Chapter 19 Dyspnea
Mechanisms of Dyspnea
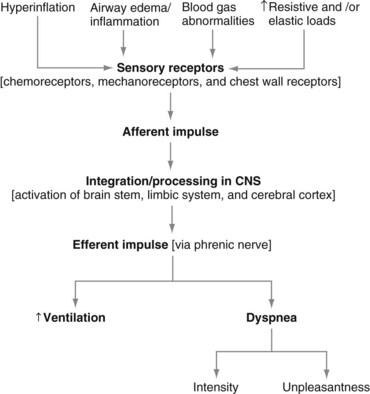
The neurophysiologic pathways that mediate the control of breathing (to supply oxygen, to eliminate carbon dioxide, and to maintain acid-base balance) also are relevant to the mechanisms of dyspnea (Figure 19-1). In simple terms, nerve fibers (sensory receptors) send electrical signals (afferent impulses) to the spinal cord, which in turn transmits these signals to the brain.
The Language of Dyspnea
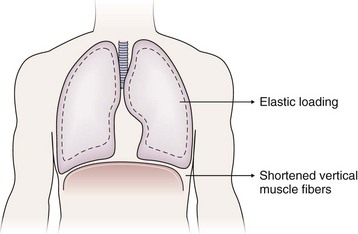
The sense of respiratory work or effort commonly is reported by patients with various conditions including asthma, COPD, interstitial lung disease, and neuromuscular disease. The descriptor “work/effort” of breathing difficulty probably is related to activation of respiratory muscle afferents imposed by mechanical loads (airway narrowing → added resistance; parenchymal edema/infiltrates → added elastance) imposed by certain diseases as well as respiratory muscle weakness. For example, in patients with COPD, the lungs typically hyperinflate during the performance of physical tasks. This dynamic hyperinflation results in two major consequences that contribute to dyspnea: (1) an added elastic load and (2) functional weakening of the diaphragm by shortening of the vertical muscle fibers (Figure 19-2). Although patients with acute asthma initially may experience chest tightness, they typically report that the increased work or effort of breathing develops as airway narrowing progresses as a result of subsequent lung hyperinflation.
Acute Dyspnea
Initial Evaluation
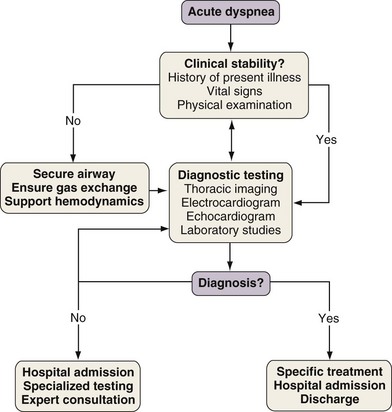
With any clinical encounter, the focus of the initial assessment is on whether or not the patient is stable (Figure 19-3). If this evaluation reveals evidence of hemodynamic insult or lability, hypotension may need to be treated promptly with intravenous fluids, vasopressors, and/or vasodilators. Airway patency and adequacy may be threatened by a depressed level of consciousness, aspiration, or trauma. Endotracheal intubation may become necessary in such instances and when gas exchange derangements cannot be rectified by supplemental oxygen or noninvasive positive-pressure ventilation. Once these basic support elements are addressed, diagnostic testing can safely proceed.
Differential Diagnosis
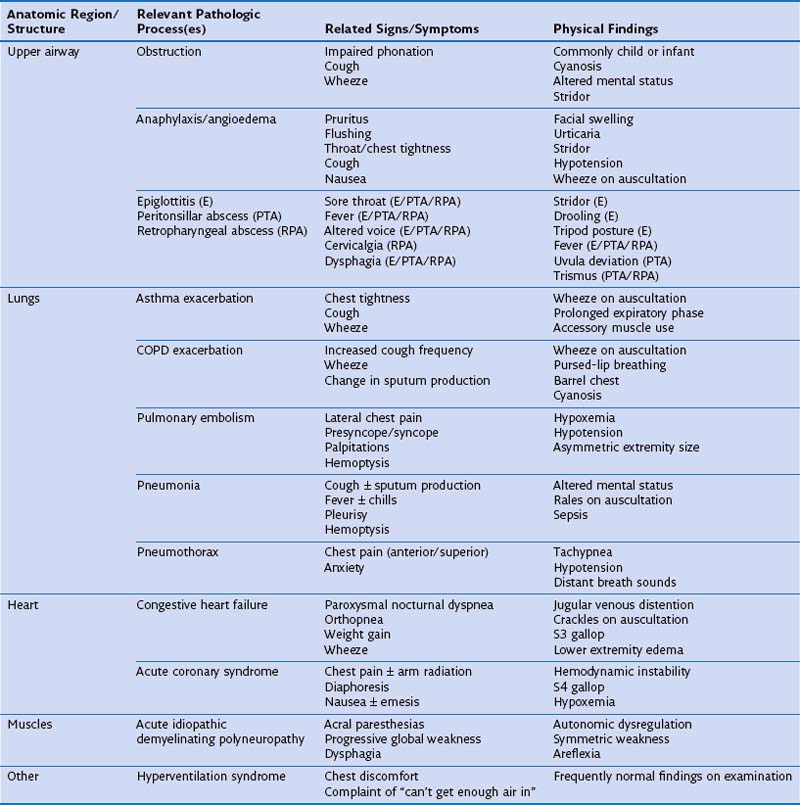
One approach to the differential diagnosis for acute dyspnea is to consider how processes in certain anatomic regions contribute to this symptom (Table 19-1). Obstruction is the most common mechanism for dyspnea arising from upper airway problems. Stridor, a variably high-pitched, harsh inspiratory noise caused by turbulent airflow, often can be heard in the context of an aspirated foreign body and edema of the epiglottis and laryngeal soft tissues. Prompt evaluation and management of upper airway blockage are critical, because the airway is endangered. Exacerbations of asthma and COPD typically are manifested as bronchospasm, wheeze, and cough. Sputum production is common to exacerbations of COPD associated with acute bronchitis and pneumonia, but its physical characteristics alone are not useful in predicting a causative pathogen. Pleuritic chest pain is sharp, incisive, breath-taking discomfort caused by irritation of the parietal pleural nerve supply along the thoracic cavity. This type of pain can accompany pulmonary embolism, pneumonia with or without pleural effusion, and pneumothorax.
Treatment
Further details on the treatment of acute dyspnea caused by the aforementioned processes are presented elsewhere in this book. Two interventions, oxygen therapy and noninvasive positive-pressure ventilation (NIPPV), merit a brief discussion here because of their application to several of these processes. Table 19-2 presents a list of processes that cause acute dyspnea and their specific therapies.
Table 19-2 Specific Therapies for Acute Dyspnea by Etiologic Condition
| Etiologic Condition | Therapeutic Intervention(s)/Agent(s) |
|---|---|
| Aspirated foreign body | Endotracheal intubation |
| Fiberoptic or rigid bronchoscopy with removal | |
| Anaphylaxis/angioedema | Antihistamines |
| Subcutaneous epinephrine | |
| Systemic corticosteroids | |
| Epiglottitis (E) | Endotracheal intubation (PTA/RPA) |
| Peritonsillar abscess (PTA) | Broad-spectrum antibiotics (PTA/RPA) |
| Retropharyngeal abscess (RPA) | Incision and drainage (PTA/RPA) |
| Asthma exacerbation | Supplemental oxygen |
| Inhaled β2-adrenergic agonists by nebulizer or MDI | |
| Systemic corticosteroids | |
| COPD exacerbation | Supplemental oxygen |
| Inhaled β2-adrenergic agonists by nebulizer or MDI | |
| Inhaled anticholinergic agents by nebulizer or MDI | |
| Systemic corticosteroids | |
| Antibiotics for purulent sputum | |
| Noninvasive positive-pressure ventilation | |
| Pulmonary embolism | Systemic anticoagulation |
| Catheter-based thromboembolectomy | |
| Pneumonia | Supplemental oxygen |
| Antibiotics | |
| Airway clearance techniques | |
| Immunization | |
| Pneumothorax | Thoracostomy tube placement |
| Supplemental oxygen | |
| Congestive heart failure | Supplemental oxygen |
| Diuretics | |
| Systemic vasodilators | |
| Inotropic agents | |
| Acute coronary syndrome | Percutaneous transluminal coronary angioplasty ± stent |
| Antiplatelet agents | |
| Lipid-lowering therapies | |
| Diuretics | |
| Acute idiopathic demyelinating polyneuropathy | Mechanical ventilatory support |
| Intravenous immunoglobulin infusion | |
| Systemic corticosteroids | |
| Hyperventilation syndrome | Anxiolytic medications |
| Psychiatric evaluation |
COPD, chronic obstructive pulmonary disease; MDI, metered dose inhaler.
Chronic Dyspnea
Differential Diagnosis
Causes of chronic dyspnea are listed in Table 19-3. Conditions such as COPD and CHF (see Table 19-1) may have both acute and chronic phases. Relatively few studies have tried to identify those diseases that most commonly underlie chronic dyspnea in the outpatient setting. A prospective evaluation of 85 patients at a university-based pulmonary clinic showed that asthma (29%), COPD (14%), interstitial lung disease (14%), and cardiomyopathy (10%) explained two thirds of cases. Although the study investigators emphasized a rational diagnostic approach that incorporated the responses to disease-specific therapies, an average of 6.2 tests were conducted per patient, and no participants had pulmonary vascular disease or muscle weakness. Another investigation of 72 consecutive patients with chronic dyspnea and unrevealing history, physical examination, chest radiograph, and spirometry data found that 36% had pulmonary disease, 14% had cardiac disease, and 19% had primary hyperventilation. Only 3% had an extrathoracic reason for their breathlessness.
| Anatomy | Processes | Risk Factors |
|---|---|---|
| Upper airway | Vocal cord dysfunction | Head, neck, and lung cancer |
| Neck surgery | ||
| Head trauma | ||
| Endotracheal intubation | ||
| Viral infection | ||
| Psychiatric disorder | ||
| Subglottic stenosis | Endotracheal intubation | |
| ANCA-positive vasculitis | ||
| Partially obstructing lesion(s) | Cancer | |
| Granulomatous inflammation | ||
| Thyroid | Thyrotoxicosis | Autoimmunity |
| Hypothyroidism | Viral infection | |
| Blood | Anemia | Chemotherapy |
| Chronic kidney disease | ||
| Chronic gastrointestinal blood loss | ||
| Heart | Systolic heart failure | Myocardial infarction |
| Diastolic heart failure | Hypertension, obesity | |
| Pericardial disease | Pericarditis | |
| Valvular disease | Mitral and aortic insufficiency | |
| Intracardiac shunt | Patent foramen ovale, ventricular septal defect | |
| Lungs | ||
| Airways | COPD | Tobacco abuse, α1-antitrypsin deficiency |
| Asthma | Atopy, genetic predisposition | |
| Cystic fibrosis | Heritable genetic defect | |
| Parenchyma | Interstitial lung disease | Idiopathic interstitial pneumonia |
| Pneumotoxic drug reaction | ||
| Connective tissue disease | ||
| Vasculature | Pulmonary arterial hypertension | Idiopathic pulmonary arterial hypertension |
| Chronic venous thromboembolism | ||
| Vasculitis | ||
| Obesity-hypoventilation syndrome | ||
| Arteriovenous malformation | ||
| Pleura | Pleural effusion | Systolic heart failure |
| Cancer | ||
| Infection | ||
| Hepatic hydrothorax | ||
| Thorax | Kyphoscoliosis | Congenital |
| Osteoporosis | ||
| Respiratory muscles | Mechanical loading | Morbid obesity |
| Pregnancy | ||
| Dyskinesia/dystonia | ||
| Neurodegenerative disease | ||
| Integrated | Deconditioning | Sedentary lifestyle |
| Nutritional deficiency | ||
| Obesity | ||
| Other | Hyperventilation syndrome | Anxiety |
ANCA, antineutrophil cytoplasmic antibodies; COPD, chronic obstructive pulmonary disease.
Dyspnea often affects patients without obvious heart or lung disease; therefore, the clinician must consider various etiologic conditions. Some of these processes, listed in Table 19-3, warrant further discussion, because dyspnea could be overlooked as a presenting symptom. Moderate to severe anemia can limit tissue oxygen delivery but does not lead to oxyhemoglobin desaturation. Thus, the mechanism by which a low hemoglobin concentration provokes dyspnea probably is related to impaired muscle energetics and a compensatory increase in ventilation and cardiac output. Patients with many forms of advanced cancer indeed experience significant, albeit temporary, dyspnea relief from blood transfusions and erythropoietic growth factor support. Respiratory muscle weakness has been implicated as the cause of chronic dyspnea in patients with hypothyroidism and thyrotoxicosis, on the basis of responses to specific medical therapy. Limited data are available regarding the prevalence of dyspnea among patients with selected neuromuscular diseases. Roughly 40% of patients with amyotrophic lateral sclerosis, 23% of patients with post-poliomyelitis, and 12% of patients with multiple sclerosis complain of dyspnea. Both weight gain and a sedentary lifestyle are common causes of exertional dyspnea in those residing in developed countries.
Diagnostic Testing
Details from the history and physical examination should inform the selection of diagnostic tests that further refine decision-making. One approach involves categorizing these modalities according to their ability to investigate relevant anatomy and physiology (Table 19-4). Various guidelines on management of COPD recommend that the diagnosis be determined by a value of 70% or less for the ratio of postbronchodilator forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC). However, the FEV1/FVC ratio declines with aging, and the use of a “fixed” ratio may lead to overdiagnosis of COPD among patients older than 60 years of age. We concur with the American Thoracic Society–European Respiratory Society recommendation that airflow obstruction be diagnosed by a FEV1/FVC value below the lower limit of normal for the specific patient.
| Test | Diagnostic Utility |
|---|---|
| Spirometry | Diagnose and quantify airflow obstruction and restriction |
| Flow-volume loop | Diagnose upper airway obstruction |
| Single-breath diffusion capacity for carbon monoxide (DLCO) | Reduced in emphysema, interstitial lung disease, and pulmonary hypertension |
| Can be reduced in anemia | |
| Can be increased in alveolar hemorrhage, asthma | |
| Lung volume determination | Confirms restrictive lung diseases |
| Bronchoprovocation testing | Diagnose airway hyperreactivity |
| Maximal inspiratory and expiratory mouth pressures | Evaluate neuromuscular weakness |
| Chest computed tomography | Interstitial lung disease |
| Airway caliber well delineated; can identify endobronchial lesions | |
| Excellent characterization of pleural space and mediastinum | |
| Echocardiography | Diagnose and quantify ventricular function |
| Evaluate valvular incompetence, pericardium | |
| Cardiopulmonary exercise testing | Diagnose cardiac dysfunction, ventilatory limitation, oxygen desaturation, deconditioning, psychogenic dyspnea |
| Complete blood count | Diagnose anemia |
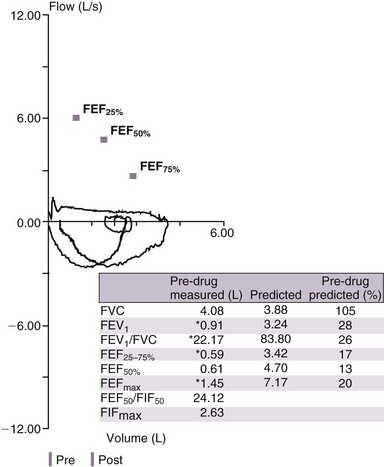
A low FVC may be due to air trapping in a patient with airflow obstruction or may point to a restrictive lung process that can be confirmed by measurement of lung volumes. Analysis of the flow-volume loop can demonstrate evidence of upper airway obstruction (Figure 19-4). In cases of suspected asthma, a negative result on direct bronchoprovocation testing essentially rules out this diagnosis. However, false-positive results can be seen in normal persons as well as those with sarcoidosis or vocal cord dysfunction. Measurement of maximal inspiratory mouth pressure is sensitive for detection of respiratory muscle weakness.
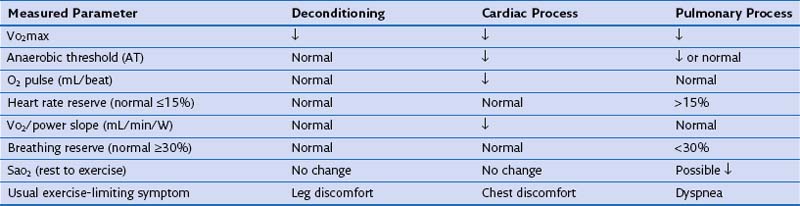
The etiology of chronic dyspnea will sometimes remain elusive despite a careful history and physical examination and multiple diagnostic endeavors. Then, cardiopulmonary exercise testing should be considered to provoke the patient’s dyspnea with comprehensive assessment of the oxygen transport system on an integrated level. Pulmonary gas exchange, cardiac function, and metabolic activity can be measured noninvasively (Table 19-5). Patients also are asked to rate dyspnea and leg discomfort throughout the exercise test. The results of cardiopulmonary exercise testing usually are able to distinguish cardiac dysfunction and ventilatory limitation but cannot always discriminate between cardiac disease and deconditioning.
Davenport PW, Vovk A. Cortical and subcortical central neural pathways in respiratory sensations. Respir Physiol Neurobiol. 2009;167:72.
Gibson NS, Sohne M, Gerdes VE, et al. The importance of clinical probability assessment in interpreting a normal D-dimer in patients with suspected pulmonary embolism. Chest. 2008;134:789.
Horton R, Rocker G. Contemporary issues in refractory dyspnoea in advanced chronic obstructive pulmonary disease. Curr Opin Support Palliat Care. 2010;4:56.
Lansing RW, Gracely RH, Banzett RB. The multiple dimensions of dyspnea: review and hypotheses. Respir Physiol Neurobiol. 2009;167:53.
Mahler DA, Fierro-Carrion G, Baird JC. Evaluation of dyspnea in the elderly. Clin Geriatr Med. 2003;19:19.
Mahler DA, Selecky PA, Harrod CG, et al. American College of Chest Physicians consensus statement on the management of dyspnea in patients with advanced lung or heart disease. Chest. 2010;137:674.
Ohar JA, Sadeghnejad A, Meyers DA, et al. Do symptoms predict COPD in smokers? Chest. 2010;137:1345.
Pratter MR, Curley FJ, Dubois J, et al. Cause and evaluation of chronic dyspnea in a pulmonary disease clinic. Arch Intern Med. 1989;149:2277.
Von Leupoldt A, Dahme B. Cortical substrates for the perception of dyspnea. Chest. 2005;128:345.
Wang CS, FitzGerald JM, Schulzer M, et al. Does this dyspneic patient in the emergency department have congestive heart failure? JAMA. 2005;294:1944.