Chapter 20 Dural Herniation and Cerebrospinal Fluid Leaks
 Videos corresponding to this chapter are available online at www.expertconsult.com.
Videos corresponding to this chapter are available online at www.expertconsult.com.
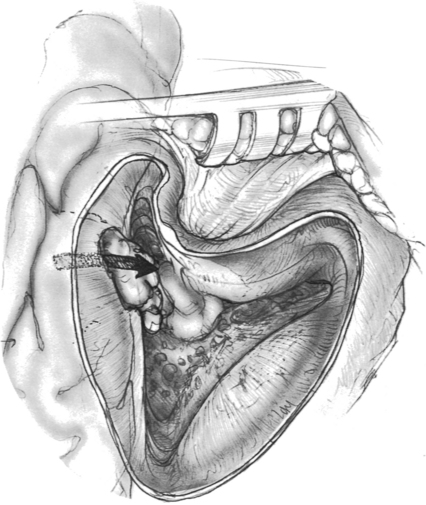
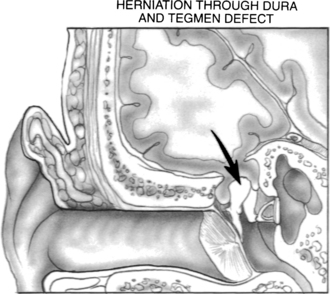
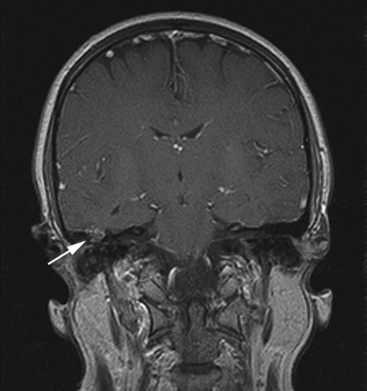
An encephalocele occurs when brain tissue herniates through a dural defect of the skull. Temporal bone encephaloceles manifest either as a mass or cerebrospinal fluid (CSF) in the middle ear or mastoid or both (Figs. 20-1 and 20-2). Encephaloceles have also been called dural herniations, brain herniations, brain prolapse, and meningoencephalocele. They have been documented since the early 1900s.1,2 They were associated with chronic suppurative otitis media and transmastoid surgery, but, with a decreasing incidence of otogenic intracranial complications in modern-day patients, they have become uncommon. Contemporary otologic literature describes a myriad of signs and symptoms that are frequently not associated with suppurative disease.3
Other causes include congenital cranial base defects, spontaneous hernias, and trauma. Iurato and associates4 reviewed 139 cases of temporal bone encephaloceles, and found that 59% occurred as a complication of mastoid surgery, 21% were spontaneous or idiopathic, 9% were a complication of chronic otitis media or chronic mastoiditis, and 9% resulted from trauma. More recently, Scurry and coworkers5 reported encephalocele formation associated with morbid obesity in eight patients. The middle cranial fossa is the most common site of occurrence; encephaloceles rarely originate from the posterior fossa.6
PATHOGENESIS
Basic knowledge of the embryology of the temporal bone is helpful in the understanding of formation of dural herniation and CSF leaks. There are four ossification centers that form the temporal bone: squamous, tympanic, petrous, and mastoid. Pneumatization follows ossification and continues into adulthood and consists of a process of marrow resorption, mucosal advancement, and bone remodeling. Disturbances in the normal ossification or pneumatization process may lead to encephalocele formation. Portions of the petrous and squamous ossification centers of the temporal bone ultimately form the roof of the middle ear and mastoid, areas that are the most likely to form encephalocele.7
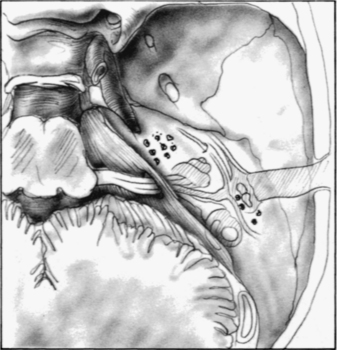
Many authors have reported multiple tegmen defects (Fig. 20-3). Ahern and Thulen8 noted a 6% incidence of multiple defects in the tegmen in 94 consecutive cadaveric specimens. Ferguson and colleagues6 noted a 22% incidence of multiple tegmen defects in 27 preserved dried temporal bones. Lang9 found tegmen defects in 20% of 70 temporal bones. Bilateral spontaneous encephaloceles could be predicted from such studies, and have been reported by Iurato and colleagues.10
The high incidence of tegmen defects is contrasted by the low incidence of spontaneous encephalocele and CSF leak formation. This observation lends credibility to the widely held belief that bony defects are necessary, but not solely responsible, for the formation of encephaloceles. The other factor that is necessary for encephalocele formation is a pathologic process at the dural level. Inflammatory process, chronic suppurative otitis media, and transmastoid surgery are the most common factors in the dura. Other factors, such as benign intracranial hypertension, obesity, and aging, also have been suggested.5,11
The association with chronic suppurative otitis media and transmastoid surgery is well documented in the literature.4,12,13 In the preantibiotic era, meningeal complications from chronic suppurative otitis media were common. The incidence of encephalocele during this period was increased by transmastoid trephination of peridural and brain abscesses. Encephalocele can occur in the face of chronic suppurative otitis media with or without surgery, with or without cholesteatoma.14,15 Paparella and coworkers16 reported 10 cases of encephalocele all associated with chronic otitis media, 6 of which had not had previous surgery, and 2 of which had cholesteatoma. These authors speculated that the inflammatory response led to destruction of the tegmen, followed by extension to adjacent dura and brain, resulting in herniation. Encephalocele formation can form when a dural tear occurs at the site of a bone dehiscence from a drill, scalpel, or cautery.12 Dural injuries may go unrecognized during the surgery; however, if recognized at the time of surgery, they should be repaired to prevent an immediate or delayed problem. Although chronic suppurative otitis media and transmastoid surgery are still the most common causes for encephalocele and CSF leak, a decreasing incidence has been cited because of improved surgical techniques, including the operating microscope and the use of drills versus gouges and chisels.17
CSF leak secondary to head trauma is well documented. Dura in the skull base that is adherent to adjacent bone tears along fracture lines. CSF leak usually resolves spontaneously or with lumbar drainage within 1 to 2 weeks. Savva and coworkers18 managed 26 of 29 patients with CSF otorrhea secondary to head injury successfully with conservative treatment. In contrast, only 1 of 53 patients with surgically induced or spontaneous CSF leak was successfully managed conservatively. Bony dehiscence and dural injury from head trauma can lead to either immediate or delayed encephalocele formation.
Spontaneous encephalocele and CSF leak has been attributed to multiple factors. Arachnoid granulations of the temporal bone may be a cause of spontaneous encephalocele and CSF fistula formation. Normally, arachnoid granulations protruding into the lumen of venous structures are involved in the resorption of CSF. When not associated with venous structures and surrounded by bone, they may enlarge and cause bone erosion as a result of intermittent subarachnoid pressure associated with age and physical activity. Gacek19 observed in temporal bones that in the tegmen tympani and mastoideum 22% had tissue on the dural surface, and 9% had tissue in the posterior fossa.
Rao and colleagues11 reported a series of 10 patients with spontaneous CSF leak with an average age of 58 and an increased incidence in women. They hypothesized that the increased incidence in women suggested an association with benign intracranial hypertension, although many of their patients did not have clinical findings. Scurry and colleagues5 reported an association of morbid obesity in eight patients with spontaneous CSF leak. They also posited a theory that obesity ultimately resulted in increased intracranial pressure and subsequent CSF leakage and encephalocele formation. Many of their patients did not have findings consistent with increased intracranial pressure, which questions the idea that it plays a significant role.
DIAGNOSIS
Diagnosis of encephalocele and CSF leak is primarily clinical with supportive radiologic and laboratory information. Clinical findings may or may not include a history of chronic suppurative otitis media or trauma. Middle ear effusion with conductive hearing loss is the most common presenting symptom.19–21 A subsequent myringotomy and tube results in a persistent clear, odorless, watery type of discharge.22–24 Leakage may vary with various maneuvers that increase intracranial pressure, such as supine positioning, Valsalva maneuver, and compression of the jugular vein.25 Leaning forward may elicit rhinorrhea.
A mass in the ear canal or behind the tympanic membrane is a rarely described finding.26,27 A more common finding is a soft compressible mass in the mastoid cavity arising off the tegmen that may mimic a blue dome cyst or cholesterol granuloma.28,29 Less common presentations include meningitis and seizures.2,6,17,28,30–36 Tension pneumocephalus is another uncommon presentation of temporal bone encephalocele.37 Air enters the intracranial space through the bone defect when the extracranial pressure exceeds the intracranial pressure. Air accumulates when a valve phenomenon allows air to enter the intradural space, but does not allow the air to escape. Tension pneumocephalus has been reported after insufflation of the external auditory canal.38,39
If enough fluid can be collected, various tests can help determine whether the fluid is consistent with CSF. A glucose value 60% of serum levels, a protein concentration of less than 200 mg/dL, and chloride level greater than normal serum levels are consistent with CSF. β2-Transferrin has replaced glucose, protein, and chloride as a more sensitive and specific test for CSF.40 β2-Transferrin is a protein specific to CSF that is produced by neuraminidase activity in the brain. Zapalac and coworkers41 retrospectively reviewed 52 cases of CSF leak and found the test to be 98% sensitive. The high sensitivity, specificity, and ability to detect small amounts in specimens contaminated with blood or mucus has made β2-transferrin a powerful diagnostic tool.42
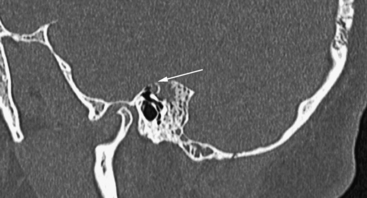
Computed tomography (CT) is ideally suited to imaging the bony anatomy of the temporal bone. High-resolution axial and coronal CT scans are needed to define defects in the tegmen tympani or mastoideum (Fig. 20-4). CT is limited in its ability to distinguish the various soft tissue densities in the mastoid. CT with intrathecal injection of metrizamide can show CSF leak fistulas for diagnosis and localization. Other radiopaque agents used for intrathecal injection, such as fluorescein and indigo carmine, have been reported.43 The main limitation is that small or intermittent leaks can lead to false-negative testing.
Magnetic resonance imaging (MRI) is superior to CT in differentiating encephalocele from other soft tissue densities in the temporal bone, such as effusion, cholesteatoma, and other lesions (Fig. 20-5).44 Bone detail is lacking, however.
Shetty and colleagues45 prospectively studied the accuracy and sensitivity of high-resolution CT and MRI in localizing fistulas in patients with CSF rhinorrhea. CT revealed an accuracy of 93% and a sensitivity of 92%. MRI cisternography showed an accuracy of 89% and a sensitivity of 87%. Zapalac and coworkers41 retrospectively examined 52 patients with skull base CSF leak fistulas and determined that the most cost-efficient algorithm for diagnosis is an initial β2-transferrin, followed by CT for preoperative localization. MRI cisternography was reserved for cases in which CT was nonlocalizing.
TREATMENT
Most CSF leaks after head trauma resolve with conservative management.18 However, CSF leaks from encephaloceles need surgical intervention. A high incidence of unsuspected encephaloceles was found in the series by Kamerer and Caparosa.12 They urged that all tissue adjacent to dural defects be submitted for histologic confirmation. Herniated brain is nonfunctional and can be amputated with bipolar cautery without neurologic sequelae. Histologically, it is consistent with neural tissue, scarring, and inflammation.
A basic concept of encephalocele repair involves a multilayered closure including a soft tissue repair of the dura combined with a repair of the bone defect.46 Soft tissue repairs without bone repair result in recurrence in some cases.11 The dura can be repaired primarily with suture. Alternatively, fascia or dural substitutes can be used to repair the defect. Intradural repairs have the advantage of pressure gradients of CSF and brain pushing the graft against the dural defect. The disadvantage is increased intradural dissection and possible brain parenchyma or vascular injury. Extradural repairs have worked consistently and eliminate the intradural dissection and risk.47 Extradural graft repairs have the theoretical disadvantage of the graft being pushed away by CSF pressure; however, extradural graft migration has not been a source of failure, presumably because it is secured by being wedged between the bone repair and the expanding brain.
Various materials have been used for the bone repair. Commonly, a piece of cortical bone is used to plug the bone defect.17,28,48 Some pliable materials, such as cartilage, can be insinuated intracranially through a transmastoid approach.49,50 Hydroxyapatite bone cement is a synthetic material that was approved by the U.S. Food and Drug Administration for repair of CSF leaks in 1996. Constantino and associates51 reported 21 patients with CSF leaks from various sites in the head and neck. Kveton and Goravalingappa52 reported on its use in the temporal bone, and specifically reported it being used successfully for encephalocele repair through a transmastoid approach. In addition, in the long-term 10-year follow-up study, Kveton and Coelho53 showed good stability with low infection and extrusion rates. The hydroxyapatite cement needs to be mixed with sodium phosphate or citric acid to begin the setting process. When they are mixed thoroughly together over a 30 to 40 second period, the cement is in a semisolid state that can be shaped and molded to the defect. The cement sets up and hardens over 4 to 5 minutes even in wet environments. Care should be taken not to allow the cement to come into contact or drain onto the ossicular chain to prevent conductive hearing loss. Other synthetic materials have been used in the past, such as silicone elastomer (Silastic) sheeting, methyl methacrylate, and mesh, but may have a higher incidence of infection or extrusion.
Three surgical approaches have been described: transmastoid, middle fossa craniotomy, and combined transmastoid/middle fossa approach. The transmastoid approach is best suited for isolated tegmen mastoideum, tympani, and posterior fossa defects. Anterior medial sites are not accessible through this approach if hearing is to be preserved. A wide mastoidectomy is performed with large exposure of the tegmen and its dura. The presigmoid bone dura should also be exposed and examined. When the dura bony defect is isolated, the encephalocele is amputated with the bipolar cautery. The dural defect can be repaired with fascia that is insinuated through the bone defect intracranially but extradural.46 Cartilage or bone cement can be used to reconstruct the bone defect. In canal wall down mastoid cavity situations, alloplastic materials should not be used for reconstruction during encephalocele repair because there is a higher incidence of infection and extrusion.
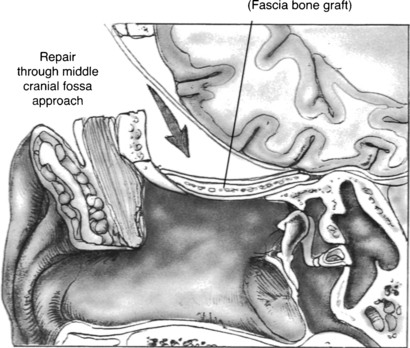
The preferred approach by many neurotologists for many reasons is a combined mastoid and middle fossa craniotomy (Figs. 20-6 and 20-7). Surgical identification of the encephalocele is often easier from the mastoid side. Middle fossa dural dissection from the skull base floor can result in dural tears, making it difficult to distinguish the encephalocele process from iatrogenic dural tears. The middle fossa craniotomy allows a wide exposure for dural and bony defect repair, which is especially important with multiple defects that may be otherwise missed through a transmastoid approach. Lesions that herniate through the tegmen tympani into the epitympanum can be removed through a combined approach without disturbing the ossicular chain.
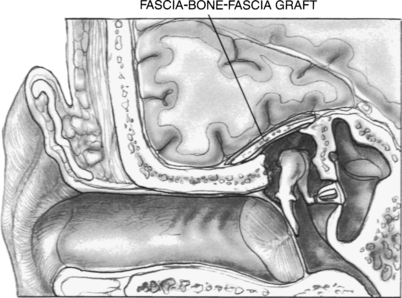
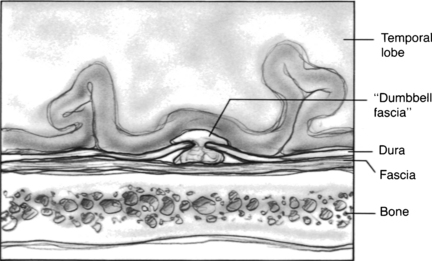
FIGURE 20-6 Tegmen defect repaired with fascia-bone-fascia technique via middle fossa craniotomy approach.
The combined mastoid and middle fossa approach begins with a mastoidectomy. When the defect or defects are identified from the mastoid side, and the encephalocele is amputated, the skin incision can be extended onto the temporal area to expose the squamous surface of the temporal bone. A fascia graft from the temporalis muscle is obtained. A temporal craniotomy is performed. The temporal lobe is elevated, and the dual defect is identified. The bony defect is easily identified in the floor of the middle fossa, especially because it can now be seen communicating into the mastoid cavity. The defect is repaired with a dural repair (Fig. 20-8), bony defect repair, and another layer of soft tissue. Anterior medial petrous apex leaks can be approached with a middle fossa approach without mastoidectomy.
1. Caboche H. De la hernie cerebral dans les interventions intracraniennes dirigees contre les moyennes suppurees. Ann d Mal de Poriell du Laryngx. 1902;28:278-294.
2. Canfield R.B. Some conditions associated with the loss of cerebrospinal fluid. Ann Otol Rhinol Laryngol. 1913;22:604.
3. Brown N.E., Grundfast K.M., Jabre A., et al. Diagnosis and management of spontaneous cerebrospinal fluid-middle ear effusion and otorrhea. Laryngoscope. 2004;114:800-805.
4. Iurato S., Ettorre G.C., Selvini C. Brain herniation into the middle ear: Two idiopathic cases treated by a combined intracranial-mastoid approach. Laryngoscope. 1989;99:950-954.
5. Scurry W.C., Ort S.A., Peterson W.M. Idiopathic temporal bone encephaloceles in the obese patient. Otolaryngol Head Neck Surg. 2007;136:961-965.
6. Ferguson B.J., Wilkins R.H., Hudson W. Spontaneous CSF otorrhea from tegmen and posterior fossa defects. Laryngoscope. 1986;96:635-644.
7. Proctor B., Nielson E., Proctor C. Petrosquamosal suture and lamina. Otolaryngol Head Neck Surg. 1981;89:482-495.
8. Ahern C., Thulen C.A. Lethal intracranial complication following inflation of the external auditory canal in treatment of serous otitis media and due to defects in the petrous bone. Acta Otolaryngol (Stockh). 1965;60:407-421.
9. Lang D.V. Macroscopic bony deficiency of the tegmen tympani in adult temporal bones. J Laryngol Otol. 1983;97:685-688.
10. Iurato S., et al. Histopathology of spontaneous brain herniations into the middle ear. Acta Otolaryngol (Stockh). 1992;112:328-333.
11. Rao A.K., Merenda D.M., Wetmore S.J. Diagnosis and management of spontaneous cerebrospinal fluid otorrhea. Otol Neurotol. 2005;26:1171-1175.
12. Kamerer D.B., Caparosa R.J. Temporal bone encephalocele—diagnosis and treatment. Laryngoscope. 1982;92:878-882.
13. Neely J.G., Kuhn J.R. Diagnosis and treatment of iatrogenic cerebrospinal fluid leak and brain herniation during or following mastoidectomy. Laryngoscope. 1985;95:1299-1300.
14. Alberti P.W.R.M., Dawes J.D.K. Cerebrospinal otorrhea and chronic ear disease. J Laryngol Otol. 1961;75:123-135.
15. Dedo H.H., Sooy F.A. Endaural encephalocele and cerebrospinal fluid otorrhea: A review. Ann Otol Rhinol Laryngol. 1970;79:168-177.
16. Paparella M.M., Meyerhoff W.L., Oliviera C.A. Mastoiditis and brain hernia (mastoiditis cerebri). Laryngoscope. 1978;88:1097-1106.
17. Glasscock M.E., Dickins J.R.E., Jackson C.G., et al. Surgical management of brain tissue herniation into the middle ear and mastoid. Laryngoscope. 1979;89:1743-1754.
18. Savva A., Taylor M.J., Beatty C.W. Management of cerebrospinal fluid leaks involving the temporal bone: Report on 92 patients. Laryngoscope. 2003;113:50-56.
19. Gacek R.R. Evaluation and management of temporal bone arachnoid granulations. Arch Otolaryngol Head Neck Surg. 1992;118:327-332.
20. Gavilan J., Trujillo M., Gavilan C. Spontaneous encephalocele of the middle ear. Arch Otolaryngol Head Neck Surg. 1984;110:205-207.
21. Levy R.A., Platt N., Aftalion B. Encephalocele of the middle ear. Laryngoscope. 1971;81:126-130.
22. Dysart B.R. Spontaneous cerebrospinal fluid otorrhea—a report on a case with successful surgical repair. Trans Am Laryngol Rhinol Otol Soc. 1959;62:381-387.
23. Adams G.L., McCoid G., Weisbeski D. Cerebrospinal fluid otorrhea presenting as serous otitis media. Minn Med. 1982;65:410-415.
24. Briant T.D.R., Bird R. Extracranial repair of cerebrospinal fluid fistula. J Otolaryngol. 1982;11:191-197.
25. Lalwani A.K. Temporal bone encephalocele. In: Jackler R.K., Brackmann D.E., editors. Neurotology. St Louis: Mosby; 1994:1149-1155.
26. Lalwani A.K., Jackler R.J. Endaural encephalocele. Otolaryngol Head Neck Surg. 1992;106:309-310.
27. Jahrsdoerfer R.A., Richtsmeier W.J., Cantrell R.W. Spontaneous CSF otorrhea. Arch Otolaryngol. 1981;107:257-2261.
28. Graham M.D. Surgical management of dural and temporal lobe herniation into the radical mastoid cavity. Laryngoscope. 1982;92:329-331.
29. Baron S.H. Herniation of the brain into the mastoid cavity: Post-surgical, postinfectional, or congenital. Arch Otolaryngol Head Neck Surg. 1969;90:127-133.
30. Kline O.R. Spontaneous cerebrospinal otorrhea. Arch Otolaryngol Head Neck Surg. 1933;18:34-39.
31. Mealey J.Jr. Chronic cerebrospinal fluid otorrhea—report of a case associated with chronic infection of the ear. Neurology. 1961;11:996-998.
32. Bowes A.K., Wiet R.J., Monsell E.M., et al. Brain herniation and space occupying lesions eroding the tegmen tympani. Laryngoscope. 1987;97:1172-1175.
33. Feenstra L., Sanna M., Zini C., et al. Surgical treatment of brain herniation into the middle ear and mastoid. Am J Otol. 1985;6:311-315.
34. Moore G.F., Nissen A.J., Yonkers A.J. Potential complications of unrecognized cerebrospinal fluid leaks secondary to mastoid surgery. Am J Otol. 1984;5:317-323.
35. Hyson M., Andermann F., Olivier A., Melenson D. Occult encephaloceles and temporal bone epilepsy: Developmental and acquired lesions in the middle fossa. Neurology. 1984;34:363-366.
36. Kemink J.L., Graham M.D., Kartush J.M. Spontaneous encephalocele of the temporal bone. Arch Otolaryngol Head Neck Surg. 1986;112:558-561.
37. Cartwright M.J., Eisenberg M.B. Tension pneumocephalus associated with rupture of a middle fossa encephalocele. J Neurosurg. 1992;76:292-295.
38. Fairman H.D., Brown N.J., Hallpike C.S. Air embolism as a complication of inflation of the tympanum through the external auditory, meatus. Acta Otolaryngol. 1968;66:65-71.
39. Fisnes K.A. Lethal intracranial complication following air insufflation with a pneumatic otoscope. Acta Otolaryngol. 1973;75:436-438.
40. Oberascher G. A modern concept of cerebrospinal fluid diagnosis in oto- and rhinorrhea. Rhinology. 1988;26:89-103.
41. Zapalac J.S., Marple B.F., Schwade N.D. Skull base cerebrospinal fluid fistulas: A comprehensive diagnostic algorithm. Otolaryngol Head Neck Surg. 2002;126:669-676.
42. Rauch S.D. Transferrin microheterogeneity in human perilymph. Laryngoscope. 2000;110:545-552.
43. Dedo H., Sooy F. Endaural encephalocele and cerebrospinal fluid otorrhea. Ann Otol Rhinol Laryngol. 1970;79:168-177.
44. Kaseff L.G., Seidenwurm D.J., Nieberding P.H., et al. Magnetic resonance imaging of brain herniation into the middle ear. Am J Otol. 1992;13:74-77.
45. Shetty P.G., Shroff M.M., Sahani D.V., et al. Evaluation of high-resolution CT and MR cisternography in the diagnosis of cerebrospinal fluid fistula. AJNR Am J Neuroradiol. 1998;19:633-639.
46. Lundy L.B., Graham M.D., Kartush J.M., et al. Temporal bone encephalocele and cerebrospinal fluid leaks. Am J Otol. 1996;17:461-469.
47. Chen D.A. Contemporary diagnosis and management of temporal bone encephalocele. In: Arriaga M.A., Day J.D., editors. Neurosurgical Issues in Otolaryngology: Principles and Practice of Collaboration. Philadelphia: Lippincott Williams & Wilkins; 1999:189-192.
48. Bartels L., Luk L.J., Balis C.. Endaural brain hernia: Repair using mastoid cortical bone. Am J Otol. Suppl, 1985, 121-125.
49. Adkins W.Y., Osguthorpe J.D. Mini-craniotomy for management of CSF otorrhea from tegmen defects. Laryngoscope. 1983;93:1038-1039.
50. Jahn A.F. Endaural brain hernia: Repair using conchal cartilage. J Otolaryngol. 1981;10:471-475.
51. Constantino P.D., Chaplin J.M., Wolpoe M.E., et al. Applications of fast setting hydroxyapatite cement: Cranioplasty. Otolaryngol Head Neck Surg. 2000;15:409-412.
52. Kveton J.F., Goravalingappa R. Elimination of temporal bone cerebrospinal fluid otorrhea using hydroxyapatite cement. Laryngoscope. 2000;110(10 Pt 1):1655-1659.
53. Kveton J.F., Coelho D.H. Hydroxyapatite cement in temporal bone surgery: A 10 year experience. Laryngoscope. 2004;114:33-37.