25. Duchenne’s Dystrophy
Definition
Duchenne’s dystrophy is a chronic, progressive, severe pseudohypertrophic muscular dystrophy—the most commonly occurring type. It is characterized by increasing weakness of pelvic and shoulder girdle musculature, pseudohypertrophy of the muscles, then atrophy and lordosis. People with the disease have a peculiar, particular swinging gait (waddling gait) with the legs kept wide apart.
Diagnostic Criteria for Duchenne’s Dystrophy
• Absence of bowel or bladder dysfunction
• Hyperlordosis with wide-based gait
• Hypertrophy of weak muscles
• Progressive course over time
• Reduced muscle contractility and electrical stimulation in advanced disease
• Sensory distribution or febrile illness
• Weakness, with onset in the legs
Signs and Symptoms of Rhabdomyolysis
• Hyperphosphatemia
• Hyperuricemia
• Lactic acidosis
• Loss of P wave or sine wave
• Peaked T waves
• Prolonged P-R and QRS intervals
• Rising creatinine phosphokinase levels
• Severe hyperkalemia
• Tea-colored urine
Incidence
This form of muscular dystrophy occurs at the rate of 1:3300 to 1:3500 births, and affects males much more often than females.
Etiology
Duchene’s dystrophy is the result of a sex-linked, recessive inheritance. A mutation in the dystrophin gene is found on the X-chromosome at the Xp21 stripe. The resultant loss of dystrophin is the first step in an interdependent series of events that includes the loss of other portions of the dystrophin-associated glycoprotein complex, breakdown of the sarcolemma with influx of calcium ions, phospholipase activation, and oxidative cellular injury. The process culminates in myonecrosis. With progression of the myonecrosis, and thus the disease, dead muscle fibers are removed by macrophages. The subsequent void is filled by both fatty and connective tissue to such an extent that the muscle appears to grow and is deceptively healthy in outward appearance, which accounts for the pseudohypertrophy.
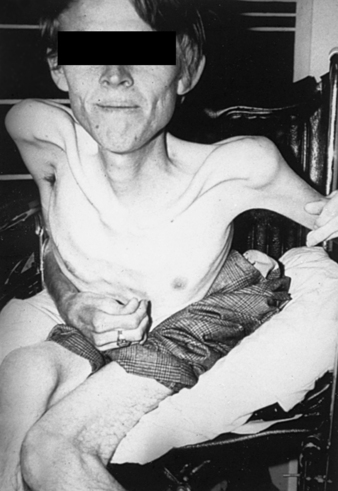 |
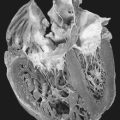
| Duchenne’s Dystrophy. An individual with late-stage Duchenne muscular dystrophy, showing severe muscle loss. |
Signs and Symptoms
• Atelectasis development
• Calf muscle pseudohypertrophy
• Contractures of iliotibial bands, hip flexors, and heel cords
• Episodes of pneumonia
• Gower sign (child that pushes on knees to stand up)
• Gradual, progressive diminution of deep tendon reflexes until they disappear completely
• Kyphoscoliosis
• Signs of nocturnal hypoxemia (lethargy, early morning headache)
• Waggling gait (particularly 3- to 6-year-old boys)
• Waning forced vital capacity
Medical Management
Duchenne’s dystrophy remains an incurable disease. Its symptoms can be treated and the progression retarded, but it cannot be reversed. Prednisone is the only medication that produces any benefits that modify the progression of the disease. The muscle wasting that is so debilitating can be retarded by administration of prednisone at 0.05 to 0.75 mg/kg/day in divided doses on alternating days. Benefits from treatment with prednisone may become clinically evident within a month of initiation of the treatment. The observed benefit may last as long as 3 years.
The remainder of the medical management of Duchenne’s dystrophy is geared toward managing symptoms and delaying the inevitable for as long as possible. Daily exercises of joints and muscle stretching delay the onset of contractures. The stretching exercises are combined with judicious application of braces, such as foot/ankle or knee/ankle/foot orthoses, to work synergistically to help the patient maintain the ability to stand, whether mobile or not, and delay the onset of contractures and scoliosis. The onset of wheelchair dependency initiates the development of greater debilities, such as contractures, scoliosis, and degradation of muscle strength. These debilities contribute to pulmonary function impairment and gastrointestinal dysmotility.
Confinement to a wheelchair hastens, somewhat, the development of Duchenne’s dystrophy. Onset of the sedentary life of muscle weakness contributes to the manifestation of complications associated with the disease. Chronic steroid use weakens the skin, making it friable and highly susceptible to breakdown and ulceration. The skin breakdown and ulceration dramatically increases the potential for infection and sepsis. Progression of the muscle weakness reduces pulmonary function. Scoliosis and hyperlordosis, the result of contractures within the spinal column, lead to development of restrictive pulmonary disease. Loss of function of the accessory muscles of breathing, the intercostals, and sternocleidomastoids contributes further to the loss of pulmonary function and the possible development of atelectasis and pneumonia. Continuous positive airway pressure (CPAP) or bi-level positive airway pressure (BiPAP) are two minimally invasive methods of pulmonary support. Development of atelectasis and pneumonia can be thwarted by frequent daily use of incentive spirometry. Broad-spectrum antibiotics may be required if pneumonia does develop. As the disease progresses, pulmonary function continues to decline, often producing increasing restrictions and ultimately creating the necessity for a tracheostomy to reduce some of the work of breathing.
Dystrophin is also found significantly in cardiac and brain tissues. As such, loss of dystrophin in these two tissues produces cardiac dysfunction and mental developmental delays. Pseudohypertrophy may also occur in the heart leading to cardiac fibrosis. Cardiac fibrosis, in turn, results in reduced cardiac output with ensuing pulmonary congestion that spirals down to fulminate congestive heart failure. The cardiac fibrosis also affects the cardiac conduction system, which may be evidenced by tall R waves in the V 1 lead, deep Q waves in the AVL, AVR, and AVF leads, shortened PR interval, and sinus tachycardia. Lethal dysrhythmias may occur.
Loss of dystrophin from cerebral tissue leads to developmental delays and diminished intellectual development. There is a shift to the left with regard to intelligence quotient (IQ) distribution, with a mean IQ being about 83 for these patients.
Complications
See Becker’s dystrophy (p. 45).
Anesthesia Implications
• Atelectasis
• Cardiac conduction abnormalities
• Cardiac fibrosis
• Congestive heart failure
• Contractures
• Cushingoid habitus
• Developmental delays
• Diminished intellectual development
• Gastric ulcers
• Infection
• Muscle atrophy
• Muscle weakness
• Pneumonia
• Pressure sores
• Sepsis
• Weight gain