Chapter 41 DRUGS AND LIVER DISEASE
INTRODUCTION
The liver also has a sophisticated series of metabolic enzymes, which, in general, convert drugs from lipophilic substances to hydrophilic substances that can be more easily eliminated. The cytochrome P450 enzymes, which play the major role in drug metabolism, may produce reactive metabolites during this process and these are thought to be the mechanism by which many adverse hepatic reactions occur.
RISK FACTORS FOR DRUG HEPATOTOXICITY
Age: older persons appear to be more susceptible to drug-induced hepatotoxicity.
Gender: women are more affected by the toxic adverse effects on the liver than men.
PRESENTATION
Drug-related hepatotoxicity can mimic clinically and histologically almost any type of liver disease (Table 41.1). Presenting symptoms are similarly diverse, from asymptomatic elevations of liver function tests to coma secondary to fulminant hepatic failure. Anorexia and tiredness can be the presentations of hepatitic reactions while itch, dark urine and jaundice occur in more severe cholestatic syndromes.
| Condition | Drug |
|---|---|
| Acute hepatocellular necrosis | Isoniazid, cloxacillin, halothane, methyldopa, paracetamol |
| Fatty liver | Tetracycline, valproic acid, corticosteroids, non-steroidal antiinflammatory drugs, perhexiline, amiodarone |
| Granulomatous reactions | Hydralazine, allopurinol, carbamazepine |
| Acute cholestasis | Oral contraceptive steroids, anabolic androgens, chlorpromazine, flucloxacillin |
| Chronic cholestasis | Chlorpromazine, flucloxacillin, amitriptyline |
| Chronic hepatitis/necrosis | Methyldopa, nitrofurantoin, dantrolene |
| Fibrosis and cirrhosis | Methotrexate |
| Vascular disorders | Oral contraceptive steroids, anabolic androgens, azathioprine |
Physical findings
Patients with suspected hepatic adverse reactions need to be closely examined for jaundice and signs of chronic liver disease such as spider naevi and palmar erythema. The size and consistency of the liver is important as it may suggest other causes for the liver dysfunction such as cirrhosis or secondary tumours.
DIAGNOSIS
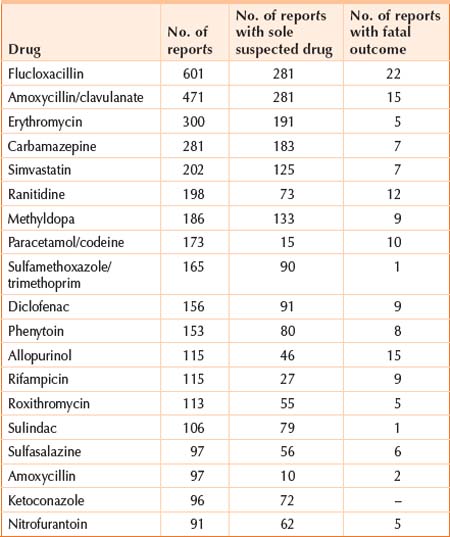
The diagnosis of hepatic drug reactions should be considered in all patients presenting with abnormal liver function tests. The diagnosis is usually made when other causes of liver dysfunction are excluded, such as viral hepatitis, autoimmune hepatitis and bile duct obstruction. All drugs, including alternative medicines, should be considered as potential causes of liver abnormalities. Although some drugs more commonly produce adverse hepatic reactions, Table 41.2 lists the drugs with hepatotoxicity most commonly reported in Australia to ADRAC (Adverse Drug Reactions Advisory Committee). Table 41.3 lists the complementary medicines most commonly reported with hepatotoxicity. Clinicians cannot be expected to remember all drugs that produce adverse hepatic reactions, but there are a number of reference texts that can be consulted to see if the reaction has been reported before.
TABLE 41.3 Most commonly reported complementary medicines with hepatotoxicity
| Medicine | No. of reports | No. of reports with sole suspected drug |
| Kombucha tea | 8 | 7 |
| Echinacea | 7 | 4 |
| Evening primrose oil | 5 | 3 |
| Valerian | 3 | – |
Finally, hepatic failure can occur secondary to drug use, and referral to a liver transplant unit should be made in these situations.
SUMMARY
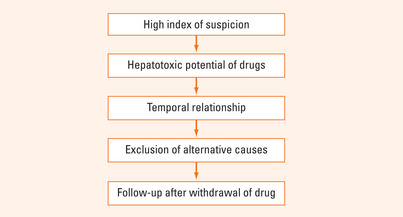
It can be difficult to diagnose drug-induced hepatotoxicity as it can mimic almost any type of liver disease. It is vital to take a detailed drug history and to have a high index of suspicion (Figure 41.1). A temporal relationship between the taking of the drug and clinical signs and symptoms may be an important clue. Exclusion of other causes is mandatory.
Treatment is the immediate stoppage of the suspected causative drug.