Drugs affecting the central nervous system
After reading this chapter, the reader will be able to:
1. Define key terms pertaining to drugs that affect the central nervous system (CNS)
2. Describe the multiple functions of the CNS
3. Recognize various effects of medications on the CNS and their ability to modulate neurotransmitters
4. Comprehend psychiatric medications, including their classification, use, and side-effect profiles
5. Recognize the effects of alcohol on the CNS during acute intoxication, chronic use, and after abrupt withdrawal
6. Distinguish physiologic and psychological bases of pain and the classes of analgesics used to treat pain
7. Recognize indications for the use of both local and general anesthesia
8. Describe the concept of conscious sedation and indications and guidelines for use
9. Distinguish drugs that stimulate the CNS and respiratory system, and describe the indications for application
The most widely used drugs, both therapeutic and recreational, are agents affecting the central nervous system. Humans are intrinsically concerned with and perhaps even defined by the processes of thinking and feeling. These processes originate within the brain. Thoughts and feelings, although poorly understood, reside primarily with neurochemical interactions and balance in the brain. Drugs that affect the central nervous system (CNS) are used for their effects on perception and mood. Although the gross anatomy of the brain has been elegantly described, the complex interaction of various brain areas and individual neurons is less well understood.
Several diseases are apparently related to loss of particular neurons with specific neurotransmitters. Parkinson disease is caused by a loss of dopamine-containing neurons in the substantia nigra area of the midbrain. This condition is characterized by resting tremor; rigidity; bradykinesia, or slowness in initiating movement; gait disturbances; and postural instability. Treatment of Parkinson disease involves increasing the amount of dopamine contained in and released from the remaining neurons.1,2 Some forms of depression are believed to be caused by reduced activity of norepinephrine neurons in the brain, particularly neurons in the locus caeruleus.3 There seems to be a decrease in the preganglionic augmentation effects of serotonin and in direct stimulatory effects of norepinephrine. Treatment is to restore more normal activity of the norepinephrine neurons by inhibiting the reuptake of serotonin by modulating neurons, enhancing the amount of norepinephrine released, and increasing the duration of its effects in the synapse.
Neurotransmitters
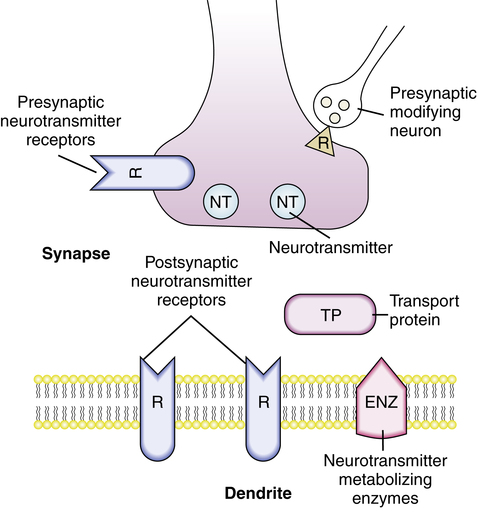
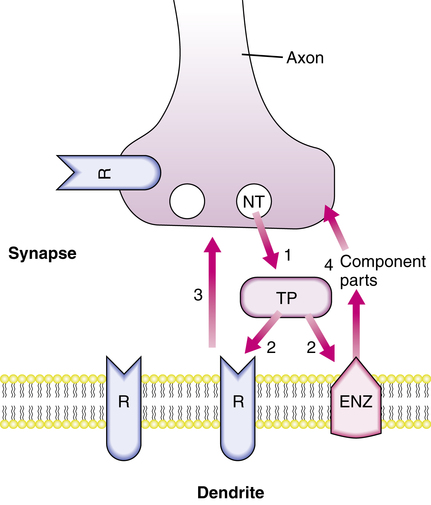
Each neuron releases predominantly one type of neurotransmitter from its axon to synapse with the next neuron. If enough receptors are activated on the postsynaptic membrane, electrical depolarization occurs, and a signal is passed to the next neuron. The functional anatomy and components of neurotransmission are illustrated in Figures 20-1 and 20-2. Released neurotransmitters are bound to and transported by proteins in the synapse, taken back up by the releasing nerve terminal, repackaged into vesicles, and recycled. Bound neurotransmitters are unavailable for receptor interactions, and alterations in the transport proteins in amount or affinity affect the signal propagation potential. Some of the released neurotransmitter is metabolized by membrane-bound enzymes on the postsynaptic cell membrane. The resulting constituent components are taken up presynaptically and used as precursors for neurotransmitter synthesis. Receptors on both the presynaptic membrane and the postsynaptic membrane specific for the released chemicals and for other chemicals from modulating and neighboring neurons affect the activity of the neuron.
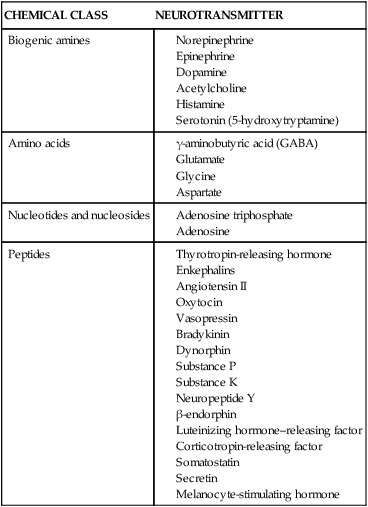
Chemicals that behave as neurotransmitters are listed in Table 20-1. The effect of the neurotransmitter released is determined by many factors, including the amount of neurotransmitter released, type and quantity of transport proteins, previous release of neurotransmitters, presence of modifying substances, efficiency of reuptake processes, and activities of modulating interneurons. Specifics of this transmission modulation system differ for various brain areas, mental functions, and neurotransmitters. CNS-active drugs may have effects on specific parts of a neurotransmitter system or have generalized effects on brain function. Augmentation or inhibition of neurotransmission can result from drug interaction at any of the sites illustrated in Figures 20-1 and 20-2.
TABLE 20-1
Central Nervous System Chemicals That Function as Neurotransmitters
| CHEMICAL CLASS | NEUROTRANSMITTER |
| Biogenic amines |
Psychiatric medications
Antidepressants
Depression is one of the most common psychiatric disorders and a major cause of worldwide disability. In the United States, the 1-month prevalence of a major depressive episode has been estimated to involve more than 2% of the population.4 The Global Burden of Disease Study found unipolar depression to be the fourth leading cause of worldwide disability, even after excluding deaths from suicide.5 The prevalence of major depressive disorder may be increasing, and it is predicted that unipolar major depression will be the second leading cause of disability worldwide by 2020.6
Depressive disorder has multiple etiologies, including biologic, psychological, and social factors. Serotonin and norepinephrine have been shown to be important neurotransmitters, and their relative deficiency has been linked to depression. For more than a decade, selective serotonin reuptake inhibitors (SSRIs) have been the first line of medical treatment for major depressive disorder. These drugs are preferred because they are safer and more tolerable than older medications such as tricyclic antidepressants (TCAs) and monoamine oxidase inhibitors (MAOIs). In addition, newer drugs target both norepinephrine and serotonin; they are called serotonin norepinephrine reuptake inhibitors. These drugs and their side effects are listed in Tables 20-2 and 20-3.
TABLE 20-2
Drugs Used to Treat Depression
| CLASS | GENERIC DRUG | U.S. BRAND NAME |
| Selective serotonin reuptake inhibitors (SSRIs) | Citalopram | Celexa |
| Fluoxetine | Prozac, Prozac Weekly, Sarafem | |
| Fluvoxamine | Luvox, Luvox CR | |
| Paroxetine | Paxil, Paxil CR, Pexeva | |
| Sertraline | Zoloft | |
| Escitalopram oxalate | Lexapro | |
| Serotonin and norepinephrine reuptake inhibitors | Venlafaxine | Effexor, Effexor XR |
| Duloxetine | Cymbalta | |
| Desvenlafaxine | Pristiq | |
| Serotonin receptor antagonist | Nefazodone | Nefazodone |
| Dopamine reuptake inhibitor | Bupropion | Wellbutrin, Wellbutrin SR, Wellbutrin XL, Zyban |
| Tricyclic antidepressants (TCAs) | Amitriptyline | Amitriptyline |
| Amoxapine | Amoxapine | |
| Clomipramine | Anafranil | |
| Desipramine | Norpramin | |
| Doxepin | Sinequan | |
| Imipramine HCl | Tofranil | |
| Imipramine pamoate | Tofranil-PM | |
| Nortriptyline | Aventyl, Pamelor | |
| Protriptyline | Vivactil | |
| Trimipramine | Surmontil | |
| Tetracyclic antidepressants | Maprotiline | Maprotiline |
| Mirtazapine | Remeron, Remeron SolTab | |
| Monoamine oxidase inhibitors (MAOIs) | Phenelzine | Nardil |
| Tranylcypromine | Parnate | |
| Isocarboxazid | Marplan | |
| Herbal remedy | St. John’s wort (Hypericum perforatum) | St. John’s wort |
| Miscellaneous drugs | Trazodone | Desyrel |
TABLE 20-3
Incidence of Side Effects of Commonly Used Antidepressants
| MEDICATION | SEDATION | AGITATION | ANTICHOLINERGIC EFFECTS* | POSTURAL HYPOTENSION | GASTROINTESTINAL UPSET | SEXUAL DYSFUNCTION | WEIGHT GAIN | WEIGHT LOSS |
| Serotonin and Norepinephrine Reuptake Inhibitors | ||||||||
| Tricyclics (Tertiary Amines) | ||||||||
| Amitriptyline | ++++ | 0 | ++++ | +++ | + | + | ++ | 0 |
| Doxepin | ++++ | 0 | ++++ | +++ | + | + | + | 0 |
| Imipramine | ++++ | 0 | ++++ | +++ | + | + | + | 0 |
| Tricyclics (Secondary Amines) | ||||||||
| Desipramine | +++ | 0 | +++ | ++ | + | + | + | 0 |
| Nortriptyline | +++ | 0 | +++ | ++ | + | + | + | 0 |
| Bicyclic | ||||||||
| Venlafaxine† | ++ | + | ++ | 0 | +++ | ++ | 0 | + |
| Selective Serotonin Reuptake Inhibitors | ||||||||
| Citalopram | 0 | 0 | + | 0 | ++ | + | + | + |
| Fluoxetine | + | ++ | + | 0 | ++ | ++ | + | + |
| Paroxetine | ++ | 0 | + | 0 | ++ | ++ | + | + |
| Sertraline | + | + | + | 0 | ++ | ++ | + | + |
| Serotonin Norepinephrine Reuptake Inhibitor | ||||||||
| Duloxetine | ++ | + | ++ | + | ++ | + | + | + |
| Norepinephrine Reuptake Inhibitor, Dopamine Reuptake Inhibitor | ||||||||
| Bupropion | + | ++ | ++ | 0 | ++ | 0 | + | ++ |
| Serotonin Antagonists and Reuptake Inhibitors | ||||||||
| Nefazodone | ++ | 0 | ++ | + | + | 0 | 0 | 0 |
| Trazodone | ++++ | 0 | ++ | + | + | 0 | + | + |
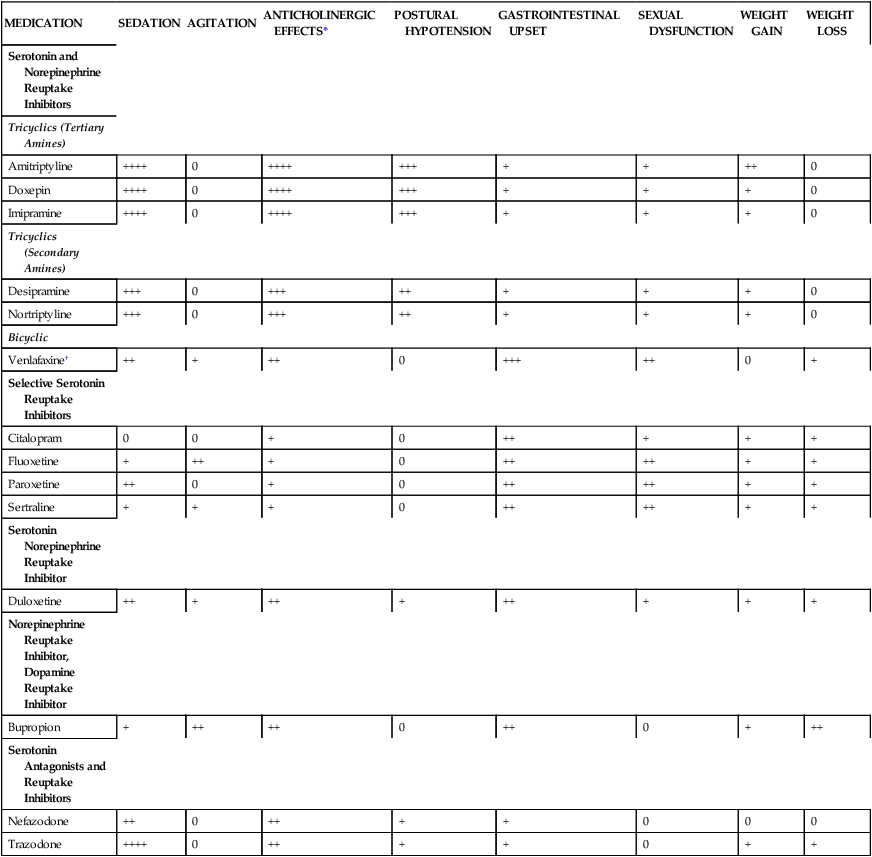
*Side effects may include dry mouth, dry eyes, blurred vision, constipation, urinary retention, tachycardia, or confusion.
†Venlafaxine may cause a dose-related elevation in diastolic blood pressure; monitoring of blood pressure is recommended.
From Whooley MA, Simon GE: Managing depression in medical outpatients, N Engl J Med 343:1947, 2000.
Mood stabilizers
Mood stabilizers are used primarily for bipolar disorder. This affective disorder involves alternating episodes of depression and mania or hypomania. Mania is characterized by at least 1 week of elevated or irritable mood and at least three of the following: inflated self-esteem or grandiosity, decreased need for sleep, being more talkative than usual, rapid thoughts or the subjective experience that one’s thoughts are racing, distractibility, an increase in goal-directed behavior, or excessive involvement in pleasurable activities that have a high potential for painful consequences.7 Hypomania is similar to mania but less intense and of shorter duration.7
Medical treatment of any degree of bipolar disorder must begin with a mood stabilizer. These drugs include lithium; anticonvulsants such as valproic acid, carbamazepine, gabapentin, and lamotrigine; and antipsychotics, which are discussed subsequently. Except for lithium, the main side effect of these drugs is sedation. Lithium has a narrow therapeutic window and consequently must be used judiciously. Lithium can cause tremor, cognitive slowing, hypothyroidism, renal insufficiency, leukocytosis, polyuria, and polydipsia. Lithium toxicity can result in coma.8 Table 20-4 lists common mood stabilizers.
TABLE 20-4
Drugs Used as Mood Stabilizers
| GENERIC DRUG | BRAND NAME |
| Carbamazepine | Tegretol, Tegretol-XR, Epitol, Carbatrol, Equetro, Teril |
| Lamotrigine | Lamictal, Lamictal XR, Lamictal CD, Lamictal ODT |
| Lithium | Lithobid, Eskalith |
| Valproic acid | Depakene, Depakote, Depakote ER, Depakote CP, Stavzor |
CD, Chewable; CP, delayed release; ER, XR, extended release; ODT, orally disintegrating.
Antipsychotics
Psychotic disorders are characterized by impaired reality testing. They include schizophrenia spectrum disorders and psychosis associated with depression or mania. Pharmacotherapy is generally used to increase dopamine in the brain. These drugs are most efficacious for active psychotic symptoms, such as hallucinations and abnormal thought processes. Older drugs, such as thorazine, thioridazine, and haloperidol, had numerous side effects, which affected compliance. These side effects included extrapyramidal symptoms such as cogwheel rigidity, acute dystonia, oculogyric crisis, and cholinergic side effects. Newer agents, such as risperidone, olanzapine, and quetiapine, are more tolerable. Table 20-5 lists common antipsychotics.
TABLE 20-5
Drugs Used in Management of Psychotic Disorders
| CLASS | GENERIC DRUG | BRAND NAME |
| Phenothiazines | Chlorpromazine | — |
| Fluphenazine | — | |
| Perphenazine | — | |
| Prochlorperazine | — | |
| Trifluoperazine | — | |
| Thioxanthene | Thiothixene | Navane |
| Butyrophenones | Droperidol | Inapsine |
| Haloperidol | Haldol | |
| Miscellaneous agents | Clozapine | Clozaril, FazaClo ODT |
| Lithium | Lithobid, Eskalith | |
| Olanzapine | Zyprexa, Zydis | |
| Pimozide | Orap | |
| Quetiapine | Seroquel, Seroquel XR | |
| Risperidone | Risperdal, Consta | |
| Ziprasidone | Geodon | |
| Aripiprazole | Abilify | |
| Paliperidone | Invega | |
| Iloperidone | Fanapt |
Drugs for alzheimer dementia: cholinesterase inhibitors
Alzheimer dementia is associated with cognitive deficits secondary to decreased acetylcholine levels within the brain. Cholinesterase inhibitors improve cognition and function in patients with Alzheimer disease. These drugs include donepezil, tacrine, galantamine, and rivastigmine. The use of these drugs is sometimes limited by gastrointestinal side effects, which include nausea, vomiting, diarrhea, and hepatotoxicity, especially with tacrine.9 These drugs are listed in Table 20-6.
TABLE 20-6
Drugs Used in Treatment of Dementia
| CLASS | GENERIC DRUG | BRAND NAME |
| Cholinesterase inhibitors | Donepezil | Aricept, Aricept ODT |
| Galantamine | Razadyne, Razadyne ER | |
| Rivastigmine | Exelon | |
| Tacrine | Cognex | |
| Memantine | Namenda |
Anxiolytics
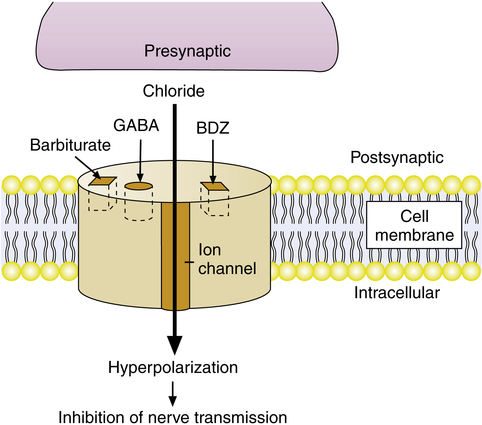
Benzodiazepines are agents that have been used to reduce anxiety under a variety of circumstances. Anxiolytics are also used as amnestics, preventing conversion of short-term experience into permanent memory. By themselves, they cause no change in respiration; however, these agents may augment the respiratory depression induced by opioids. They have little effect on cardiac function and are very safe agents from this standpoint. Benzodiazepines are excellent induction agents when providing general anesthesia and are useful in preventing unpleasant recall during uncomfortable interventions. They may be used as somnifics. These agents are used to terminate seizures, and they elevate seizure threshold. Benzodiazepines exert their effects by binding to benzodiazepine receptors in the γ-aminobutyric acid (GABA) receptor complex on neurons, increasing the GABA chloride channel permeability, which hyperpolarizes the neuron, making depolarization less likely (Figure 20-3). A specific antagonist, flumazenil (Romazicon), can reverse the sedative effects of the benzodiazepines.
Several other drugs are used to treat anxiety and insomnia. Some of these are listed with the benzodiazepines in Table 20-7. Their mechanisms of action are not related to interactions with the benzodiazepine receptor or the GABA system. Some of the drugs listed in Table 20-7 are used to promote sleep; these and other nonrelated sleep-inducing agents are listed in Table 20-8. Although they induce sleep, benzodiazepines and other drug classes interfere with the normal sleep cycles by reducing the amount of time spent in rapid eye movement (REM) sleep.
TABLE 20-7
Drugs Used to Treat Anxiety and Insomnia
| CLASS | GENERIC DRUG | U.S. BRAND NAME |
| Benzodiazepines | Alprazolam | Xanax, Xanax XR, Niravam |
| Clorazepate dipotassium | Tranxene, Gen-Xene | |
| Chlordiazepoxide | Librium | |
| Diazepam | Valium, Diastat | |
| Estazolam | — | |
| Flurazepam | Dalmane | |
| Lorazepam | Ativan | |
| Midazolam | Midazolam | |
| Oxazepam | — | |
| Temazepam | Restoril | |
| Triazolam | Halcion | |
| Benzodiazepine antagonist | Flumazenil | Romazicon |
| Other anxiolytics | Buspirone HCl | BuSpar |
| Doxepin HCl | Sinequan, Zonalon | |
| Hydroxyzine | Vistaril | |
| Meprobamate | Meprobamate |
TABLE 20-8
Medications Used to Induce Sleep
| CLASS | GENERIC DRUG | BRAND NAME |
| Benzodiazepines | Estazolam | — |
| Flurazepam HCl | Dalmane | |
| Quazepam | Doral | |
| Temazepam | Restoril | |
| Triazolam | Halcion | |
| Barbiturates | Secobarbital | Seconal |
| Pentobarbital | Nembutal | |
| Butabarbital | Butisol | |
| Antihistamines | Cyproheptadine | Cyproheptadine |
| Diphenhydramine | Benadryl | |
| Hydroxyzine | Vistaril | |
| Miscellaneous | Chloral hydrate | Somnote |
| Dexmedetomidine | Precedex | |
| Ethanol (alcohol) | ||
| Eszopiclone | Lunesta | |
| Ramelteon | Rozerem | |
| Zaleplon | Sonata | |
| Zolpidem | Ambien, Ambien CR, Edluar |
Other hypnotics
Difficulty sleeping is a common clinical complaint that frequently results in the prescription of a hypnotic. In addition to the short-acting benzodiazepines and barbiturates mentioned earlier, several other sedatives are used for inducing sleep. All of these agents disrupt sleep patterns and may not improve overall well-being. Hypnotics to induce sleep are generally recommended for brief periods (1 or 2 weeks). However, some patients continue to take these medications for years. A new drug, eszopiclone, is approved for long-term use. Medications used for sleep enhancement are listed in Table 20-8.
Pain treatment
Besides the difficulty of estimating the amount of pain, many factors alter patient responses to a given degree of discomfort. Physiologic, social, and psychological factors profoundly alter patient perception and tolerance of pain.10,11 The meaning of pain to the individual can affect the reported pain and the response of the pain to treatment. It is helpful to view the pain experience as composed of at least two components: (1) the sensation of pain as mediated by the CNS receiving nociceptive input from peripheral pain receptors and (2) suffering, the negative, personal emotional response to the pain experience. The integration and expression of these two components produce the pain behavior, which influences the patient’s analgesic requirements. Medications may be directed at the origin, integration, or interpretation of the pain experience. Combinations of medications often are more effective than a single approach to this common problem.
Another factor that must be taken into account when assessing pain is that patients have poor pain memories. With time, the ability to recall the severity and characteristics of pain diminishes; this applies to the effects of treatment as well. Patients asked whether past pain treatment was effective almost always report improvement in pain, even if objective evaluation at the time documents no change or even worsening pain.12 Antidepressants combined with analgesics are used to treat chronic pain states. These agents may be effective by modifying the depressed mood that accompanies chronic discomfort.
Although there are external clues to the presence and magnitude of a person’s pain, personal reports are the only way to judge the presence and magnitude of pain. Visual or numerical analogue pain scales are the most commonly employed methods for estimating the magnitude of pain. The simplest and most common pain scale employed is an 11-point scale, with 10 being the worst imaginable pain and 0 being totally without pain. Patients are asked to rate their pain from the worst imaginable pain (10) to no pain at all (0). These scales seem to have internal and external validity.13–15 The numerical rating is convenient and recognizable, and it lends itself to frequent repetition and consistent reporting. In children, a series of smiling and frowning faces, such as the Wong/Baker Rating Scale, may be used to allow the child to report the degree of pain.16 These scales help in assessing the adequacy of analgesia and create a shorthand way for patients to communicate their need for additional analgesia to the bedside caregiver.
Caregivers must integrate visual or numerical analogue pain scale reports, patient pain behaviors, and vital signs with their own biases17 regarding the degree of pain that should be present to decide whether to administer additional analgesic drugs.18 Caregivers apparently often deliver inadequate amounts of analgesics.19 Inappropriate expectations of the degree of pain in both the patient and the caregiver contribute to this reluctance to administer potent analgesics. Acute pain remains undertreated in many patients.
Nonsteroidal antiinflammatory drugs
Nonsteroidal antiinflammatory drugs (NSAIDs) are analgesics frequently used to treat moderate pain (Table 20-9). NSAIDs work by affecting the hypothalamus and by inhibiting the production of inflammatory mediators, primarily prostaglandins, at the peripheral site of the painful stimulus. The salicylates are the oldest member of this class and have been known for more than 100 years for their effects as antipyretics. Aspirin is a common component of over-the-counter (OTC) analgesics and cold remedies. Aspirin decreases the synthesis of prostaglandin by irreversibly inhibiting two enzymes: cyclooxygenase-1 and cyclooxygenase-2 (COX-1 and COX-2). COX-1 is located primarily on tissues, including blood vessels, kidney, and gastric mucosa, and COX-2 is associated primarily with inflammation. In contrast to aspirin, others in this group reversibly inhibit these enzymes. Although selective COX-2 inhibitors are thought to cause fewer gastrointestinal side effects, there are no clinical trials clearly showing this.20
TABLE 20-9
Nonsteroidal Antiinflammatory Drugs
| CLASS | GENERIC DRUG | BRAND NAME |
| Nonspecific Cyclooxygenase Inhibitors | ||
| Salicylates | Aspirin | Bayer |
| Choline salicylate | Arthropan | |
| Diflunisal | Diflunisal | |
| Magnesium salicylate | ||
| Salsalate | Amigesic, Disalcid | |
| Sodium salicylate | ||
| Aniline derivative | Acetaminophen | Tylenol |
| Indoles | Etodolac | |
| Indomethacin | Indocin, Indocin SR | |
| Sulindac | Clinoril | |
| Propionic acid derivatives | Ibuprofen | Advil |
| Fenoprofen | Nalfon | |
| Flurbiprofen | Ansaid | |
| Ketoprofen | ||
| Naproxen | Aleve, Anaprox, Naprelan, Naprosyn | |
| Oxaprozin | Daypro | |
| Piroxicam derivative | Piroxicam | Feldene |
| Miscellaneous | Diclofenac | Voltaren, Voltaren-XR, Cataflam |
| Ketorolac | ||
| Meclofenamate | ||
| Mefenamic acid | Ponstel | |
| Nabumetone | ||
| Tolmetin | Tolectin | |
| Ziconotide | Prialt | |
| COX-2 Inhibitors | ||
| Celecoxib | Celebrex | |
| Meloxicam | Mobic | |
| Rofecoxib | Vioxx* | |
| Valdecoxib | Bextra† |
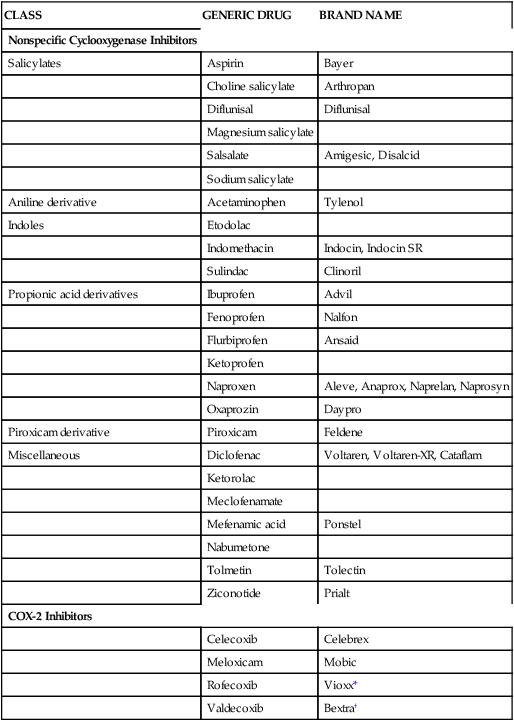
COX-2, Cyclooxygenase-2; SR, sustained release; XR, extended release.
Gastric irritation and ulceration are major problems with administering NSAIDs. Renal injury can result from prolonged use and high doses of these medications. NSAIDs also inhibit platelet aggregation, and this compounds the problem of gastrointestinal bleeding. The antiplatelet effects are used therapeutically either after or to prevent cardiac thrombosis. Aspirin use in childhood febrile illness has been associated with an increased incidence of Reye syndrome, an often fatal increase in intracranial pressure associated with massive hepatic dysfunction.21,22 Allergic reactions to NSAIDs are common. Rashes, urticaria, angioneurotic edema, asthma, and anaphylaxis have been reported.
Acetaminophen (Tylenol), although a weak inhibitor of the cyclooxygenase system, has no significant antiinflammatory effects but is effective in relieving mild to moderate pain. It does not inhibit platelets or cause gastric ulcers. In large doses, acetaminophen can cause lethal hepatic necrosis. Because it is used in many nonprescription cold preparations, accidental overdose from combined dosing during self-medication occasionally occurs. An overdose of acetaminophen can be treated with oral acetylcysteine as described on Chapter 9.
Opioid analgesics
Opioids are listed in Table 20-10. Some have pure agonist effects, acting as the endogenous mediators at the receptors, and others antagonize the endogenous mediators but have a small agonist effect (mixed drugs or agonist-antagonist drugs). There are several strictly antagonist agents. These drugs are used to reverse the analgesic and respiratory depressive effects of the opioids. The most serious side effect of opioid antagonists is respiratory depression, which is mediated by decreased sensitivity of the respiratory center to elevations in arterial carbon dioxide pressure (Paco2). Miosis (small pupils) is pathognomonic for opioid drug administration and is a consequence of effects on the sympathetic nervous system. Constipation results from opioid depression of motility of the stomach and intestines. Nausea and vomiting are a direct effect on the brainstem effectors. Cough suppression results from a direct central effect of the opioid.
TABLE 20-10
| EFFECT AT OPIOID RECEPTOR | GENERIC DRUG | BRAND NAME |
| Agonist | Morphine | Avinza, Kadian, MS Contin |
| Opium | Paregoric | |
| Codeine | — | |
| Alfentanil | Alfenta | |
| Dihydrocodeine | Available only in combination with other agents | |
| Fentanyl | Sublimaze, Actiq, Duragesic | |
| Heroin | — | |
| Hydrocodone | Available only in combination with other agents | |
| Hydromorphone | Dilaudid | |
| Levorphanol | Levo-Dromoran | |
| Meperidine | Demerol | |
| Methadone | Dolophine, Methadose | |
| Oxycodone | Roxicodone, OxyContin | |
| Oxymorphone | Opana, Opana ER | |
| Propoxyphene | Darvon | |
| Remifentanil | Ultiva | |
| Sufentanil | Sufenta | |
| Tramadol | Ultram, Ultram ER, Ryzolt | |
| Mixed agonist-antagonist | Buprenorphine | Buprenex, Subutex |
| Butorphanol | Stadol | |
| Nalbuphine | ||
| Pentazocine | Talwin | |
| Antagonist | Naloxone | |
| Naltrexone | ReVia, Vivitrol |
Opioid inhalation
Opioids are occasionally administered by inhalation. Inhaled opioids have been suggested to be more effective than systemic opioids for decreasing the sensation of dyspnea in patients with advanced respiratory failure. Opioid receptors have been found in lung tissue, but their exact function in modifying the sensation of dyspnea has not been determined. Inhaled (nebulized) opioids may affect dyspnea by a central mechanism because these drugs are rapidly absorbed from the lung. No controlled studies have shown improved effectiveness of opioids when administered by inhalation; however, this route may be an alternative when intravenous access is unavailable.23 Patients with terminal cancer without lung disease have been given systemic doses of analgesics through this route with good clinical effect.
Local anesthetics
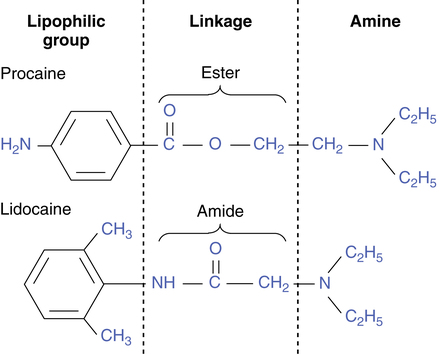
Pain treatment can be achieved by blocking transmission of the pain impulse from the damaged area. Local anesthetics are used to interrupt these nervous signals. Local anesthetics produce nerve conduction block by blocking sodium channels. These are located all along the cell, including the axon. When depolarization occurs, the impulse is propagated down the axon by an abrupt increase in the membrane sodium permeability. When the drug binds to and occludes the channel pore, sodium is unable to enter the cell, and propagation of the electrical impulse is stopped. All local anesthetics consist of a lipophilic part and a hydrophilic, amine part connected by either an amide or ester linkage; this is illustrated in Figure 20-4. Table 20-11 lists several common agents. Sodium channel blockade makes some of these drugs useful in terminating cardiac conduction abnormalities in addition to providing analgesia. Some evidence suggests that systemic administration or inhalation may also enhance bronchodilation in asthma and suppress irritant tracheal cough responses. At toxic levels, CNS excitation occurs, and frank seizures may result. Epinephrine is often added to a local anesthetic for vasoconstriction to delay its absorption, prolonging its effect and decreasing blood levels and potential toxicity. Bupivacaine is very cardiotoxic, and a toxic dose may result in profound and prolonged cardiac depression or arrest.
TABLE 20-11
| CLASS | GENERIC DRUG | BRAND NAME |
| Esters | Benzocaine | Hurricaine, Solarcaine |
| Chloroprocaine | Nesacaine | |
| Procaine | Novocain | |
| Amides | Bupivacaine | Marcaine, Sensorcaine |
| Lidocaine | Xylocaine | |
| Mepivacaine | Carbocaine, Polocaine | |
| Prilocaine | Citanest | |
| Ropivacaine | Naropin |
Epidural analgesia
Continuing epidural infusions for analgesia have improved postoperative pain therapy. There is evidence that patient outcome may also be improved with epidural infusions of local anesthetics, opioids, or both24,25; this is especially true for very ill patients.26–29 The quality of analgesia and the ability to eliminate pain in many body areas are superior with local anesthetic infusion compared with systemic analgesics. Epidural infusions are common in some surgical procedures, especially in the delivery of newborns by cesarean section. Minimal effects on normal sensory and motor function can be achieved with very dilute local anesthetics. Addition of opioids to the mixture permits even less local anesthetic to be infused. If sympathetic blockade produces unacceptable hypotension, local anesthetics can be eliminated completely, with significant analgesia obtained with opioid infusion alone. Pain modulation from epidural opioids occurs at receptors at the spinal cord segmental level. The major side effects of epidural analgesia using local and opioid infusions are listed in Box 20-1.
Combinations of analgesic classes
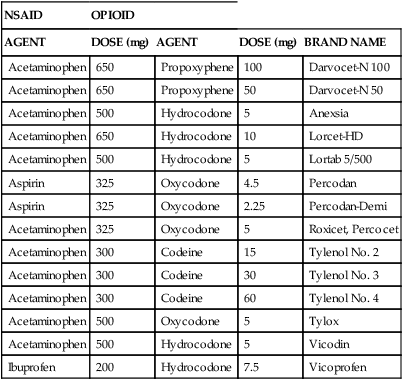
Another strategy to improve analgesia and to reduce the likelihood of opioid overdose is to combine several different classes of analgesics. Prescription combinations of NSAIDs and opioids are widely available (Table 20-12). The concept of attacking pain at several places is useful, but the fixed combinations of drugs with different effects, toxicities, and half-lives make titration to an individual patient’s needs difficult with these agents. Use of the separate agents, independently titrated, may improve this problem, but it is more difficult for patients to take numerous medications.
TABLE 20-12
Examples of Combinations of Nonsteroidal Antiinflammatory Drugs (NSAIDs) and Opioid Analgesics
| NSAID | OPIOID | |||
| AGENT | DOSE (mg) | AGENT | DOSE (mg) | BRAND NAME |
| Acetaminophen | 650 | Propoxyphene | 100 | Darvocet-N 100 |
| Acetaminophen | 650 | Propoxyphene | 50 | Darvocet-N 50 |
| Acetaminophen | 500 | Hydrocodone | 5 | Anexsia |
| Acetaminophen | 650 | Hydrocodone | 10 | Lorcet-HD |
| Acetaminophen | 500 | Hydrocodone | 5 | Lortab 5/500 |
| Aspirin | 325 | Oxycodone | 4.5 | Percodan |
| Aspirin | 325 | Oxycodone | 2.25 | Percodan-Demi |
| Acetaminophen | 325 | Oxycodone | 5 | Roxicet, Percocet |
| Acetaminophen | 300 | Codeine | 15 | Tylenol No. 2 |
| Acetaminophen | 300 | Codeine | 30 | Tylenol No. 3 |
| Acetaminophen | 300 | Codeine | 60 | Tylenol No. 4 |
| Acetaminophen | 500 | Oxycodone | 5 | Tylox |
| Acetaminophen | 500 | Hydrocodone | 5 | Vicodin |
| Ibuprofen | 200 | Hydrocodone | 7.5 | Vicoprofen |
Chronic pain syndromes
The characteristics of neuropathic pain include evidence of a primary injury; pain involving (but not confined to) a body area with a sensory deficit; burning, electric, or shooting character to the pain; dysesthesias in the area; pain spreading beyond the cutaneous nerve distribution; sympathetic hyperactivity; and allodynia, hyperpathia, and hyperalgesia. In some complex regional pain syndromes, autonomic deregulation results in skin changes, edema, and nail and hair loss. This syndrome may lead to severe suffering and incapacitation. Once established, neuropathic pain is poorly responsive to analgesic treatment, but the pain may respond to sympathetic interruption or α-receptor blockade.30 Modification of the initial pain input possibly may decrease the incidence or reduce the severity of the syndrome that develops over time.
Anesthesia
• Pleasant and rapid induction and emergence
• Rapid changes of depth of anesthesia to match surgical demands
• Skeletal muscle relaxation to facilitate surgical exposure
The first and most common anesthetic agents are gases and volatile liquids (Table 20-13). Dosage and potency are compared by using the concept of minimal alveolar concentration (MAC), which is the amount necessary to achieve the anesthetic state. This is a statistical concept, similar to the ED50 (effective dose for 50% of subjects to respond), based on the measured agent concentration in exhaled gas (which is in equilibration with the blood) sufficient to prevent movement on surgical incision in half of the subjects. The mechanisms by which anesthetic gases and vapors exert their effects are poorly understood but may be receptor-mediated (the GABA receptor being a top candidate) or may be more diffuse, temporary disruption of nerve cell communication. The facts that anesthetic vapor potency is linearly related to fat solubility and that anesthetics can be reversed by high pressures (50 to 100 atm) suggest that cell wall swelling from the agent dissolving in the lipid membrane is an important contributor to the anesthetic state.
TABLE 20-13
Gases and Volatile Liquids Used to Produce General Anesthesia
| CLASS | AGENT | COMMON OR BRAND NAME |
| Gases | Nitrous oxide | Laughing gas |
| Liquids | Halothane | — |
| Isoflurane | Forane | |
| Enflurane | Ethrane | |
| Sevoflurane | Ultane | |
| Desflurane | Suprane |
Volatile anesthetics by themselves achieve some of the characteristics of the ideal anesthetic in that depth of anesthesia can be changed rapidly, induction and emergence are rapid (with some agents), and there are few toxic concerns. These agents do not reliably provide muscle relaxation. Neuromuscular blockers and other adjuvant drugs are often titrated to create the desired anesthetic state and to prevent potent agent overdose. Neuromuscular blockers are discussed in Chapter 18. Their use in anesthesia includes facilitation of tracheal intubation (often a short-acting agent) and surgical relaxation, necessary for intrathoracic, intraabdominal, and other procedures. Pharmacologic reversal of long-acting neuromuscular blocking agents is also discussed in Chapter 18.
Because volatile anesthetics provide little analgesia, narcotic and nonnarcotic analgesics are often a part of the anesthetic mixture. Analgesics may reduce the amount of volatile agent necessary to achieve anesthesia. Induction of general anesthesia is usually facilitated by a rapidly effective sedative-hypnotic agent, although inhalation induction with a newer volatile agent (sevoflurane) is rapid and not unpleasant. Table 20-14 lists commonly used anesthetic induction agents.
TABLE 20-14
| CLASS | GENERIC DRUG | BRAND NAME |
| Barbiturates | Methohexital | Brevital |
| Benzodiazepines | Diazepam | Valium |
| Lorazepam | Ativan | |
| Midazolam | Versed | |
| Miscellaneous agents | Etomidate | Amidate |
| Ketamine | Ketalar | |
| Propofol | Diprivan | |
| Fospropofol disodium | Lusedra |
Conscious sedation
Institutional standards for safe and effective provision of conscious sedation are required by the Joint Commission on Accreditation of Healthcare Organizations and other regulatory agencies. These standards must be adhered to throughout the institution, whether sedation is provided by a nurse, respiratory therapist (RT), anesthesiologist, or other health care provider. Many concerned groups have developed guidelines for providing safe conscious sedation. RTs should understand sedative and analgesic pharmacology and may actively participate in provision of conscious sedation.31–33 Because most of the serious complications of conscious sedation relate to airway compromise, RTs are uniquely qualified to safeguard patients and improve outcomes during conscious sedation.
Standards for providing conscious sedation
Most guidelines for conscious sedation and many clinical reports differentiate several levels of sedation. Often a clear distinction is drawn between conscious and deep sedation.34 However, the progression from conscious sedation to deep sedation to general anesthesia is difficult to control clinically, and each deeper level implies increased risks and mandates more intensive monitoring and an increased level of support. The definitions of these states and suggested requirements for monitoring are given in Table 20-15.
TABLE 20-15
Levels of Sedation and Recommendations for Monitoring
| LEVEL OF SEDATION | DEFINITION | SUGGESTED MONITORS |
| Conscious sedation | Minimally depressed level of consciousness, retaining patient’s ability to maintain airway independently and continuously and to respond to physical stimulation and verbal commands |
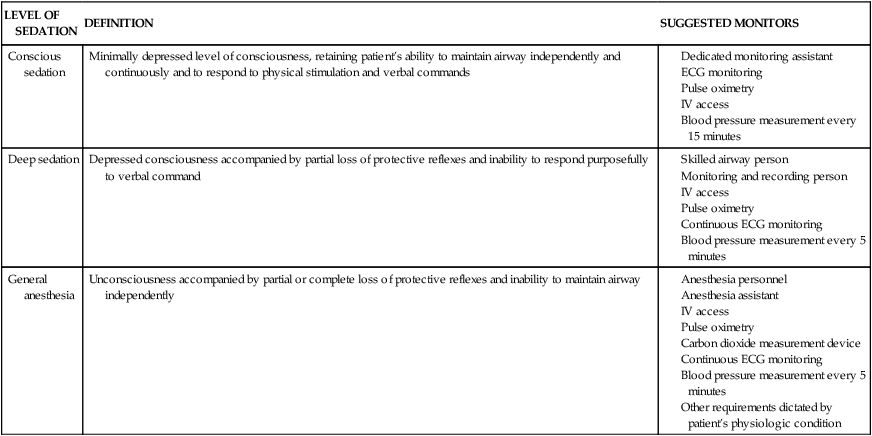
ECG, Electroencephalogram; IV, intravenous.
All published conscious sedation standards insist on the presence of more than one person during the period of sedation (at least the operator and a monitoring assistant). Several guidelines suggest that deep sedation and general anesthesia are indistinguishable and that at least three qualified people must be continually present during the sedation period.35 The standards also suggest that one person must have, as sole responsibility, continual monitoring of the patient and recording of vital signs. When providing conscious sedation, it is necessary to assess continuously and ensure oxygenation, ventilation, and temperature maintenance.
Although some conscious sedation guidelines suggest how to monitor these vital functions, the decision to use a particular device and frequency of repeated observations is left to the responsible clinician.36 What is not left to the discretion of the clinician is the number of personnel necessary and that they must be specially qualified and assigned only to monitor one patient’s vital functions and the progress of sedation. Resuscitation equipment must be immediately available with individuals trained to use it. Competency in providing conscious sedation requires a didactic understanding of the pharmacology of the drugs discussed in this chapter and a performance-based competency including intravenous therapy, monitor use, and supervised clinical practice.37
Central nervous system and respiratory stimulants
In contrast to most of the sedative drugs discussed in this chapter, some drugs can increase activity of the brain rather than depress it. Such drugs are termed analeptic drugs. If the effects are primarily on the respiratory center, the agent may be a respiratory or ventilatory stimulant. Stimulant drugs are used for treatment of narcolepsy, attention-deficit hyperactivity disorder (ADHD), obesity, and, to a lesser extent, respiratory failure. Some of these drugs are listed in Table 20-16. Most stimulant drugs are sympathomimetics, acting directly on α and β receptors. Their abuse potential is great, and their side effects are predictable. They interfere with sleep and are used (and abused) to promote wakefulness and weight loss.
TABLE 20-16
Central and Peripheral Nervous System–Stimulating Drugs
| CLASS | USE | GENERIC DRUG | BRAND NAME |
| Sympathomimetics | Diet | Benzphetamine | Didrex |
| Diethylpropion | Tenuate | ||
| Phendimetrazine | Bontril | ||
| Phentermine | Adipex-P | ||
| Sibutramine | Meridia | ||
| Diet and CNS stimulant | Amphetamine | Adderall | |
| Diet and CNS stimulant | Methamphetamine | Desoxyn | |
| CNS stimulant | Dextroamphetamine | Dexedrine | |
| Xanthines | CNS stimulant | Aminophylline | |
| Caffeine | |||
| Progestational agent | CNS stimulant | Medroxyprogesterone acetate | Provera |
| Respiratory stimulant | Peripheral chemoreceptor stimulant | Doxapram | Dopram |
| Miscellaneous | ADHD | Dexmethylphenidate | Focalin |
| Methylphenidate | Ritalin, Methylin, Concerta, Daytrana |
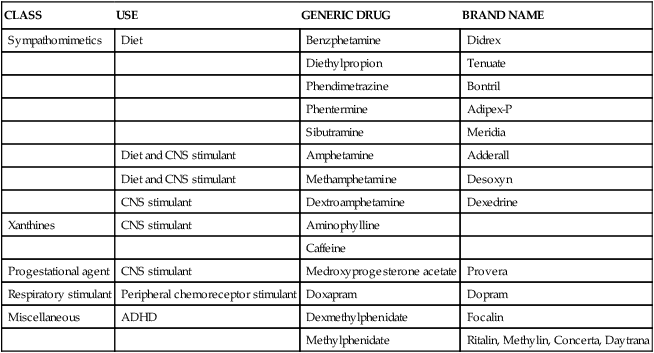
ADHD, Attention-deficit hyperactivity disorder; CNS, central nervous system.