Dry Eye Disease
Epidemiology and Pathophysiology
Introduction
Epidemiology is that branch of medical study which concerns itself with the frequency, distribution, determinants and assessments associated with disease. In this chapter we shall cover major studies and results, concentrating on the most recent ones, which affect clinical understanding of dry eye disease (DED). For a comprehensive review of this field, including an assessment of symptom questionnaires, the reader is referred to the 2007 Report of the International Dry Eye Workshop (DEWS), chapter on the epidemiology of dry eye disease.1 As noted in the above referenced publication, there has been a significant increase in both interest in and studies of the prevalence and incidence of DED, particularly since the 1990s. At the first Tear Film and Ocular Surface Society international meeting in 1992, a call for a ‘consensus conference’ was made. This resulted in the National Eye Institute/Industry workshop report, which determined that there was a paucity of data concerning prevalence and incidence of DED. New methodologies in the field of epidemiology and statistical analysis have been employed to expand our knowledge in this regard.
Clinicians have long recognized that complaints relating to DED are common in clinical practice. In an attempt to apply scientifically valid methodologies to this enquiry, an agreed definition is essential. The DEWS report, building on an earlier text, defined DED as ‘a multifactorial disease of tears and ocular surface that results in symptoms of discomfort, visual disturbance and tear instability with potential damage to the ocular surface. It is accompanied by increased osmolarity of the tear film and inflammation of the ocular surface’.2 Armed with this widely agreed definition, many studies have been published. These studies address different population groups throughout the world. They agree that DED is a very common condition, increasing in frequency with advancing age. The estimates of prevalence (proportion of a population with the disease at a given time), range from about 5% to over 30%. These differences may be related to different population characteristics but primarily to differences in the characterization of subjects with disease.
Nomenclature
Various names have been attached to dry eye. These include keratoconjunctivitis sicca (KCS), dry eye syndrome, and dysfunctional tear syndrome (DTS). KCS is a traditional name implying dryness and inflammation of the ocular surface. Its use began to wane with the recognition that all cases of the condition do not demonstrate a lack of tears and with recognition of the role of inflammation in the pathogenesis of the disease process. Dry eye or dry eye syndrome became the predominant terms in use throughout the English-speaking world and even in non-English-speaking countries, in which the term is widely recognized. With publication of the Delphi report in 2006,3 a new term, dysfunctional tear syndrome, was proposed. The issue was put to a vote at the Tear Film and Ocular Surface Society meeting prior to publication of the DEWS report. A majority of the expert participants preferred the term ‘dry eye’ or ‘dry eye disease,’ citing its wide recognition, not only by clinicians and researchers but also by patients.4 The latter term emphasizes the increasingly well-characterized pathophysiological processes of a discrete disease. Both DED and DTS are currently in use.
Risk Factors for Dry Eye Disease
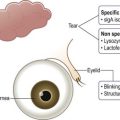
There are a number of risk factors for the development of DED (Fig. 11.1). Reference 1 lists these factors and stratifies them based on levels of evidence. The most obvious factors associated with DED are increasing age, female gender and hormonal changes. Connective tissue disease is associated with more severe forms of DED, e.g. Sjögren’s syndrome and graft-versus-host disease. Dry eye disease occurs with greater frequency in women, particularly in the menopausal and postmenopausal years. The preponderance of evidence has demonstrated that androgen insufficiency is a principal risk factor for both lacrimal gland involvement and meibomian gland dysfunction (MGD). The female : male ratio decreases with advancing age as large segments of males with MGD become affected. The distribution of subtypes of DED, based on glands primarily affected is discussed in the section on pathophysiology later.
Impact on Visual Function
A recent recognition is the impact that DED has on visual function. A hallmark of DED is tear film instability.2 This is manifest in a rapid tear breakup time, during the inter-blink interval. This effect is not apparent in standard high-contrast visual acuity testing, in which the subject can blink and transiently re-form the tear film. However, within several seconds in dry eye patients, the tear film breaks up causing a significant loss of resolving power and a drop in acuity. A Japanese study has documented that inter-blink visual acuities of 20/60 and greater are common.5,6 Patients rarely recognize the connection between their visual problem and dry eye, usually complaining of decreased reading time, or ocular fatigue. This is probably caused by repeated blinking in an unconscious effort to clear vision. Other parameters of visual loss in DED, such as contrast sensitivity and Shack Hartmann aberrometry measurements may be also affected.7,8 These features of DED lead to the substantial degradation of quality of life measures in the disease. In one study, the equivalent burden of having moderate to severe dry eye was similar to that of moderate to severe angina.9
Role of Symptoms in DED
The literature states that dry eye is a symptom-driven disease.1 This implies that symptoms are a requisite component of the diagnosis. While symptoms certainly play a major role in the disease, recent studies have noted two seemingly paradoxical observations, i.e. that many patients with early/mild disease have symptoms greater than any objective findings, and that in a subset of patients with severe disease there is a paucity of symptoms. The latter is thought to be due to a decrease in sensory perception associated with inflammation; the former has been linked to a hyperalgesia, associated with early nerve ending response to injury. In a recent presentation of an observational study, only 60–70% of patients with DED were reported to have symptoms (as measured by the Ocular Surface Disease Index).10 The remaining 30–40% do not present with measurable symptoms. Reliance on symptoms alone for diagnosis would therefore, seem unwise.
Pathophysiology of Dry Eye Disease
Although there are numerous risk factors for the development of dry eye disease, the final common expression of the pathophysiology includes tear film hyperosmolarity and instability. The concept of a functional lacrimal unit has been developed in which the secretory glands (lacrimal, meibomian and mucin-producing surface cells), the entire ocular surface, and the lids are linked by a neural network, which responds to external stimuli to maintain a stable tear film and underlying ocular surface, necessary for subserving clear vision.11 This unit has recently been enlarged to include the nasolacrimal duct, which has receptors that respond to volume changes.
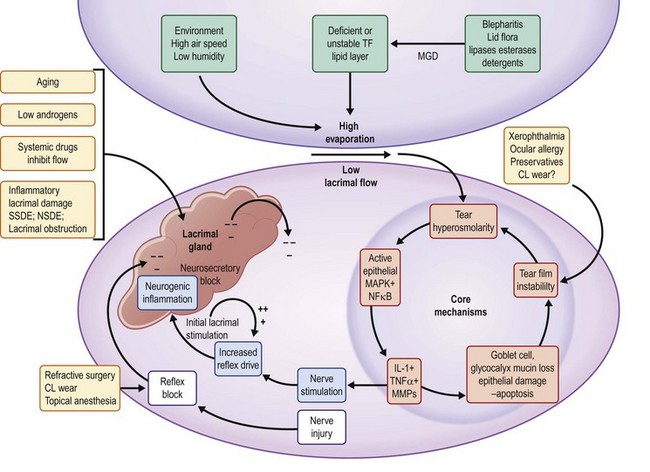
A key concept to understanding the development of DED is the breakdown in this unit leading to an unstable, concentrated (hyperosmolar) tear film. In Table 11.1, this concept is illustrated in a graphic, which the entry points or risk factors in the periphery lead to the core central mechanisms.2 As these mechanisms (e.g. lacrimal hyposecretion and/or meibomian gland dysfunction) come into play, there is a mutually reinforcing cycle of dysfunction leading to inflammation in the ocular surface and lacrimal glands. This vicious cycle concept has been developed by Baudouin12 and is illustrated in Figure 11.1.
Table 11.1
Risk Factors for Dry Eye Disease
| Mostly Consistent1 | Suggestive2 | Unclear3 |
| Older age | Asian race | Cigarette smoking |
| Female sex | Medications | Hispanic ethnicity |
| Postmenopausal estrogen therapy | Tricyclic antidepressants | |
| Omega-3 and Omega-6 fatty acids | Selective serotonin reuptake inhibitors | Anticholinergics |
| Medications | Diuretics | Anxiolytics |
| Antihistamines | Beta-blockers | Antipsychotics |
| Connective tissue disease | Diabetes mellitus | Alcohol |
| LASIK and refractive excimer laser surgery | HIV/ HTLVl infection | Menopause |
| Radiation therapy | Systemic chemotherapy | Botulinum toxin injection |
| Hematopoietic stem cell transplantation | Large incision ECCE and penetrating keratoplasty | |
| Isotretinoin | Acne | |
| Vitamin A deficiency | Low humidity environments | Gout |
| Hepatitis C infection | Sarcoidosis | Oral contraceptives |
| Androgen deficiency | Ovarian dysfunction | Pregnancy |
1Mostly consistent evidence implies the existence of at least one adequately powered and otherwise well-conducted study published in a peer-reviewed journal, along with the existence of a plausible biological rationale and corroborating basic research or clinical data.
2Suggestive evidence implies the existence of either: (1) inconclusive information from peer-reviewed publications or (2) inconclusive or limited information to support the association, but either not published or published somewhere other than in a peer-reviewed journal.
3Unclear evidence implies either directly conflicting information in peer-reviewed publications or inconclusive information but with some basis for a biological rationale.
(This table was published in Report of the International Dry Eye Workshop (DEWS) The Ocular Surface 2007;5:99. Reproduced with permission from Elsevier, the publisher.)
Principal Causative Factors
Tear hyperosmolarity is considered the central mechanism in DED, leading to inflammation at the ocular surface with resulting tissue damage and symptoms.2 The hyperosmolar state is the result of either an insufficient secretion of fluid from the lacrimal glands (low aqueous flow) and/or excessive evaporation of the tear film. Normal tear osmolarity averages around 295 mOsmol/L which is isotonic with blood. Tear osmolarity over the ocular surface is greater than that in other tear compartments (e.g. the menisci), because of the larger surface area of the interpalpebral space, allowing for evaporative water loss between blinks. Indirect evidence of this comes from studies exposing the ocular surface in humans to increasing concentrations of solutions until ocular irritation occurs.13 It is also probable that, were it possible to sample tears from the preocular surface, the values would change over the ocular surface as the lid moves over the surface. There is a transfer of fluid from this preocular surface compartment to the tear menisci at their nasal extent with each complete blink. The menisci serve as a compartment of mixed tears and in normal subjects, is remarkably stable. As disease develops, the osmolarity in the tear menisci rises. While not the same as that over the ocular surface, there is a relationship that permits use of menisci to evaluate and monitor activity on the surface.
Hyperosmolarity affects the ocular surface in multiple ways. Hyperosmolarity initiates a cascade of inflammatory events mediated by at least two separate pathways, leading to the recruitment and activation of inflammatory cells on the ocular surface and the production of inflammatory cytokines and chemokines (see below). Associated with these changes is an increase in surface cell death (apoptosis), changes in mucin production and a loss of lubrication between the lid and the ocular surface.2
The lacrimal functional unit is capable of responding to these changes by increasing aqueous tear production in an attempt to lower the osmolarity. This may result in a transiently lower tear osmolarity but in aqueous tear deficient dry eye (ADDE). The lacrimal glands lack the secretory capacity to maintain this effort. In evaporative dry eye (EDE), most commonly caused by meibomian gland dysfunction (MGD), the excessive evaporative tear loss is associated with a thin or defective tear lipid layer which stimulates reflex tearing.14 Prolonged overstimulation of the lacrimal gland results in a neurogenic inflammation in the lacrimal gland, characterized by increased autoantigen expression, T-cell targeting and release of inflammatory cytokines into the tear film. These lead to compromise of the gland and a mixed or hybrid form of the disease. Other compensatory mechanisms may include increased blinking and squinting. Tear osmolarity has a linear relationship with increasing severity of dry eye10 and is the best most reliable metric for the diagnosis of DED.15
Tear Film Instability
The tear film should provide for a complete tear layer throughout the blink interval. When break-up of the tear film occurs prior to the next blink, there is a discontinuity that degrades the visual image and produces irritation. The ratio of the TBUT to the inter-blink interval has been called the ocular protection index.16 Values less than 1 are considered pathological.
Disturbances in the mucin cover of the cornea are responsible for rapid break-up. Tear break-up can occur over areas of corneal irregularity and are more rapid and extensive. Damage to the mucin layer or glycocalyx have been reported with the use of preservative-containing eye drops, particularly those containing benzalkonium chloride, which is a constituent of many glaucoma medications. Up to three-quarters of patients with long-term use of topical glaucoma medications exhibit symptoms and signs of DED, and the frequency increases with the number of medications used.17 Contact lens wear has also been associated with tear instability.
The Role of Inflammation
Laboratory Studies
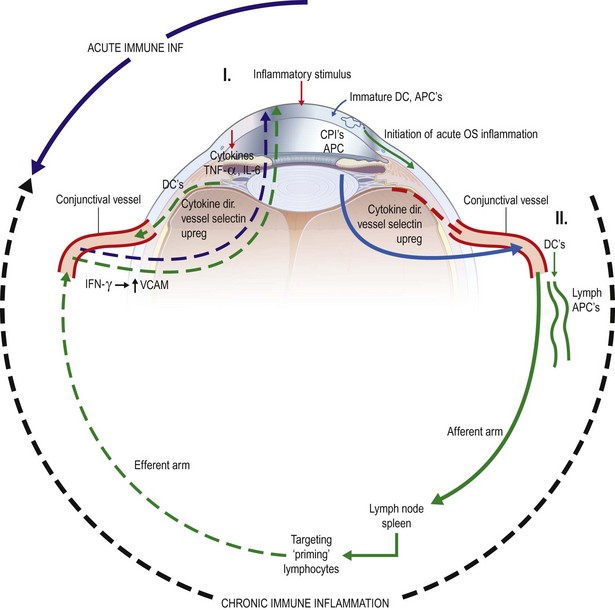
Both in vitro studies and animal models of dry eye disease have been utilized to study DED. Initial studies in canines demonstrated that topical treatment with cyclosporine A could effectively treat dry eye.18 The use of human conjunctival cell lines has demonstrated that inflammatory cytokines can up-regulate markers of inflammation, such as HLA-DR and ICAM-1. Studies utilizing mouse models, which usually involve initiation of disease by environmental stress, with or without systemic scopolamine injections, show inflammation of the conjunctiva and T-cell activation, which are seen in human DED.19 Figure 11.2 from Reference 19 illustrates the mechanisms thought to be involved in acute and chronic immune-associated inflammation in DED.
Human Studies
Histopathological studies, measurement of inflammatory cytokines, chemokines and immunomodulatory molecules in tears, and tests for expression of cell surface antigens, all support the role of inflammation in human dry eye. Inflammation leads to tissue damage and is an intimate part of the vicious cycle of mutually reinforcing events in DED.2 All of these markers of inflammation, which are seen in moderate to severe disease, are present to a lesser extent in mild to moderate cases. Many of the studies of inflammation in DED have employed patients suffering from Sjögren’s syndrome, graft-versus-host disease and other systemic conditions associated with autoimmune disease. In these cases, inflammation may play a primary role with autoimmune attack of the lacrimal gland as an early feature. In cases of dry eye associated with hormonal and aging changes, inflammation may play a more downstream role as a consequence of tear hyperosmolarity.
Role of Androgens in DED
There is an extensive literature on the role of androgen deficiency in the pathogenesis of DED. Both low levels of systemic androgen and high levels of estrogen are risk factors for the development of DED. Biologically active androgens support the secretory activities of the lacrimal and meibomian glands. In addition, there are a number of associations between DED and other diseases characterized by decreased androgen activity, including complete androgen insensitivity syndrome, premature ovarian failure, and patients undergoing treatment with antiandrogen compounds for prostate cancer. A comprehensive review of this subject is published.20
Distribution of Subtypes of Dry Eye Disease
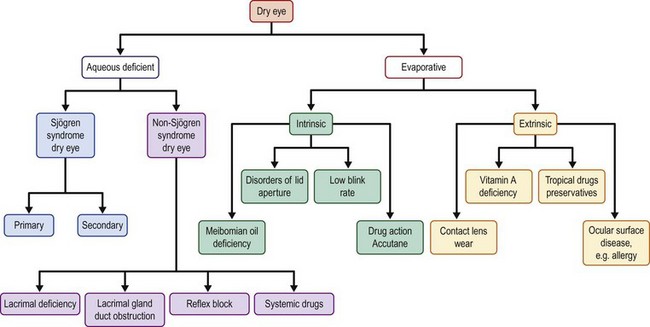
As noted earlier, DED based on the glandular structures primarily involved has been divided into two mechanistic subtypes, i.e. aqueous tear deficient dry eye (ADDE) and evaporative dry eye (EDE). Although there are a number of conditions leading to the latter, the predominant factor is meibomian gland dysfunction (MGD). Aqueous deficiency has commonly been treated as synonymous with DED, with MGD occupying a position as a co-morbid condition. In recent years, there has been increased interest in the role of MGD in the pathogenesis of DED and its integral role in the lacrimal functional unit. Figure 11.3 from (Reference 2) depicts a comprehensive description of the mechanistic division of the different components of the ocular surface.
Studies in Asia, Europe, and more recently in the US, have documented the high prevalence of MGD, particularly in populations over the age of 50. MGD has become a major focus of research, and a comprehensive monograph on this subject was published in 2011. Early reports of the high prevalence of MGD in Asian populations led to the impression that MGD was more common in Asians.21 However, recent studies in Europe and the US have reported similarly high prevalences.22,23 In the most recent report of 224 subjects, MGD alone far outweighs ADDE alone, 79 to 24, with 57 subjects showing evidence of a combined form of DED.24 With increasing severity, there is a marked increase in the combined form of the disease, as initial compensatory mechanisms fail, compromising the other structures of the lacrimal functional unit. Most cases of Sjögren-associated DED are a mixed form of the disease. The recently published report of the MGD workshop thoroughly treats this topic.25 A large population-based study in Spain has reported that more subjects with clear objective signs of MGD are asymptomatic than those with symptoms.26
Conclusion
The key factors in this disease are twin hallmarks of the disease, i.e. tear film instability and an elevated tear osmolarity. These two facets are closely linked and are responsible for many of the observed changes in DED. Of the many tests that have been used to diagnose the condition, most exhibit variability over time and between eyes. In addition, the individual tests do not correlate well with each other, nor do they correlate well with patient-reported symptoms. In several recently published studies these observations were analyzed. The most commonly used objective tests are quite variable over a 3 month period of observation in dry eye patients. In addition, patient symptoms as measured by the Ocular Surface Disease Index (OSDI) are variable and do not correlate well with objective signs. Indeed, recent findings point out that many patients with DED do not report symptoms, undermining the notion that DED is defined as a symptomatic disease. The variability seen is thought to be due to tear film instability.27 The lack of correlation between signs of disease (i.e., Schirmer test, tear break-up times, corneal and conjunctival staining, lid margin scoring) suggests that each test provides important but independent information, that may become positive at different stages of the disease. For example, fewer than 50% of patients with mild to moderate disease demonstrate any corneal staining. Another characteristic of patients with mild to moderate disease is conflict between various signs of disease. This has led to the development of composite index of disease severity, incorporating all of the commonly performed tests.10 The contribution of each test at different stages of disease can then be evaluated. Of all the tests used, only tear osmolarity demonstrates a linear relationship to increasing severity of DED.
References
1. The epidemiology of dry eye disease. Report of the epidemiology subcommittee of the International Dry Eye Workshop. No authors listed. Ocular Surf. 2007;5:93–107.
2. The definition and classification of dry eye disease. Report of the definition and classification subcommittee of the international dry eye workshop. No authors listed. Ocular Surf. 2007;5:75–92.
3. Behrens, A, Doyle, JJ, Stern, M, et al. Dysfunctional tear syndrome syndrome: a Delphi approach to treatment recommendations. Cornea. 2006;25:900–907.
4. Baum, J, Foulks, G, Lemp, MA. What’s in a name? Cornea. 2006;25:871–872.
5. Kaido, M, Dogru, M, Ishida, R, et al. Concept of functional visual acuity and its application. Cornea. 2007;26(9 Suppl. 1):S29–S35.
6. Goto, E, Yagi, Y, Matsumoto, Y, et al. Impaired functional visual acuity of dry eye patients. Amer J Ophthalmol. 2002;133:181–186.
7. Montes-Mico, R, Caliz, A, Alio, JL. Wavefront analysis of higher order aberrations in dry eye patients. J Refract Surg. 2004;20:243–247.
8. Thibos, LN, Hong, X. Clinical applications of the Shack Hartmann aberrometer. Optom Vis Sci. 1999;76:817–825.
9. Schiffman, RM, Walt, JG, Jacobsen, G, et al. Utility assessment among patients with dry eye disease. Ophthalmology. 2003;110:1412–1419.
10. Sullivan, BD, Whitmer, D, Nichols, KK, et al. An objective approach to dry eye disease severity. Invest Ophthalmol Vis Sci Dec. 2010;51:6125–6130.
11. Beuerman, RW, Mircheff, A, Pflugfelder, SC, et al. The lacrimal functional unit. In: Pflugfelder SC, Beuerman RW, Stern ME, eds. Dry eye and ocular surface disorders. New York: Marcel Dekker, 2004.
12. Baudouin, C. The vicious cycle in dry eye syndrome: a mechanistic approach. J Fr Ophtalmol. 2007;30:239–246.
13. Liu, H, Begley, C, Chen, M, et al. A link between tear instability and hyperosmolarity in dry eye. Invest Ophthalmol Vis Sci. 2009;50:3671–3679.
14. Bron, AJ, Yokoi, N, Gafney, E, et al. Predicted phenotypes of dry eye: proposed consequences of its natural history. Ocul Surf Apr. 2009;7:78–92.
15. Lemp, MA, Bron, AJ, Baudouin, C, et al. Tear osmolarity in the diagnosis and management of dry eye disease. Am J Ophthalmol. 2011;151:792–798.
16. Ousler, GW, Hagberg, KW, Schindelar, M, et al. The ocular protection index. Cornea. 2008;27:509–513.
17. Leung, EW, Medeiros, FA, Weinreb, RN. Prevalence of ocular surface disease in glaucoma patients. J Glaucoma. 2008;17:50–55.
18. Kaswan, R. Characteristics of a canine model of KCS: effective treatment with topical cyclosporine. Adv Exp Med Biol. 1994;350:583–594.
19. Research in dry eye: Report of the Research Subcommittee of the International Dry Eye Workshop. No authors listed. Ocular Surf. 2007;5:179–193.
20. Sullivan, DA. Tearful relationships? Sex, hormones, the lacrimal gland and aqueous-deficient dry eye. Ocul Surf. 2004;2:92–123.
21. Uchino, M, Dogru, M, Yagi, Y, et al. The features of dry eye disease in a Japanese elderly population. Optom Vis Sci. 2006;83(11):797–802.
22. Lemp, MA, Nichols, KK. Blepharitis in the United States 2009: a survey-based perspective on prevalence and treatment. Ocul Surf. 2009;7(Suppl. 2):S1–S14.
23. Viso, E, Gude, F, Rodriguez-Ares, MT. The association of meibomian gland dysfunction and other common ocular disease with dry eye: a population-based study in Spain. Cornea. 2011;30:1–6.
24. Lemp, MA, Crews, LA, Bron, AJ, et al. Distribution of aqueous deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012;31:472–478.
25. Schaumberg, DA, Nichols, JJ, Papas, EB, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on the epidemiology of and associated risk factors for MGD. Invest Ophthalmol Vis Sci. 2011;52:1994–2005.
26. Viso, E, Rodríguez-Ares, MT, Abelenda, D, et al. Prevalence of asymptomatic and symptomatic meibomian gland dysfunction in the general population of Spain. Invest Ophthalmol Vis Sci. 2012;53:2601–2606.
27. Sullivan, BD, Crews, LA, Sönmez, B, et al. Clinical utility of objective tests for dry eye disease: variability over time and implications for clinical trials and disease management. Cornea. 2012;31:1000–1008.