 2006; 41:1854–1858.
22. Zuckerbraun, BS, Morrison, K, Gaines, B, et al. Effect of age on cervical spine injuries in children after motor vehicle collisions: Effectiveness of restraint devices. J Pediatr Surg. 2004; 39:483–486.
23. Hayes, JR, Groner, JI. Using multiple imputation and propensity scores to test the effect of car seats and seat belt usage on injury severity from trauma registry data. J Pediatr Surg. 2008; 43:924–927.
24. Arbogast, KB, Kent, RW, Menon, RA, et al. Mechanisms of abdominal organ injury in seat-belt restrained children. J Trauma. 2007; 62:1473–1480.
25. Lutz, N, Nance, ML, Kallan, MJ, et al. Incidence and clinical significance of abdominal wall bruising in restrained children involved in motor vehicle crashes. J Pediatr Surg. 2004; 39:972–975.
26. Chidester, S, Rana, A, Lowell, W, et al. Is the ‘seat belt sign’ associated with serious abdominal injuries in pediatric trauma? J Trauma. 2009; 67:s34–s36.
27. Nadler, EP, Potoka, DA, Shultz, BL, et al. The high morbidity associated with handlebar injuries in children. J Trauma. 2005; 58:1171–1174.
28. Pollack, MM, Alexander, SR, Clarke, N, et al. Improved outcomes from tertiary center pediatric intensive care: A statewide comparison of tertiary and nontertiary care facilities. Crit Care Med. 1991; 19:150–159.
29. Nakayama, DK, Copes, WS, Sacco, WJ. Differences in pediatric trauma care among pediatric and nonpediatric centers. J Pediatr Surg. 1992; 27:427–431.
30. Cooper, A, Barlow, B, DiScala, C, et al. Efficacy of pediatric trauma care: Results of a population-based study. J Pediatr Surg. 1993; 28:299–305.
31. Hall, JR, Reyes, HM, Meller, JT, et al. Outcome for blunt trauma is best at a pediatric trauma center. J Pediatr Surg. 1996; 31:72–77.
32. Hulka, F, Mullins, RJ, Mann, NC, et al. Influence of a statewide trauma system on pediatric hospitalization and outcome. J Trauma. 1997; 42:514–519.
33. Potoka, DA, Schall, LC, Gardner, MJ, et al. Impact of pediatric trauma centers on mortality in a statewide system. J Trauma. 2000; 49:237–245.
34. Potoka, DA, Schall, LC, Ford, HR. Improved functional outcome for severely injured children treated at pediatric trauma centers. J Trauma. 2001; 51:824–834.
35. Farrell, LS, Hannan, EL, Cooper, A. Severity of injury and mortality associated with pediatric blunt injuries: Hospitals with pediatric intensive care units vs. other hospitals. Pediatr Crit Care Med. 2004; 5:5–9.
36. Densmore, JC, Lim, HJ, Oldham, KT, et al. Outcomes and delivery of care in pediatric injury. J Pediatr Surg. 2006; 41:92–98.
37. Pracht, EE, Tepas, JJ, Langland-Orban, B, et al. Do pediatric patients with trauma in Florida have reduced mortality rates when treated in designated trauma centers? J Pediatr Surg. 2008; 43:212–221.
38. Knudson, MM, Shagoury, C, Lewis, FR. Can adult trauma surgeons care for injured children? J Trauma. 1992; 32:729–739.
39. Fortune, JM, Sanchez, J, Graca, L, et al. A pediatric trauma center without a pediatric surgeon: A four year outcome analysis. J Trauma. 1992; 33:130–139.
40. Rhodes, M, Smith, S, Boorse, D. Pediatric trauma patients in an ‘adult’ trauma center. J Trauma. 1993; 35:384–393.
41. Bensard, DD, McIntyre, RC, Moore, EE, et al. A critical analysis of acutely injured children managed in an adult level I trauma center. J Pediatr Surg. 1994; 29:11–18.
42. D’Amelio, LF, Hammond, JS, Thomasseau, J, et al. ‘Adult’ trauma surgeons with pediatric commitment: A logical solution to the pediatric trauma manpower problem. Am Surg. 1995; 61:968–974.
43. Partrick, DA, Moore, EE, Bensard, DD, et al. Operative management of injured children at an adult level I trauma center. J Trauma. 2000; 48:894–901.
44. Sherman, HF, Landry, VL, Jones, LM. Should level I trauma centers be rated NC-17? J Trauma. 2001; 50:784–791.
45. Aaland, MO, Hlaing, T. Pediatric trauma deaths: A three-part analysis from a nonacademic trauma center. Am Surg. 2006; 72:249–259.
46. Winthrop, AL, Brasel, KJ, Stahovic, L, et al. Quality of life and functional outcome after pediatric trauma. J Trauma. 2005; 58:468–474.
47. vanderSluis, CK, Kingma, J, Eisma, WH, et al. Pediatric polytrauma: Short-term and long-term outcomes. J Trauma. 1997; 43:501–506.
48. Burd, RS, Jang, TS, Nair, SS. Predicting hospital mortality among injured children using a national trauma database. J Trauma. 2006; 60:792–801.
49. Haddon, W. Advances in the epidemiology of injuries as a basis for public policy. Public Health Rep. 1980; 95:411–421.
50. Kokoska, ER, Keller, MS, Rallo, MC, et al. Characteristics of pediatric cervical spine injuries. J Pediatr Surg. 2001; 36:100–105.
51. Patel, JC, Tepas, JJ, Mollitt, DL, et al. Pediatric cervical spine injuries: defining the disease. J Pediatr Surg. 2001; 36:373–376.
52. Kewalramani, LS, Kraus, JF, Sterling, HM, et al. Acute spinal-cord lesions in a pediatric population: Epidemiological and clinical features. Paraplegia. 1980; 18:206–219.
53. Pang, D, Wilberger, E. Spinal cord injury without radiographic abnormality in children. J Neurosurg. 1982; 57:114–129.
54. Bohn, D, Armstrong, A, Becker, L, et al. Cervical spine injuries in children. J Trauma. 1990; 30:463–469.
55. Bosch, PP, Vogt, MT, Ward, WT. Pediatric spinal cord injury without radiographic abnormality: The absence of occult instability and lack of indication for bracing. Spine. 2002; 27:2788–2800.
56. Cooper, A, Barlow, B, DiScala, C. Mortality and truncal injury: The pediatric perspective. J Pediatr Surg. 1994; 29:33–38.
57. Peclet, MH, Newman, KD, Eichelberger, MR, et al. Thoracic trauma in children: An indicator of increased mortality. J Pediatr Surg. 1990; 25:961–966.
58. Haller, JA. Injuries of the gastrointestinal tract in children: Notes on recognition and management. Clin Pediatr. 1966; 5:476–480.
59. Rogers, CG, Knight, V, MacUra, KJ. High-grade renal injuries in children—is conservative management possible? Urology. 2004; 64:574–579.
60. Pearl, RH, Wesson, DE, Spence, LJ, et al. Splenic injury: A 5-year update with improved results and changing criteria for conservative management. J Pediatr Surg. 1989; 24:121–125.
61. Galat, JA, Grisoni, ER, Gauderer, MWL. Pediatric blunt liver injury: Establishment of criteria for appropriate management. J Pediatr Surg. 1990; 25:1162–1165.
62. Barlow, B, Niemirska, M, Gandhi, R. Ten years of experience with falls from a height in children. J Pediatr Surg. 1983; 18:509–511.
63. Selbst, SM, Baker, MD, Shames, M. Bunk bed injuries. Am J Dis Child. 1990; 144:721–723.
64. Helfer, RE, Slovis, TL, Black, M. Injuries resulting when small children fall out of bed. Pediatrics. 1977; 60:533–535.
65. Joffe, M, Ludwig, S. Stairway injuries in children. Pediatrics. 1988; 82:457–461.
66. Barlow, B, Niemirska, M, Gandhi, R, et al. Response to injury in children with closed femur fractures. J Trauma. 1987; 27:429–430.
67. Ismail, N, Bellemare, JF, Mollitt, D, et al. Death from pelvic fracture: Children are different. J Pediatr Surg. 1996; 31:82–85.
68. McIntyre, RR, Bensard, DD, Moore, EE, et al. Pelvic fracture geometry predicts risk of life-threatening hemorrhage in children. J Trauma. 1993; 35:423–429.
69. Herzenberg, JE, Hensinger, RN, Dedrick, DK, et al. Emergency transport and positioning of young children who have an injury of the cervical spine. J Bone Joint Surg Am. 1989; 71:15–22.
70. Schafermeyer, RW, Ribbeck, BM, Gaskins, J, et al. Respiratory effects of spinal immobilization in children. Ann Emerg Med. 1991; 20:1017–1019.
71. Viccellio, P, Simon, H, Pressman, BD, et al. A prospective multicenter study of cervical spine injury in children. Pediatrics. 2001; 108:e20.
72. Gausche, M, Lewis, RJ, Stratton, SJ, et al. Effect of out-of-hospital pediatric endotracheal intubation on survival and neurological outcome: A controlled clinical trial. JAMA. 2000; 283:783–790.
73. Cooper, A, DiScala, C, Foltin, G, et al. Prehospital endotracheal intubation for severe head injury in children: A reappraisal. Semin Pediatr Surg. 2001; 10:3–6.
74. Ehrlich, PF, Seidman, PS, Atallah, O, et al. Endotracheal intubations in rural pediatric trauma patients. J Pediatr Surg. 2004; 39:1376–1380.
75. Cooper, A, Barlow, B, DiScala, C, et al. Efficacy of prehospital volume resuscitation in children who present in hypotensive shock. J Trauma. 1993; 35:160.
76. Teach, SJ, Antosia, RE, Lund, DP, et al. Prehospital fluid therapy in pediatric trauma patients. Pediatr Emerg Care. 1995; 11:5–8.
77. Tepas, JJ, Mollitt, DL, Talbert, JL, et al. The pediatric trauma score as a predictor of injury severity in the injured child. J Pediatr Surg. 1987; 22:14–18.
78. Champion, HR, Sacco, WJ, Copes, WS, et al. A revision of the trauma score. J Trauma. 1989; 29:623–629.
79. Hannan, EL, Farrell, LS, Meaker, PS, et al. Predicting inpatient mortality for pediatric blunt trauma patients: A better alternative. J Pediatr Surg. 2000; 35:155–159.
80. Sasser, SM, Hunt, RC, Faul, M, et al. Recommendations of the National Expert Panel on Field Triage, 2011. MMWR. 2012; 61(RR-1):1–21.
81. Prehospital Trauma Life Support Committee of the National Association of Emergency Medical Technicians in cooperation with the Committee on Trauma of the American College of Surgeons. PHTLS: Prehospital Trauma Life Support, 7th ed. St. Louis: Mosby Elsevier; 2010.
82. American College of Surgeons Committee on Trauma. Advanced Trauma Life Support® ATLS® Student Course Manual, 9th ed. Chicago: American College of Surgeons; 2012.
83. Rees, MJ, Aickin, R, Kolbe, A, et al. The screening pelvic radiograph in pediatric trauma. Pediatr Radiol. 2001; 31:497–500.
84. Junkins, EP, Stotts, A, Santiago, R, et al. The clinical presentation of pediatric thoracolumbar fractures: A prospective study. J Trauma. 2008; 65:1066–1071.
85. Kragh, JF, Cooper, A, Aden, JK, et al. Survey of trauma registry data on tourniquet use in pediatric war casualties. Pediatr Emerg Care. 2012; 28:1361–1365.
86. Holcomb, JB, Wade, CE, Michalek, JE, et al. Increased plasma and platelet to red cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008; 248(3):447–458.
87. Dressler, AM, Finck, CM, Carroll, CL, et al. Use of a massive transfusion protocol with hemostatic resuscitation for severe intraoperative bleeding in a child. J Pediatr Surg. 2010; 45:1530–1533.
88. Patregnani, JT, Borgman, MA, Maegele, M, et al. Coagulopathy and shock on admission is associated with mortality for children with traumatic injuries at combat support hospitals. Pediatr Crit Care Med. 2012; 13:273–277.
89. Schwaitzberg, SD, Bergman, KS, Harris, BH. A pediatric model of continuous hemorrhage. J Pediatr Surg. 1988; 23:605–609.
90. Klinker, DB, Arca, MJ, Lewis, BD, et al. Pediatric vascular injuries: Patterns of injury, morbidity, and mortality. J Pediatr Surg. 2007; 42:178–183.
91. Hammer, CE, Groner, JI, Caniano, DA, et al. Blunt intraabdominal arterial injury in pediatric trauma patients: Injury distribution and markers of outcome. J Pediatr Surg. 2008; 34:916–923.
92. Anderson, SA, Day, M, Chen, NK, et al. Traumatic aortic injuries in the pediatric population. J Pediatr Surg. 2008; 43:1077–1081.
93. Heckman, SR, Trooskin, SZ, Burd, RS. Risk factors for blunt thoracic injury in children. J Pediatr Surg. 2005; 40:98–102.
94. Isaacman, DJ, Scarfone, RJ, Kost, SI, et al. Utility of routine laboratory testing for detecting intraabdominal injury in the pediatric trauma patient. Pediatrics. 1993; 92:691–694.
95. Keller, MS, Coln, CE, Trimble, JA, et al. The utility of routine trauma laboratories in pediatric trauma resuscitations. Am J Surg. 2004; 188:671–678.
96. Capraro, AJ, Mooney, D, Waltzman, ML. The use of routine laboratory studies as screening tools in pediatric abdominal trauma. Pediatr Emerg Care. 2006; 22:480–484.
97. Kincaid, EH, Chang, MC, Letton, RW, et al. Admission base deficit in pediatric trauma: A study using the National Trauma Data Bank. J Trauma. 2001; 51:332–335.
98. Randolph, LC, Takacs, M, Davis, KA. Resuscitation in the pediatric trauma population: Admission base deficit remains an important prognostic indicator. J Trauma. 2002; 53:838–842.
99. Oldham, KT, Guice, KS, Kaufman, RA, et al. Blunt hepatic injury and elevated hepatic enzymes: A clinical correlation in children. J Pediatr Surg. 1984; 19:457–461.
100. Adamson, WT, Hebra, A, Thomas, PB, et al. Serum amylase and lipase alone are not cost-effective screening methods for pediatric pancreatic trauma. J Pediatr Surg. 2003; 38:354–357.
101. Lieu, TA, Fleisher, GR, Mahboubi, S, et al. Hematuria and clinical findings as indications for intravenous pyelography in pediatric blunt renal trauma. Pediatrics. 1988; 82:216–222.
102. Brenner, DJ, Elliston, CD, Berdon, WE. Estimated risks of radiation-induced fatal cancer from pediatric CT. Am J Roentgenol. 2001; 176:289–296.
103. Fenton, SJ, Hansen, KW, Meyers, RL, et al. CT scan and the pediatric trauma patient: are we overdoing it? J Pediatr Surg. 2004; 39:1877–1881.
104. Kim, PK, Zhu, X, Houseknecht, E, et al. Effective radiation dose from radiologic studies in pediatric trauma patients. World J Surg. 2005; 29:1557–1562.
105. Brody, AS, Frush, DP, Huda, W, et al. Radiation risk to children from computed tomography. Pediatrics. 2007; 120:677–682.
106. Chwals, WJ, Robinson, AV, Sivit, CJ, et al. Computed tomography before transfer to a level I pediatric trauma center risks duplication with associated increased radiation exposure. J Pediatr Surg. 2008; 43:2268–2272.
107. Cook, SH, Fielding, JR, Phillips, JD. Repeat abdominal computed tomography after pediatric blunt trauma: missed injuries, extra costs, and unnecessary radiation exposure. J Pediatr Surg. 2010; 45:2019–2024.
108. Brunetti, MA, Mahadevappa, M, Nabaweesi, R, et al. Diagnostic radiation exposure in pediatric trauma patients. J Trauma. 2011; 70:E24–E28.
109. Wang, MY, Griffith, PR, Sterling, J, et al. A prospective population-based study of pediatric trauma patients with mild alterations in consciousness (Glasgow Coma Scale score of 13–14). Neurosurg. 2000; 46:1093–1099.
110. Kuppermann, N, Holmes, JF, Dayan, PS, et al. Identification of children at very low risk of clinically-important brain injuries after head trauma: A prospective cohort study. Lancet. 2009; 374:1160–1170.
111. Renton, J, Kincaid, S, Ehrlich, PF. Should helical CT scanning of the thoracic cavity replace the conventional chest x-ray as a primary assessment tool in pediatric trauma? An efficacy and cost analysis. J Pediatr Surg. 2003; 38:793–797.
112. Kwon, A, Sorrells, DL, Kurkchubaske, AG, et al. Isolated computed tomography diagnosis of pulmonary contusion does not correlate with increased morbidity. J Pediatr Surg. 2006; 41:78–82.
113. Keenan, HT, Hollingshead, MC, Chung, CJ, et al. Using CT of the cervical spine for early evaluation of pediatric patients with head trauma. Am J Roentgenol. 2001; 177:1405–1409.
114. Flood, RG, Mooney, DP. Rate and prediction of traumatic injuries detected by abdominal computed tomography scan in intubated children. J Trauma. 2006; 61:340–345.
115. Taylor, GA, Eichelberger, MR, O’Donnel, R, et al. Indications for computed tomography in children with blunt abdominal trauma. Ann Surg. 1991; 213:212–218.
116. Patel, JC, Tepas, JJ. The efficacy of focused abdominal sonography for trauma (FAST) as a screening tool in the assessment of injured children. J Pediatr Surg. 1999; 34:44–47.
117. Mutabagani, KH, Coley, BD, Zumberge, N, et al. Preliminary experience with focused abdominal sonography for trauma (FAST) in children: Is it useful? J Pediatr Surg. 1999; 34:48–54.
118. Pershad, J, Gilmore, B. Serial bedside emergency ultrasound in a case of pediatric blunt abdominal trauma with severe abdominal pain. Pediatr Emerg Care. 2000; 16:375–376.
119. Corbett, SW, Andrews, HG, Baker, EM, et al. ED evaluation of the pediatric trauma patient by ultrasonography. Am J Emerg Med. 2000; 18:244–249.
120. Coley, BD, Mutabagani, KH, Martin, LC, et al. Focused abdominal sonography for trauma (FAST) in children with blunt abdominal trauma. J Trauma. 2000; 48:902–906.
121. Soudack, M, Epelman, M, Maor, R, et al. Experience with focused abdominal sonography for trauma (FAST) in 313 pediatric patients. J Clin Ultrasound. 2004; 32:53–61.
122. Suthers, SE, Albrecht, R, Foley, D, et al. Surgeon-directed ultrasound for trauma is a predictor of intraabdominal injury in children. Am Surg. 2004; 70:164–168.
123. Soundappan, SV, Holland, AJ, Cass, DT, et al. Accuracy of surgeon-performed focused abdominal sonography (FAST) in blunt paediatric trauma. Injury. 2005; 36:970–975.
124. Emery, KH, McAneney, CM, Racadio, JM, et al. Absent peritoneal fluid on screening trauma ultrasonography in children: A prospective comparison with computed tomography. J Pediatr Surg. 2001; 36:565–569.
125. Holmes, JF, London, KL, Brant, WE. Isolated intraperitoneal fluid on abdominal computed tomography in children with blunt trauma. Acad Emerg Med. 2000; 7:335–341.
126. Rathaus, V, Zissin, R, Werner, M, et al. Minimal pelvic fluid in blunt abdominal trauma: The significance of this sonographic finding. J Pediatr Surg. 2001; 36:1387–1389.
127. Holmes, JF, Brant, WE, Bond, WF, et al. Emergency department ultrasonography in the evaluation of hypotensive and normotensive children with blunt abdominal trauma. J Pediatr Surg. 2001; 36:968–973.
128. Venkatesh, KR, McQuay, N. Outcomes of management in stable children with intra-abdominal free fluid without solid organ injury after blunt abdominal injury. J Trauma. 2007; 62:216–220.
129. Filiatrault, D, Longpre, D, Patriquin, H, et al. Investigation of childhood blunt abdominal trauma: A practical approach using ultrasound as the initial diagnostic modality. Pediatr Radiol. 1987; 17:373–379.
130. Baxt, C, Kassam-Adams, N, Nance, ML, et al. Assessment of pain after injury in the pediatric patient: Child and parent perceptions. J Pediatr Surg. 2004; 39:979–983.
131. Winthrop, AL, Wesson, DE, Pencharz, PB, et al. Injury severity, whole body protein turnover, and energy expenditure in pediatric trauma. J Pediatr Surg. 1987; 22:534–537.
132. Oliver, RC, Sturtevant, JP, Scheetz, JP, et al. Beneficial effects of a hospital bereavement intervention program after traumatic childhood death. J Trauma. 2001; 50:440–448.
133. Winston, FK, Kassam-Adams, N, Vivarelli-O’Neill, C, et al. Acute stress disorder symptoms in children and their parents after pediatric traffic injury. Pediatrics. 2002; 109:e90.
134. Schreier, H, Ladakokos, C, Morabito, D, et al. Posttrauma stress symptoms in children after mild to moderate pediatric trauma: A longitudinal examination of symptom prevalence, correlates, and parent-child symptom reporting. J Trauma. 2005; 58:353–363.
135. Han, PP, Holbrook, TL, Sise, MJ, et al. Postinjury depression is a serious complication in adolescents after major trauma: Injury severity and injury-event factors predict depression and long-term quality of life deficits. J Trauma. 2011; 70:923–930.
136. Roaten, JB, Partrick, DA, Nydam, TL, et al. Nonaccidental trauma is a major cause of morbidity and mortality among patients at a regional level 1 pediatric trauma center. J Pediatr Surg. 2006; 41:2013–2015.
137. Cooper, A, Floyd, T, Barlow, B, et al. Fifteen years’ experience with major blunt abdominal trauma due to child abuse. J Trauma. 1988; 28:1483–1487.
138. Wood, J, Rubin, DM, Nance, ML, et al. Distinguishing inflicted versus accidental abdominal injuries in young children. J Trauma. 2005; 59:1203–1208.
139. Duhaime, A-C, Gennarelli, TA, Thibault, LE, et al. The shaken baby syndrome: A clinical, pathological, and biomechanical study. J Neurosurg. 1987; 66:409–415.
140. Christian, CW, Block, R. Abusive head trauma in infants and children. and the Committee on Child Abuse and Neglect. Pediatrics. 2009; 123:1409–1411.
141. Chang, DC, Knight, V, Ziegfeld, S, et al. The tip of the iceberg for child abuse: The critical roles of the pediatric trauma service and its registry. J Trauma. 2004; 57:1189–1198.
142. Boyce, MC, Melhorn, KJ, Vargo, G. Pediatric trauma documentation: Adequacy for assessment of child abuse. Arch Pediatr Adolesc Med. 1996; 150:730–732.
143. Selbst, SM, Henretig, F, Fee, MA, et al. Lead poisoning in a child with a gunshot wound. Pediatrics. 1986; 77:413–416.
144. Cigdem, MK, Onen, A, Siga, M, et al. Selective nonoperative management of penetrating abdominal injuries in children. J Trauma. 2009; 67:1284–1287.
145. Chen, MK, Schropp, KP, Lobe, TE. The use of minimal access surgery in pediatric trauma: a preliminary report. J Laparoendoscop Surg. 1995; 5:295–301.
146. Gandhi, RR, Stringel, G. Laparoscopy in pediatric abdominal trauma. J Soc Laparoendoscop Surg. 1997; 1:349–351.
147. Feliz, A, Shultz, B, McKenna, C, et al. Diagnostic and therapeutic laparoscopy in pediatric abdominal trauma. J Pediatr Surg. 2006; 41:72–77.
148. Marwan, A, Harmon, CM, Georgeson, KE. Use of laparoscopy in the management of pediatric abdominal trauma. J Trauma. 2010; 69:761–764.
149. Committee on Trauma. American College of Surgeons: Resources for Optimal Care of the Injured Patient. Chicago: American College of Surgeons; 2006.
150. Committee on Trauma. American College of Surgeons: Regional Trauma Systems: Optimal Elements, Integration, and Assessment-Systems Consultation Guide. Chicago: American College of Surgeons; 2008.
151. Nance, ML, Carr, BG, Branas, CC. Access to pediatric trauma care in the United States. Arch Pediatr Adolesc Med. 2009; 163:512–518.
152. Odetola, FO, Miller, WC, Davis, MM, et al. The relationship between the location of pediatric intensive care unit facilities and child death from trauma: A county-level ecologic study. J Pediatr. 2005; 147:74–77.
153. Hunt, EA, Hohenhaus, SM, Luo, X, et al. Simulation of pediatric trauma stabilization in 35 North Carolina emergency departments: Identification of targets for performance improvement. Pediatrics. 2006; 117:641–648.
154. Mikrogianakis, A, Osmond, MH, Nuth, JE, et al. Evaluation of a multidisciplinary pediatric mock trauma code educational initiative; A pilot study. J Trauma. 2008; 64:761–767.
155. Falcone, RA, Daugherty, M, Schweer, L, et al. Multidisciplinary trauma team training using high-fidelity trauma simulation. J Pediatr Surg. 2008; 43:1065–1071.
156. Popp, J, Yochum, L, Spinella, P, et al. Simulation training for surgical residents in pediatric trauma scenarios. Connecticut Med. 2012; 76:159–162.
157. O’Connell, KJ, Farah, MM, Spandorfer, P, et al. Family presence during pediatric trauma team activation: An assessment of a structured program. Pediatrics. 2007; 120:e565–e574.
158. Dudley, NC, Hansen, KW, Furnival, RA, et al. The effect of family presence on the efficiency of pediatric trauma resuscitation. Ann Emerg Med. 2009; 53:777–784.
159. Osler, TM, Vane, DW, Tepas, JJ, et al. Do pediatric trauma centers have better survival rates than adult trauma centers? An examination of the National Pediatric Trauma Registry. J Trauma. 2001; 50:96–101.
160. Ehrlich, PF, McClellan, WT, Wesson, DE. Monitoring performance: Long-term impact of trauma verification and review. J Am Coll Surg. 2005; 200:166–172.
161. Vavilala, MS, Cummings, P, Sharar, SR, et al. Association of hospital trauma designation with admission patterns of injured children. J Trauma. 2004; 54:119–124.
162. Smith, DF, Hackel, A. Selection criteria for pediatric critical care transport teams. Crit Care Med. 1983; 11:10–12.
163. MacNab, AJ. Optimal escort for interhospital transport of pediatric emergencies. J Trauma. 1991; 31:205–209.
164. Kanter, RK, Boeing, NM, Hannan, WP, et al. Excess morbidity associated with interhospital transport. Pediatrics. 1992; 90:893–898.
165. Edge, WE, Kanter, RK, Weigle, CGM, et al. Reduction of morbidity in interhospital transport by specialized pediatric staff. Crit Care Med. 1994; 22:1186–1191.