Drugs Acting on the Respiratory System
DRUGS AFFECTING AIRWAY CALIBRE
Airway smooth muscle tone results from a balance between opposing sympathetic and parasympathetic influences (Fig. 9.1). Sympathetic activity causes bronchodilatation while cholinergic parasympathetic activity from the vagus nerve causes bronchoconstriction. Drugs which increase sympathetic influence or decrease cholinergic parasympathetic activity generally cause bronchodilatation by relaxation of airway smooth muscle, and so may be used in the management of asthma and chronic obstructive pulmonary disease (COPD). Sympathetic control is mediated at a cellular level by β2-receptors. Agonists such as adrenaline that bind to these G-protein coupled receptors (Gs) stimulate adenylate cyclase. This enzyme catalyses the conversion, within the cell, of adenosine triphosphate (ATP) to cyclic adenosine monophosphate (cAMP). Through kinase enzyme systems, cAMP relaxes airway smooth muscle. Cyclic AMP is degraded to inactive 5′-AMP by the enzyme phosphodiesterase. Drugs that increase the concentration of cAMP within the cell relax airway smooth muscle (e.g. β2-agonists, phosphodiesterase inhibitors). Conversely, drugs which reduce the level of cAMP (e.g. β2-antagonists) may cause bronchoconstriction.
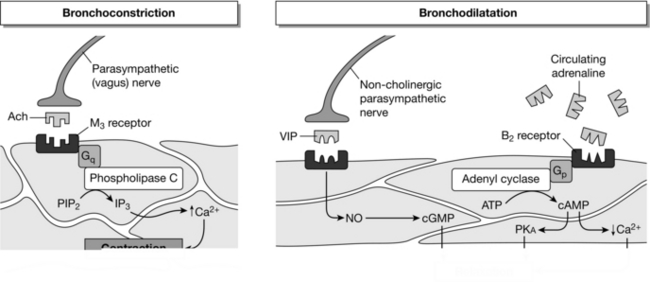
FIGURE 9.1 Summary of the three physiological systems that control airway smooth muscle contraction. See text for details. ACh, acetylcholine; Gq and Gs, G-proteins; PIP2, phosphatidylinositol biphosphate; IP3, inositol triphosphate; VIP, vasoactive intestinal peptide; NO, nitric oxide; cGMP, cyclic guanosine monophosphate; ATP, adenosine triphosphate; PKA, phosphokinase; cAMP, cyclic adenosine monophosphate.
Bronchodilators
β-Adrenergic Agonists
β-Adrenergic agonists are usually the first-line treatment for relieving bronchospasm in asthma and COPD. These drugs have additional beneficial effects in the management of asthma (Table 9.1). There are at least three subtypes of β-receptor in the body: β1 is found in cardiac tissue, β2 in pulmonary tissue and peripheral vasculature and β3 in adipose tissue. Although non- selective β-agonists such as adrenaline and ephedrine can be used as bronchodilators, their unwanted β1 action (e.g. tachycardia) has meant that β2-specific agonists are preferred. Salbutamol, a synthetic sympathomimetic amine, is the most commonly used selective β2-agonist. Although developed as a selective β2-agonist, salbutamol may have β1 side-effects in high doses or in the presence of hypoxaemia or hypercapnia. Salbutamol is a short-acting bronchodilator with a fast onset of action used for the relief of acute symptoms.
TABLE 9.1
Effects of β-Agonist Drugs on the Airways
Specific
Increase in intracellular cAMP and bronchodilatation
Non-Specific but Complementary
Inhibition of mast cell mediator release
Inhibition of plasma exudation and microvascular leakage
Prevention of airway oedema
Increased mucous secretion
Increased mucociliary clearance
Prevention of tissue damage mediated by oxygen free radicals
Decreased acetylcholine release in cholinergic nerves by an action on prejunctional β2-receptors
Route of Administration and Dose: Inhalation is usually the most appropriate route of administration of β2-agonists in order to minimize systemic side-effects. An inhaled drug may also be more effective, because it easily reaches the mast and epithelial cells of the airway which are relatively inaccessible to a drug administered systemically. Salbutamol is administered from a pressurized aerosol (100 μg per puff; 1 or 2 puffs four times daily). The effect lasts for 4–6 h. The drug may also be nebulized in inspired gases and inhaled via a face mask or added to the breathing system in patients undergoing artificial ventilation. For this purpose, a dose of 2.5–5 mg up to four times daily is used. In severe bronchospasm, up to 5 mg may be given as frequently as every 30 min initially. Side-effects are more likely when these drugs are nebulized as they deliver a larger dose of which a significant proportion is absorbed systemically.
Adverse Effects: Adverse effects of β-agonists include the following:
 tachycardia/tachyarrhythmias (β1 effect)
tachycardia/tachyarrhythmias (β1 effect)
 decreased peripheral vascular resistance and postural hypotension (β2 effect)
decreased peripheral vascular resistance and postural hypotension (β2 effect)
 muscle tremor – resulting from a direct effect on β2-receptors in skeletal muscle
muscle tremor – resulting from a direct effect on β2-receptors in skeletal muscle
 hypokalaemia caused by increased uptake of potassium ions by skeletal muscles (β2 effect)
hypokalaemia caused by increased uptake of potassium ions by skeletal muscles (β2 effect)
 metabolic effects – increases in the plasma concentrations of free fatty acids, insulin, glucose, pyruvate and lactate (β3 effects)
metabolic effects – increases in the plasma concentrations of free fatty acids, insulin, glucose, pyruvate and lactate (β3 effects)
Anticholinergic Drugs
Indications: Ipratropium is used as a second-line bronchodilator in acute exacerbations of asthma and COPD. It has an additive effect when used in combination with β-agonists. It is particularly effective in older patients with COPD. Tiotropium is prescribed to COPD patients with the aim of decreasing frequency of exacerbations; it is not useful in the treatment of acute bronchospasm.
Route of Administration and Dose: Ipratropium can be delivered from a metered dose inhaler (20–40 μg) or as a nebulizer (250–500 μg) up to four times daily. Tiotropium is delivered as a dry powder by a ‘Spiriva HandiHaler’ device; the dose is 18 μg once daily.
Adverse Effects: Dry mouth is the most commonly reported adverse effect of the antimuscarinic bronchodilators. Other less frequent side-effects include nausea, constipation and palpitations. The drugs are well known to precipitate acute urinary retention and so should be used with caution in patients with benign prostatic hypertrophy. They may also cause acute angle-closure glaucoma, particularly when given as a nebulizer (accidental instillation into the eye) with salbutamol.
Methylxanthines
 phosphodiesterase inhibition, leading to increased intracellular cAMP
phosphodiesterase inhibition, leading to increased intracellular cAMP
 adenosine receptor antagonism, preventing mast cell degranulation
adenosine receptor antagonism, preventing mast cell degranulation
 endogenous catecholamine release
endogenous catecholamine release
Indications: Methylxanthines are usually prescribed when inhaled therapies have failed or have been only partly effective. They are particularly useful in COPD. Recently, they have been shown to improve exercise tolerance in intensive care patients on a weaning programme.
Route of Administration and Dose: Theophylline may be used as a sustained release oral preparation for the prevention of acute exacerbations of COPD. The dose depends on the preparation being used and is given twice daily. Aminophylline is an intravenous preparation used for the relief of acute episodes of bronchospasm. It is a strong alkaline solution and should not be given intramuscularly or subcutaneously. A loading dose of 5 mg kg–1 should be given slowly over 20 min followed by an infusion of 0.5-0.7 mg kg–1 h–1. If the patient is already taking an oral theophylline, then the loading dose should be omitted.
There is a close relationship between the degree of bronchial dilatation and the plasma concentration of theophylline. A concentration of < 10 mg L–1 is associated with a mild effect and a concentration of > 25 mg L–1 with frequent side-effects. Consequently, the therapeutic window is narrow and the plasma concentration should be maintained within the range 10–20 mg L–1 (55–110 μmol L–1). Plasma assays should be performed 6 h after commencing an infusion or following a rate change and then every 24 h. Approximately 40% of the drug is protein-bound. Theophylline is metabolized mainly in the liver by cytochrome P450 microenzymes; 10% is excreted unchanged in urine. Factors which affect the activity of hepatic enzymes and thus the clearance of the drug are summarized in Table 9.2. The infusion rate of aminophylline should be adjusted accordingly (e.g. 1.6 times for smokers, 0.5 times for patients receiving erythromycin). The dose for obese patients should be based on ideal body weight and frequent estimation of plasma concentration is required to prevent ineffective therapy or toxicity.
TABLE 9.2
Factors Affecting the Plasma Concentration of Methylxanthines for a Given Dose
Factors Which Lower the Plasma Concentration:
Children
Smoking
Enzyme induction – rifampicin, chronic ethanol use, phenytoin, carbamazepine, barbiturates
High protein diet
Low carbohydrate diet
Factors Which Increase the Plasma Concentration:
Old age
Congestive heart failure
Enzyme inhibition – erythromycin, omeprazole, valproate, isoniazid, ciprofloxacin
High carbohydrate diet
Adverse Effects: Adverse effects of methylxanthines may be severe and are more likely to occur in patients also receiving a β-agonist bronchodilator or other sympathomimetic drugs.
 Central nervous system effects: stimulation of the CNS may lead to nausea, restlessness, agitation, insomnia, tremor and seizures. Some CNS effects (e.g. tremor) may occur even with therapeutic plasma concentrations of the drug.
Central nervous system effects: stimulation of the CNS may lead to nausea, restlessness, agitation, insomnia, tremor and seizures. Some CNS effects (e.g. tremor) may occur even with therapeutic plasma concentrations of the drug.
 Cardiovascular effects: methylxanthines have positive chronotropic and inotropic effects on the heart. Tachyarrhythmias may occur with therapeutic doses, especially in the presence of halothane.
Cardiovascular effects: methylxanthines have positive chronotropic and inotropic effects on the heart. Tachyarrhythmias may occur with therapeutic doses, especially in the presence of halothane.
 Renal effects: methylxanthines cause increased urine output which may be related to an effect on tubular function or may be an indirect effect of the increased cardiac output. They may also lead to hypokalaemia.
Renal effects: methylxanthines cause increased urine output which may be related to an effect on tubular function or may be an indirect effect of the increased cardiac output. They may also lead to hypokalaemia.
 Miscellaneous effects: methylxanthines are known to increase gastric acid secretion and promote gastro-oesophageal reflux.
Miscellaneous effects: methylxanthines are known to increase gastric acid secretion and promote gastro-oesophageal reflux.
Anti-Inflammatory Agents
Steroids are used commonly as anti-inflammatory agents. Synthetic glucocorticoids have metabolic, anti-inflammatory and immunosuppressive effects (Table 9.3). They are able to penetrate the cell membrane and interact with intracellular steroid receptors, influencing transcription of genes and protein synthesis. Steroid administration leads to reduced numbers of lymphocytes and eosinophils, reduced secretion of prostaglandins from macrophages and a reduction in endothelial permeability. Steroids also increase the sensitivity of β2-adrenoceptors to both endogenous and inhaled agonists and help prevent tachyphylaxis to adrenoceptor agonists such as salbutamol. There is some evidence that high-dose inhaled steroids help to slow the airway remodelling that results from chronic inflammation in asthma. Remodelling consists of airway smooth muscle hypertrophy, excess mucous glands and thickening of the basement membrane. It is thought to be one cause for the airway hyper-responsiveness that is characteristic of asthma.
TABLE 9.3
Sites of Action for the Anti-Inflammatory Effects of Steroids
| Site of Action | Mechanism |
| Intracellular steroid receptors | Steroid–receptor complex alters the transcription of genes leading to altered protein synthesis |
| Lipocortin | Increased lipocortin production inhibits release of arachidonic acid metabolites and platelet-activating factor from lung and macrophages |
| Eosinophils | Decrease in the number of eosinophils and inhibition of degranulation |
| T lymphocytes | Reduction in the number of T lymphocytes and the production of cytokines |
| Macrophages | Reduced secretion of leukotrienes and prostaglandin |
| Endothelial cells | Reduction of the leak between cells |
| Airway smooth muscle β2-adrenoceptors |
Increased agonist sensitivity, augmenting the effect of agonists; increase in receptor density and prevention of tachyphylaxis |
| Mucous glands | Reduced mucous secretion |
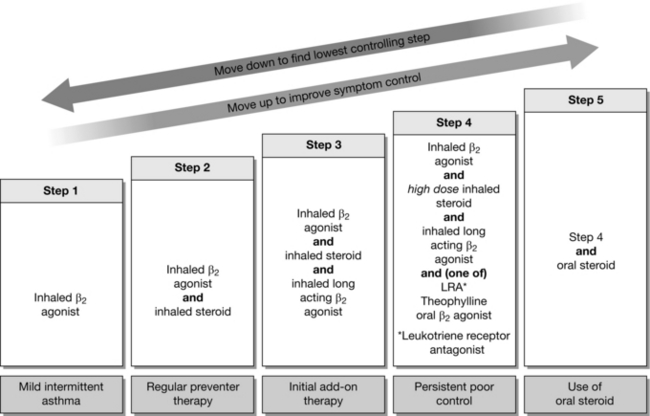
Indications: Inhaled steroids are used commonly in the management of chronic asthma to reduce the frequency of attacks. They are usually considered when patients require a β2-agonist more than twice a week, if symptoms disturb sleep more than once a week or if the patient has suffered exacerbations in the last 2 years which required a systemic corticosteroid. The addition of an inhaled steroid constitutes ‘Step 2’ of the British Thoracic Society (BTS) guidelines for the management of asthma (Fig. 9.2). Some patients with COPD may have steroid-responsive disease. Inhaled steroids are unlicensed for COPD but may be considered in patients with an FEV1 < 50% of predicted, or for patients having more than two acute exacerbations requiring oral steroids per year. Oral and intravenous steroids are useful in the management of acute asthma or COPD. They may also have a role in the treatment of sarcoidosis, interstitial lung disease and pulmonary eosinophilia.
Route of Administration and Dose: The three most commonly used inhaled steroids are shown in Table 9.4. Standard doses are those used in Step 2 of the BTS guidelines. If symptom control is still poor on Step 3 of the protocol (steroid + long acting β2-receptor agonist) then high-dose inhaled steroid constitutes Step 4.
TABLE 9.4
Doses of Inhaled Steroids. Step 2 and Step 4 Refer to British Thoracic Society Asthma Guidelines (Fig. 9.2)
| Steroid | Standard (Step 2) Dose | High (Step 4) Dose |
| Beclometasone dipropionate | 100–400 μg twice daily | 0.4–1.0 mg twice daily |
| Fluticasone dipropionate | 50–200 μg twice daily | 200–500 μg twice daily |
| Mometasone furoate | 200 μg twice daily | 400 μg twice daily |
Adverse Effects: Steroids have many local and systemic side-effects which commonly limit their use. Inhaled drugs are less likely than oral or intravenous steroids to cause systemic side-effects but may cause local irritation and predisposition to oral candidiasis. Some of the recognized side-effects of steroids are listed in Table 9.5.
TABLE 9.5
Common Adverse Effects of Steroids
Local Effects (Inhaled Steroids)
Hoarse voice
Oral/pharyngeal candidiasis
Throat irritation and cough
Systemic Effects (from Inhaled or Systemic Steroids)
Adrenergic suppression
Fluid retention
Hypertension
Peptic ulceration
Diabetes mellitus
Increased appetite
Weight gain
Bruising and skin thinning
Osteoporosis
Cataracts
Psychosis
Other Drugs
Leukotriene Receptor Antagonists
The leukotriene receptor antagonists are an additional therapy useful in the management of chronic asthma. They may be used in addition to, or in place of, a long acting β2-receptor agonist such as salmeterol in Step 3 of the BTS guidelines (Fig. 9.2). There are two drugs in this class in common clinical use: montelukast and zafirlukast. They may be particularly beneficial in exercise-induced asthma and may also be used in patients with seasonal allergic rhinitis who have concomitant asthma. Two oral drugs in this class are prescribed commonly; montelukast 10 mg is given once a day, usually in the evening, or zafirlukast 20 mg can be given twice a day. The most common adverse effects are gastrointestinal upset and abdominal pain, with headache and insomnia also reported. These drugs have been associated rarely with the development of Churg-Strauss vasculitis and patients should be told to report any rash or worsening of pulmonary symptoms.
DRUGS ACTING ON THE PULMONARY VASCULATURE
Inhaled Agents
Indications: Inhaled nitric oxide may be used in the management of critically ill patients with pulmonary hypertension or severe ![]() mismatch. It may also form part of the management of persistent pulmonary hypertension of the newborn. Given as an inhaled preparation, it only vasodilates vessels close to well ventilated areas, thereby improving
mismatch. It may also form part of the management of persistent pulmonary hypertension of the newborn. Given as an inhaled preparation, it only vasodilates vessels close to well ventilated areas, thereby improving ![]() matching (Fig. 9.3). It has negligible systemic effects because of its rapid inactivation by haemoglobin after diffusing into the pulmonary circulation.
matching (Fig. 9.3). It has negligible systemic effects because of its rapid inactivation by haemoglobin after diffusing into the pulmonary circulation.
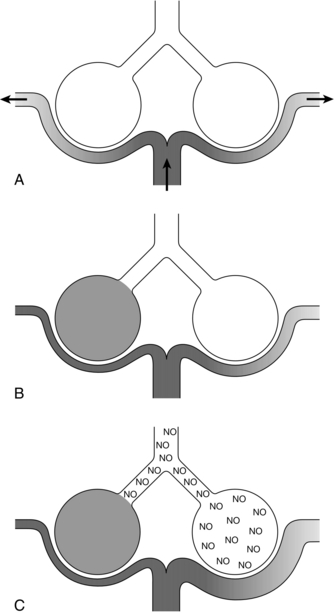
FIGURE 9.3 Schematic diagram of two alveoli representing regions of lung with different ![]() ratios. (A) Normal situation – all areas ventilated and equally perfused. (B) A region of lung is not ventilated, e.g. due to pulmonary oedema. Hypoxic pulmonary vasoconstriction reduces blood flow through this lung region; blood flow to the area which is still ventilated is unchanged, and pulmonary vascular resistance may increase. (C) Inhaled nitric oxide (NO) therapy – the NO reaches blood vessels only in the ventilated areas of lung and so vasodilates only these areas, resulting in better matching of ventilation and perfusion and reduced pulmonary vascular resistance.
ratios. (A) Normal situation – all areas ventilated and equally perfused. (B) A region of lung is not ventilated, e.g. due to pulmonary oedema. Hypoxic pulmonary vasoconstriction reduces blood flow through this lung region; blood flow to the area which is still ventilated is unchanged, and pulmonary vascular resistance may increase. (C) Inhaled nitric oxide (NO) therapy – the NO reaches blood vessels only in the ventilated areas of lung and so vasodilates only these areas, resulting in better matching of ventilation and perfusion and reduced pulmonary vascular resistance.
Route of Administration and Dose: NO in nitrogen is available in concentrations between 400 and 1000 ppm. It is usually administered in doses between 20 and 80 ppm. Most studies suggest that the maximum effect occurs at the lower end of this dose range and that higher doses risk toxicity. NO can be administered safely only to a patient whose trachea is intubated and whose lungs are ventilated using a closed circuit system. The usual apparatus for delivery involves placing an injector device in the inspiratory limb between the gas outlet of the ventilator and the humidifier. The device detects gas flow going to the patient and injects an appropriate quantity of NO to achieve the desired inspired concentration.
Adverse Effects: One of the risks of NO therapy is its oxidation to nitrogen dioxide (NO2). The rate of oxidation is directly proportional to the concentration of oxygen in the gas mixture, duration of mixing and the square of the concentration of NO. These higher oxides may react with water to form nitric and nitrous acids which can lead to lipid peroxidation, impaired mitochondrial function, prolonged bleeding time and mutagenesis. Therefore all delivery systems should measure levels of NO2. NO therapy, especially in the newborn, may lead to methaemoglobinaemia. All patients receiving NO should have the methaemoglobin concentration monitored by arterial blood gas analysis; concentrations greater than 2% should be avoided. Some patients may display a rebound phenomenon on withdrawal of NO therapy, with worsening hypoxaemia and increased pulmonary vascular resistance. For this reason, NO therapy should be reduced gradually to allow endogenous production to restart.
DRUGS MODERATING VENTILATORY CONTROL
Doxapram
Indications: Doxapram is used mostly to reverse postoperative respiratory depression following general anaesthesia. It may rarely be used in the management of acute respiratory failure or exacerbations of COPD. It is also used off-licence in the treatment of neonatal apnoea.
Route of Administration and Dose: When reversing postoperative respiratory depression, a dose of 1–1.5 mg kg− 1 is given intravenously over 30 s and repeated if necessary after an hour. In exceptional cases, an intravenous infusion may be required. Up to 4 mg kg− 1 may be used in the treatment of acute respiratory failure, alongside oxygen therapy and frequent blood gas monitoring.
Adverse Effects: Intravenous injection of doxapram may cause perianal warmth, dizziness and sweating. The drug can lead to hypertension and tachycardia. It may rarely precipitate muscle fasciculation, laryngospasm, confusion and seizures. Administration should therefore be avoided in patients with uncontrolled hypertension, ischaemic heart disease or epilepsy. There is no role for doxapram in the management of laryngospasm or other causes of complete mechanical airway obstruction.
OXYGEN THERAPY
Indications
 reduced inspired partial pressure of oxygen, e.g. altitude
reduced inspired partial pressure of oxygen, e.g. altitude
 hypoventilation, e.g. caused by narcotics
hypoventilation, e.g. caused by narcotics
 diffusion impairment, e.g. pulmonary oedema
diffusion impairment, e.g. pulmonary oedema
 ventilation/perfusion mismatch or shunt, e.g. pulmonary embolus
ventilation/perfusion mismatch or shunt, e.g. pulmonary embolus
Acute Oxygen Therapy
Low-Concentration Oxygen Therapy
Low-concentration or controlled oxygen therapy is reserved for patients at risk of type 2 (hypercapnic) respiratory failure who may be harmed by uncontrolled high concentrations of oxygen. Patients with severe COPD, bronchiectasis, cystic fibrosis, severe kyphoscoliosis or ankylosing spondylitis are included in this group, as are patients with chronic musculoskeletal weakness on home ventilation therapy. Each case must be considered in the light of clinical findings but, as a general rule, oxygen therapy should be started at 24 or 28% and gradually titrated against oxygen saturation (SpO2) or preferably arterial oxygen tension. An SpO2 of 88–92% is often acceptable in these patients and may avoid pronounced hypercapnia and respiratory arrest. Patients are advised to carry an ‘oxygen alert card’ (Fig. 9.4) which, together with previous blood gas analysis results, may help to guide therapy.
DRUGS AFFECTING MUCOCILIARY FUNCTION
The mucous found in the airways is a non-homogeneous viscoelastic substance consisting of two phases separated by a thin film of surfactant. A superficial gel-like layer lies on a more liquid or aqueous layer in contact with the epithelial cells. Secretions from goblet cells and bronchial glands maintain the normal airway surface liquid (ASL). Goblet cells secrete mucopolysaccharides which form the gel-like layer and are stimulated by irritant factors. Epithelial cells and bronchial glands secrete the low-viscosity aqueous layer and are under vagal control. Two mechanisms exist to clear mucous from the respiratory tract: mucociliary clearance and cough clearance. The former is the dominant mechanism in health and relies on the forward propulsion of the gel layer on the aqueous layer by epithelial cell cilia. The efficiency of mucociliary clearance is affected by ciliary and ASL factors. Ciliary factors include beat frequency, amplitude and cilia spacing, while ASL factors include the depth of both layers of ASL, and the elasticity of the gel layer. Optimal aqueous layer depth is essential to ensure that the tips of the cilia interact with the gel layer only on their forward stroke. Cough clearance becomes more important in various disease states associated with impaired mucociliary clearance such as cystic fibrosis. A high-velocity interaction at the air–mucous layer boundary causes wave formation in the mucous layer, leading to forward propulsion. Many physical factors influence mucociliary function (Table 9.6) and several pharmacological agents have been developed to optimise sputum clearance.
TABLE 9.6
Factors Affecting Mucociliary Function
Factors that Depress Mucociliary Function:
Extremes of temperature
Acidic environment
Smoking
Dehydration
Alcohol
Anaesthetics
Dry Gases
Factors that Optimize Mucociliary Function:
Temperature range 29–34 °C
Hydration
Humidification