Chapter 17 Drug therapy and poisoning
Drug therapy
The patient
 the symptomatic treatment of severe pain
the symptomatic treatment of severe pain
 the initiation of ‘blind’ antimicrobial therapy where delay would expose a patient to hazard or discomfort (e.g. antimicrobial therapy for a patient with a suspected lower urinary tract infection).
the initiation of ‘blind’ antimicrobial therapy where delay would expose a patient to hazard or discomfort (e.g. antimicrobial therapy for a patient with a suspected lower urinary tract infection).
 Immunization against serious microbial infections (e.g. influenza vaccination)
Immunization against serious microbial infections (e.g. influenza vaccination)
 The reduction of individual risk factors to prevent later disease (e.g. the use of antihypertensive, or lipid-lowering, agents, to reduce the chances of ischaemic heart disease and stroke)
The reduction of individual risk factors to prevent later disease (e.g. the use of antihypertensive, or lipid-lowering, agents, to reduce the chances of ischaemic heart disease and stroke)
 Oral contraceptives in sexually active women wishing to avoid pregnancy.
Oral contraceptives in sexually active women wishing to avoid pregnancy.
Co-morbidity may also significantly alter the way in which conditions are treated particularly in the elderly. Some examples are shown in Table 17.1.
Table 17.1 Examples of drugs to be avoided in people with co-morbidity
| Co-morbidity | Avoid | Effect |
|---|---|---|
|
Parkinson’s disease |
Neuroleptics |
Exacerbates Parkinsonian symptoms (including tremor) |
|
Hypertension |
Non-steroidal anti-inflammatory drugs |
Sodium retention |
|
Asthma |
Beta-blockers, adenosine |
Bronchospasm |
|
Respiratory failure |
Morphine, diamorphine |
Respiratory depression |
|
Renovascular disease |
ACE inhibitors/antagonists |
Reduction in glomerular filtration |
|
Chronic heart failure |
Trastuzumab |
Worsening of heart failure |
|
Chronic infections (e.g. tuberculosis, hepatitis C, histoplasmosis |
Cytokine modulators (e.g. etanercept) |
Increased risk of exacerbation |
Prescribing in neonates, infants, children and adolescents
 The rates of hepatic metabolism and renal excretion of drugs are reduced in neonates and infants.
The rates of hepatic metabolism and renal excretion of drugs are reduced in neonates and infants.
 Premature babies have approximately 1% of their body weight as fat (compared with 20% in adults), leading to a marked increase in plasma drug levels of fat-soluble drugs.
Premature babies have approximately 1% of their body weight as fat (compared with 20% in adults), leading to a marked increase in plasma drug levels of fat-soluble drugs.
There are other difficulties in prescribing for children:
 Many treatments have never been subject to formal trials in either children or adolescents and their benefits and risks have not, therefore, been appropriately assessed in these age groups. Efforts are being made, internationally, to redress this.
Many treatments have never been subject to formal trials in either children or adolescents and their benefits and risks have not, therefore, been appropriately assessed in these age groups. Efforts are being made, internationally, to redress this.
 For many drugs, there are no paediatric preparations or formulations. Instead, adult products are used.
For many drugs, there are no paediatric preparations or formulations. Instead, adult products are used.
 Precise oral dosing is often impossible in babies who spit out unpleasant-tasting products!
Precise oral dosing is often impossible in babies who spit out unpleasant-tasting products!
 Adverse effect profiles of medicines may be different in children compared with adults (e.g. Reye’s syndrome in children given aspirin, suicidal ideas in depressed adolescents treated with selective serotonin reuptake inhibitors).
Adverse effect profiles of medicines may be different in children compared with adults (e.g. Reye’s syndrome in children given aspirin, suicidal ideas in depressed adolescents treated with selective serotonin reuptake inhibitors).
Prescribing for the elderly
The use of drugs in the elderly is often a problem:
 Rates of hepatic drug metabolism and renal excretion decline with age. Extrapolation of drug dosages, from those appropriate in younger adults, may therefore lead to toxic plasma levels.
Rates of hepatic drug metabolism and renal excretion decline with age. Extrapolation of drug dosages, from those appropriate in younger adults, may therefore lead to toxic plasma levels.
 Changes in drug distribution due to changes in body composition, and the preferential distribution of the cardiac output to the brain, may also predispose to toxicity.
Changes in drug distribution due to changes in body composition, and the preferential distribution of the cardiac output to the brain, may also predispose to toxicity.
 Co-morbidity, often associated with polypharmacy, leads to increased opportunities for disease–drug and drug–drug interactions.
Co-morbidity, often associated with polypharmacy, leads to increased opportunities for disease–drug and drug–drug interactions.
 Concordance with treatment regimens diminishes as the number of prescribed drugs increases, and is especially poor in the face of cognitive impairment.
Concordance with treatment regimens diminishes as the number of prescribed drugs increases, and is especially poor in the face of cognitive impairment.
 Exaggerated pharmacodynamic effects of drugs acting on the central nervous, cardiovascular and gastrointestinal systems are common.
Exaggerated pharmacodynamic effects of drugs acting on the central nervous, cardiovascular and gastrointestinal systems are common.
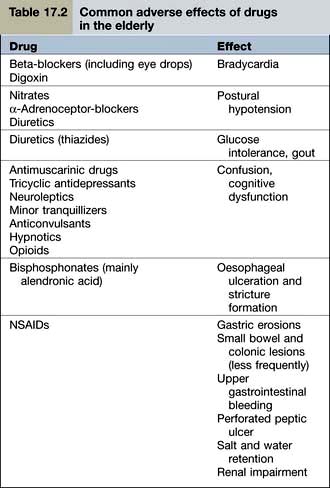
Examples of common problems encountered in the use of drugs amongst older people are shown in Table 17.2.
Drug use in pregnancy
Clinicians should be extremely cautious about prescribing drugs to pregnant women, and only essential treatments should be given. When a known teratogen is needed during pregnancy (e.g. an anticonvulsant drug or lithium), the potential adverse effects should be discussed with the parents, preferably before conception. If parents wish to go ahead with the pregnancy, they should be offered an appropriate ultrasound scan to assess whether there is any fetal damage. Some known human teratogens are shown in Table 17.3.
| Drug | Effect |
|---|---|
|
ACE inhibitors/antagonists |
Oligohydramnios |
|
Retinoids, e.g. acitretin |
Multiple abnormalities |
|
Carbimazole |
Neonatal hypothyroidism |
|
|
Abnormalities of bone growth |
|
Antiepileptics |
|
|
Carbamazepine |
Cleft palate |
|
Lamotrigine |
|
|
Phenytoin |
|
|
Valproate |
Neural tube defects |
|
NSAIDs |
Delayed closure of the ductus arteriosus |
|
Cytotoxic drugs |
Most are presumed teratogens |
|
Lithium |
Ebstein’s anomaly |
|
Misoprostol |
Moebius’s syndrome |
|
Thalidomide (and possibly lenalidomide) |
Phocomelia |
Note: All drugs should be avoided in pregnancy unless benefit clearly outweighs the risk.
The drug
Selecting the right drug involves three elements:
 The drug’s clinical efficacy for the proposed use
The drug’s clinical efficacy for the proposed use
The most common approach to assessing a drug’s efficacy is the randomized controlled trial (RCT), although other approaches (see p. 907) can be informative. The demonstration of absolute efficacy (against placebo) may, itself, be insufficient. Where there is more than one treatment for the same indication these should be compared with one another, taking account of the magnitude of their benefits, their individual adverse reaction profiles, and their costs.
The dose
Fixed dosage regimens
Drugs suitable (in adults) for prescribing at the same ‘fixed’ dose, for all patients, share common features. Efficacy is optimal in virtually all patients; and the risks of dose-related (type A) adverse reactions (see p. 904) are normally low. These drugs have a high ‘therapeutic ratio’ (i.e. the ratio between toxic and therapeutic doses). Examples of drugs prescribed at a fixed dose are shown in Table 17.4.
| Drug | Indication |
|---|---|
|
Aspirin |
Secondary prevention of myocardial infarction |
|
Clopidogrel |
|
|
Bendroflumethiazide |
Hypertension |
|
Broad spectrum penicillins |
Lower urinary tract infection |
|
Cephalosporins |
|
|
Macrolides |
Upper and lower respiratory tract infection |
|
Levonorgestrel |
Emergency contraception |
|
Ulipristal |
|
|
Oestrogen antagonists |
Secondary prevention of breast cancer |
|
Aromatase inhibitors |
|
|
Vaccines |
e.g. Diphtheria, pertussis, mumps, measles, rubella, influenza, etc. |
Pharmacokinetics
 destroyed in the gastrointestinal tract
destroyed in the gastrointestinal tract
 able to cross the gastrointestinal epithelium
able to cross the gastrointestinal epithelium
 metabolized by the liver before reaching the systemic circulation (so-called presystemic or ‘first pass’ metabolism). First pass metabolism can be avoided by the intravascular (i.v.), intramuscular (i.m.) or sublingual routes.
metabolized by the liver before reaching the systemic circulation (so-called presystemic or ‘first pass’ metabolism). First pass metabolism can be avoided by the intravascular (i.v.), intramuscular (i.m.) or sublingual routes.
Liver drug metabolism occurs in two stages:
 Phase I is the modification of a drug, by oxidation, reduction or hydrolysis. Of these, oxidation is the most frequent route and is largely undertaken by a family of isoenzymes known as the cytochrome P450 system (see p. 902). Inhibition or induction of cytochrome P450 isoenzymes are major causes of drug interactions (Table 17.5).
Phase I is the modification of a drug, by oxidation, reduction or hydrolysis. Of these, oxidation is the most frequent route and is largely undertaken by a family of isoenzymes known as the cytochrome P450 system (see p. 902). Inhibition or induction of cytochrome P450 isoenzymes are major causes of drug interactions (Table 17.5).
 Phase II involves conjugation with glucuronate, sulphate, acetate or other substances to render the drug more water soluble and therefore able to be excreted in the urine.
Phase II involves conjugation with glucuronate, sulphate, acetate or other substances to render the drug more water soluble and therefore able to be excreted in the urine.
|
Inducers |
Carbamazepine |
|
Hyperforina |
|
|
Nifedipine |
|
|
Non-nucleoside reverse transcriptase inhibitors (NNRTIs) |
|
|
Omeprazole |
|
|
Phenobarbital |
|
|
Phenytoin |
|
|
Rifampicin |
|
|
Ritonavir (see p. 180) |
|
|
Inhibitors |
Allopurinol |
|
Amiodarone |
|
|
Cimetidine |
|
|
Erythromycin, clarithromycin |
|
|
Fluoxetine, paroxetine |
|
|
Grapefruit juice (contains flavonoids) |
|
|
Imidazoles |
|
|
Quinolones |
|
|
Saquinavir |
|
|
Sulphonamides |
a Hyperforin is one of the ingredients of the herbal product known as St John’s wort used by herbalists to treat depression. Although it is marketed as a licensed medicine, it is a reminder that drug interactions can occur with alternative, as well as conventional, medicines.
Genetic causes of altered pharmacokinetics
Variability in the genes that encode drug-metabolizing enzymes (Table 17.6) is a major determinant of the inter-individual differences in the therapeutic and adverse responses to drug treatment. The most common involve polymorphisms of the cytochrome P450 family of enzymes, CYP. The first to be discovered was the polymorphism in the hydroxylation of the antihypertensive agent debrisoquin (CYP2D6). Defective catabolism was shown to be a monogenetically inherited trait, involving 5–10% of Caucasian populations, and leading to an exaggerated hypotensive response.
Table 17.6 Some genetic polymorphisms involving drug metabolism
| Enzyme | Drug |
|---|---|
|
P450 |
|
|
Cytochrome CYP1A2 |
Amitriptyline |
|
Clozapine |
|
|
Cytochrome CYP3A4 |
Amlodipine |
|
Ciclosporin |
|
|
Nifedipine |
|
|
Sildenafil |
|
|
Simvastatin |
|
|
Protease inhibitors |
|
|
Tacrolimus |
|
|
Cytochrome CYP2C9 |
Warfarin |
|
Glipizide |
|
|
Losartan |
|
|
Phenytoin |
|
|
Cytochrome CYP2D6 |
Beta-blockers |
|
Codeine |
|
|
Risperidone |
|
|
SSRIs |
|
|
Tramadol |
|
|
Venlafaxine |
|
|
Cytochrome CYP2C19 |
Clopidogrela |
|
Cyclophosphamide |
|
|
Diazepam |
|
|
Lansoprazole |
|
|
Omeprazole |
|
|
Plasma pseudocholinesterase |
Succinylcholine |
|
Mivacurium |
|
|
Thiopurine methyltransferase |
Azathioprine |
|
Mercaptopurine |
|
|
UDP-glucuronosyl transferase |
Irinotecan |
|
N-acetyl transferase |
Isoniazid |
CYP, cytochrome; SSRIs, Selective serotonin reuptake inhibitors.
a Clopidogrel is a prodrug and impaired metabolizers have a reduced response.
Individual differences in the activity of thiopurine methyltransferase (TPMT) determine the doses of mercaptopurine and azathioprine that are used. TMPT activity is therefore undertaken routinely in children undergoing treatment for acute lymphatic leukaemia and people with Crohn’s disease (see p. 233).
This may, however, become possible in the future, and would contribute – in part – to the prospect of ‘personalized prescribing’ (see p. 899).
Other causes of altered pharmacokinetics
Rates of hepatic drug clearance can also be influenced by environmental factors including diet, alcohol consumption and concomitant therapy with drugs capable of inducing or inhibiting (Table 17.5) drug metabolism. Hepatic drug clearance also decreases with age. By contrast, renal drug clearance does not show substantial variation between healthy individuals although it declines with age and in people with intrinsic renal disease.
Pharmacodynamics
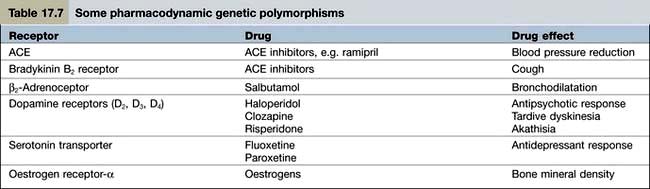
Pharmacodynamics is the study of what the drug does to the body. Pharmacodynamic sources of variability in the intensity of drug action are at least partly due to drug receptor polymorphisms (Table 17.7). At present, the pharmacodynamic tests used in clinical practice to target therapy are largely confined to the expression of:
Monitoring the effects of treatment
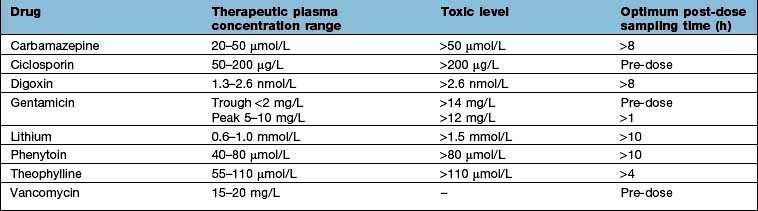
Measuring plasma drug concentrations
For a few drugs, dosages can be effectively monitored by reference to their plasma concentrations (Table 17.8). This technique is only useful, however, if both the following criteria are fulfilled:
Affordability
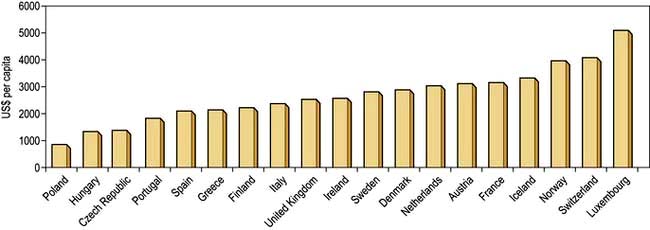
The money available for healthcare varies widely across the world and there are marked differences (Fig. 17.1). All healthcare systems try to provide their populations with the highest standards of care within the resources they have at their disposal. The expenditure of large sums on a few people may deprive many of cost-effective remedies – a phenomenon known as the ‘opportunity cost’. The differences in healthcare expenditure shown in Figure 17.1 can be very largely accounted for by their differences in national wealth as reflected by their gross domestic products.
Adverse drug reactions
Classification
Two types of ADR are recognized.
| Type of reaction and drug | Adverse reaction |
|---|---|
|
Type A (augmented) |
|
|
ACE inhibitors |
Hypotension |
|
ACE antagonists |
Hypotension |
|
Anticoagulants |
Gastrointestinal bleeding |
|
Antipsychotics |
Acute dystonia/dyskinesia |
|
Cytotoxic agents |
Bone marrow dyscrasias |
|
Erythromycin |
Nausea, vomiting |
|
Glucocorticosteroids |
Hypoadrenalism |
|
Insulin |
Hypoglycaemia |
|
Tricyclic antidepressants |
Dry mouth |
|
Type B (bizarre) |
|
|
Benzylpenicillin |
Anaphylaxis |
|
Broad-spectrum penicillins |
Maculopapular rash |
|
Carbamazepine |
Toxic epidermal necrolysis |
|
Carbamazepine |
Hepatotoxicity |
|
Isotretinoin |
Depression |
ACE, angiotensin-converting enzyme; SSRIs, selective serotonin reuptake inhibitors.
a In children and adolescents.
Type B (idiosyncratic) reactions (Table 17.9) have no resemblance to the recognized pharmacological or toxicological effects of the drug. They are:
Diagnosis
 Appropriate time interval. The time interval between the administration of a drug and the suspected adverse reaction should be appropriate. Acute anaphylaxis usually occurs within a few minutes of administration, whilst aplastic anaemia will only become apparent after a few weeks (because of the life-span of erythrocytes). Drug-induced malignancy, however, will take years to develop.
Appropriate time interval. The time interval between the administration of a drug and the suspected adverse reaction should be appropriate. Acute anaphylaxis usually occurs within a few minutes of administration, whilst aplastic anaemia will only become apparent after a few weeks (because of the life-span of erythrocytes). Drug-induced malignancy, however, will take years to develop.
 Nature of the reaction. Some conditions (maculopapular rashes, angio-oedema, fixed drug eruptions, toxic epidermal necrolysis) are so typically iatrogenic that an adverse drug reaction is very likely.
Nature of the reaction. Some conditions (maculopapular rashes, angio-oedema, fixed drug eruptions, toxic epidermal necrolysis) are so typically iatrogenic that an adverse drug reaction is very likely.
 Plausibility. Where an event is a manifestation of the known pharmacological property of the drug, its recognition as a type A adverse drug reaction can be made (e.g. hypotension with an antihypertensive agent, or hypoglycaemia with an antidiabetic drug). Unless there have been previous reports in the literature, the recognition of type B reactions may be very difficult. The first cases of depression with isotretinoin, for example, were difficult to recognize as an ADR even though a causal association is now acknowledged.
Plausibility. Where an event is a manifestation of the known pharmacological property of the drug, its recognition as a type A adverse drug reaction can be made (e.g. hypotension with an antihypertensive agent, or hypoglycaemia with an antidiabetic drug). Unless there have been previous reports in the literature, the recognition of type B reactions may be very difficult. The first cases of depression with isotretinoin, for example, were difficult to recognize as an ADR even though a causal association is now acknowledged.
 Exclusion of other causes. In some instances, particularly suspected hepatotoxicity, an iatrogenic diagnosis can only be made after the exclusion of other causes of disease.
Exclusion of other causes. In some instances, particularly suspected hepatotoxicity, an iatrogenic diagnosis can only be made after the exclusion of other causes of disease.
 Results of laboratory tests. In a few instances, the diagnosis of an adverse reaction can be inferred from the plasma concentration (Table 17.8). Occasionally, an ADR produces diagnostic histopathological features. Examples include putative reactions involving the skin and liver.
Results of laboratory tests. In a few instances, the diagnosis of an adverse reaction can be inferred from the plasma concentration (Table 17.8). Occasionally, an ADR produces diagnostic histopathological features. Examples include putative reactions involving the skin and liver.
 Results of dechallenge and rechallenge. Failure of remission when the drug is withdrawn (i.e. ‘dechallenge’) is unlikely to be an ADR. The diagnostic reliability of dechallenge, however, is not absolute: if the ADR has caused irreversible organ damage (e.g. malignancy) then dechallenge will result in a false-negative response. Rechallenge, involving re-institution of the suspected drug to see if the event recurs, is often regarded as an absolute diagnostic test. This is, in many instances, correct but there are two caveats. First, it is rarely justifiable to subject a patient to further hazard. Second, some adverse drug reactions develop because of particular circumstances which may not necessarily be replicated on rechallenge (e.g. hypoglycaemia with an antidiabetic agent).
Results of dechallenge and rechallenge. Failure of remission when the drug is withdrawn (i.e. ‘dechallenge’) is unlikely to be an ADR. The diagnostic reliability of dechallenge, however, is not absolute: if the ADR has caused irreversible organ damage (e.g. malignancy) then dechallenge will result in a false-negative response. Rechallenge, involving re-institution of the suspected drug to see if the event recurs, is often regarded as an absolute diagnostic test. This is, in many instances, correct but there are two caveats. First, it is rarely justifiable to subject a patient to further hazard. Second, some adverse drug reactions develop because of particular circumstances which may not necessarily be replicated on rechallenge (e.g. hypoglycaemia with an antidiabetic agent).
Management
Specific therapy is sometimes required for ADRs such as bleeding with warfarin (vitamin K), acute dystonias (benztropine) or acute anaphylaxis (see Emergency Box 3.1, p. 69).
FURTHER READING
Pirmohamed M. The applications of pharmacogenetics to prescribing: what is currently known? Clin Med 2009; 9:493–495.
Relling M, Giacomini KM. Pharmacogenetics. In: Brunton LL, Lazo JS, Parker KL, eds. Gilman & Goodman’s The Pharmacological Basis of Therapeutics, 11th edn. New York: McGraw-Hill; 2006:93–115.
Wilkinson GR. Drug metabolism and variability among patients in drug response. N Engl J Med 2005; 352:2211–2221.
Woodcock J, Lesko LJ. Pharmacogenetics – tailoring treatment for outliers. N Engl J Med 2009; 360:811–813.
Evidence-based medicine
Randomized controlled trials
 In a superiority trial the study treatment is usually compared to placebo or to current standard practice.
In a superiority trial the study treatment is usually compared to placebo or to current standard practice.
 In an equivalence trial the treatment under study is compared to another treatment for the same condition.
In an equivalence trial the treatment under study is compared to another treatment for the same condition.
Assessing randomized controlled trials
Were the treated and control groups comparable?
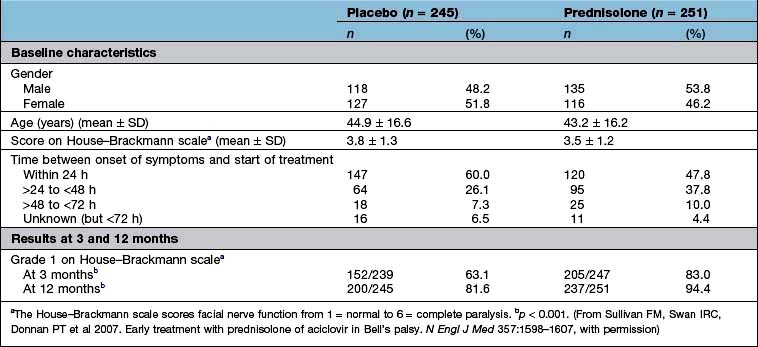
Were they similar in their ‘baseline’ characteristics? Were they, for example, of similar age, severity and duration of illness? If not, are the differences likely to influence the results? Has the statistical analysis (using analysis of covariance, or Cox’s proportional hazards model) (see below) tried to adjust for them? Table 17.10 shows some of the baseline characteristics of a trial comparing prednisolone with placebo in the treatment of Bell’s palsy (idiopathic facial paralysis).
Outcomes
There are two ways to look at the outcomes of an RCT.
 Per protocol analysis: this includes only those who completed the study.
Per protocol analysis: this includes only those who completed the study.
 Intention-to-treat analysis: this includes all patients from the time of randomization.
Intention-to-treat analysis: this includes all patients from the time of randomization.
Ideally, there should be no difference but in reality the results of a per protocol analysis are usually more advantageous to a treatment than an intention-to-treat analysis. The reason is that the intention-to-treat analysis will take account of patients who have withdrawn from the trial because of intolerance of the treatment or adverse drug reactions. It is therefore a much more robust approach. The results of the intention-to-treat analysis, in the trial of prednisolone in Bell’s palsy, are shown in Table 17.10. The trial results indicate, with a high probability, that treatment of Bell’s palsy with prednisolone will increase the chances of a full recovery of facial nerve function.
Analysis of a superiority trial
 Any difference may still be due to chance; and it is often better to await the results of at least two independent studies before adopting a new treatment.
Any difference may still be due to chance; and it is often better to await the results of at least two independent studies before adopting a new treatment.
 A trial may show no ‘statistically significant’ difference, when one in fact exists, because too few patients have been included, in other words the trial lacked sufficient ‘power’. The ‘power’ of a study (the number of patients needed in each treatment group to detect a predefined difference) should have been defined at the outset. If the study was underpowered, the results of the study should be interpreted with extreme care.
A trial may show no ‘statistically significant’ difference, when one in fact exists, because too few patients have been included, in other words the trial lacked sufficient ‘power’. The ‘power’ of a study (the number of patients needed in each treatment group to detect a predefined difference) should have been defined at the outset. If the study was underpowered, the results of the study should be interpreted with extreme care.
 A statistically significant difference may not, necessarily, be clinically relevant.
A statistically significant difference may not, necessarily, be clinically relevant.
 Effect size. The results of the well-designed trial in Table 17.10 show, very convincingly, that the treatment of Bell’s palsy with prednisolone increases the chances of complete recovery of facial nerve function, at 12 months, from 81.6% to 94.4%. This is a far more convincing description of the benefits of treatment than the p value.
Effect size. The results of the well-designed trial in Table 17.10 show, very convincingly, that the treatment of Bell’s palsy with prednisolone increases the chances of complete recovery of facial nerve function, at 12 months, from 81.6% to 94.4%. This is a far more convincing description of the benefits of treatment than the p value.
 Another expression of the benefit of a treatment such as prednisolone can be derived from the number needed to treat (NNT). This is an estimate of the numbers of patients needed to be treated with a drug to achieve one positive result. In the study shown in Table 17.10, the NNT to enable one patient with Bell’s palsy to regain normal facial nerve function, after prednisolone treatment, is eight.
Another expression of the benefit of a treatment such as prednisolone can be derived from the number needed to treat (NNT). This is an estimate of the numbers of patients needed to be treated with a drug to achieve one positive result. In the study shown in Table 17.10, the NNT to enable one patient with Bell’s palsy to regain normal facial nerve function, after prednisolone treatment, is eight.
Controlled observational trials
Historical controlled trials
 There should be a biologically plausible basis for the observed benefits.
There should be a biologically plausible basis for the observed benefits.
 There should be no appropriate treatment that could be reasonably used as a control.
There should be no appropriate treatment that could be reasonably used as a control.
 The condition should have a known and predictable natural history.
The condition should have a known and predictable natural history.
 The treatment should not be expected to have adverse effects that would compromise its potential benefits.
The treatment should not be expected to have adverse effects that would compromise its potential benefits.
 There should be a reasonable expectation that the magnitude of the therapeutic effects of treatment will be large enough to make the interpretation of its benefits unambiguous.
There should be a reasonable expectation that the magnitude of the therapeutic effects of treatment will be large enough to make the interpretation of its benefits unambiguous.
Case–control studies
A case–control design allows an estimation of the odds ratio (OR), which is the ratio of the probability of an event occurring to the probability of the event not occurring (Box 17.1).
Box 17.1
Estimation of odds ratio
| Cases | Controls | |
|---|---|---|
|
Risk factor present |
a |
c |
|
Risk factor absent |
b |
d |
|
The odds ratio (OR) = (a ÷ b) / (c ÷ d) |
||
Evaluation of new drugs
New drugs are subjected to a vigorous programme of preclinical and clinical testing before they are licensed for general use (Table 17.11) and are also monitored for safety following licensing. Doctors are recommended to fill in yellow cards when they suspect an adverse reaction has taken place.
|
Phase I: Healthy human subjects (usually men) |
Phase II: First assessment in patients
Phase III: Use in wider patient population
Phase IV: Post-marketing surveillance
Statistical analyses
The average
 The mean is the average of a distribution of values that are grouped symmetrically around the central tendency.
The mean is the average of a distribution of values that are grouped symmetrically around the central tendency.
 The median is the middle value of a sample. It is used, particularly, where the values in a sample are asymmetrically distributed around the central tendency.
The median is the middle value of a sample. It is used, particularly, where the values in a sample are asymmetrically distributed around the central tendency.
 The mode is the interval, in a frequency distribution of values, that contains more values than any other.
The mode is the interval, in a frequency distribution of values, that contains more values than any other.
In a symmetrically distributed population, the mean, median and mode are the same.
Correlation
 If r = 1, there is complete and direct concordance between the two variables
If r = 1, there is complete and direct concordance between the two variables
 The 1 in 20 rule is a convention and does not exclude the possibility that a presumed association is due to chance
The 1 in 20 rule is a convention and does not exclude the possibility that a presumed association is due to chance
 The fact that there is an association between two variables does not necessarily mean that it is causal. For example, a correlation between blood pressure and weight, with r = 0.75 and p < 0.05, does not mean that weight has a direct effect on blood pressure (or vice versa).
The fact that there is an association between two variables does not necessarily mean that it is causal. For example, a correlation between blood pressure and weight, with r = 0.75 and p < 0.05, does not mean that weight has a direct effect on blood pressure (or vice versa).
Expressions of benefit and harm
There are three ways in which the outcomes, in clinical studies, are expressed:
Continuous outcomes. Studies such as that in Table 17.10 may report outcomes using one or more continuous scales. In this study of the effects of prednisolone in the treatment of Bell’s palsy, the House–Brackmann measure of facial nerve function was used as the outcome measure. Conventional tests of statistical significance using Student’s t-test, for example, can be calculated to assess whether the null hypothesis should be rejected.
Current information sources
 The Summary of Product Characteristics (SmPCs) produced by manufacturers but vetted by drug regulatory authorities (for the UK, these are the Medicines and Healthcare Products Regulatory Agency and the European Medicines Agency).
The Summary of Product Characteristics (SmPCs) produced by manufacturers but vetted by drug regulatory authorities (for the UK, these are the Medicines and Healthcare Products Regulatory Agency and the European Medicines Agency).
 The relevant national formulary. Many countries have their own formularies, for example the UK has the British National Formulary (BNF), produced jointly by the British Medical Association and the Royal Pharmaceutical Society.
The relevant national formulary. Many countries have their own formularies, for example the UK has the British National Formulary (BNF), produced jointly by the British Medical Association and the Royal Pharmaceutical Society.
 Guidance produced by the Technology Appraisals Guidance series from the UK’s National Institute for Health and Clinical Excellence (NICE).
Guidance produced by the Technology Appraisals Guidance series from the UK’s National Institute for Health and Clinical Excellence (NICE).
Poisoning
Self-poisoning can kill
A third of patients admitted with an overdose in the UK state that they are unaware of the toxic effects of the substance involved; the majority take whatever drug is easily available at home (Box 17.2). Studies reveal that:
 Acute overdoses often involve more than one agent; alcohol is the most commonly implicated second agent.
Acute overdoses often involve more than one agent; alcohol is the most commonly implicated second agent.
 There is often a poor correlation between the drug history and the toxicological analytical findings. Therefore, a patient’s statement about the type and amount of drug ingested cannot always be relied upon.
There is often a poor correlation between the drug history and the toxicological analytical findings. Therefore, a patient’s statement about the type and amount of drug ingested cannot always be relied upon.
Box 17.2
Prevention of self-poisoning
Patients usually take what is readily available at home.
 Only small amounts of drugs should be bought
Only small amounts of drugs should be bought
 Foil-wrapped drugs are less likely to be taken in overdose
Foil-wrapped drugs are less likely to be taken in overdose
 Keep drugs and liquids in their original containers
Keep drugs and liquids in their original containers
 Child-resistant drug containers should be used
Child-resistant drug containers should be used
 Doctors should be careful in prescribing all drugs
Doctors should be careful in prescribing all drugs
 Prescriptions for any susceptible patient (e.g. the depressed) must be monitored carefully
Prescriptions for any susceptible patient (e.g. the depressed) must be monitored carefully
 Household products should be labelled and kept safely away from children
Household products should be labelled and kept safely away from children
The approach to the patient
History
More than 80% of adults are conscious on arrival at hospital and the diagnosis of self-poisoning can usually be made from the history (Table 17.12). In the unconscious patient a history from friends or relatives is helpful, and the diagnosis can often be inferred from the medicine containers or a ‘suicide note’ brought by the paramedics. In any patient with an altered level of consciousness, acute poisoning must always be considered in the differential diagnosis.
Examination
 Level of consciousness: the Glasgow Coma Scale should be used (see p. 1092)
Level of consciousness: the Glasgow Coma Scale should be used (see p. 1092)
 Ventilation: pulse oximetry can be used to measure oxygen saturation. The displayed reading may be inaccurate when the saturation is below 70%, there is poor peripheral perfusion and in the presence of carboxyhaemoglobin and methaemoglobin. Only measurement of arterial blood gases will indicate the presence both of hypercapnia and hypoxia
Ventilation: pulse oximetry can be used to measure oxygen saturation. The displayed reading may be inaccurate when the saturation is below 70%, there is poor peripheral perfusion and in the presence of carboxyhaemoglobin and methaemoglobin. Only measurement of arterial blood gases will indicate the presence both of hypercapnia and hypoxia
 Pupil size and reaction to light
Pupil size and reaction to light
If the patient is unconscious, the following should also be checked:
 Cough and gag reflex: present or absent.
Cough and gag reflex: present or absent.
 Temperature: measured with a low-reading rectal thermometer if the ear temperature suggests it is low.
Temperature: measured with a low-reading rectal thermometer if the ear temperature suggests it is low.
Some of the physical signs that may aid identification of the agents responsible for poisoning are shown in Table 17.13. The cluster of features on presentation may be distinctive and diagnostic. For example, sinus tachycardia, fixed dilated pupils, exaggerated tendon reflexes, extensor plantar responses and coma suggest tricyclic antidepressant poisoning (Table 17.13 and Table 17.14).
| Features | Likely poisons |
|---|---|
|
Constricted pupils (miosis) |
Opioids, organophosphorus insecticides, nerve agents |
|
Dilated pupils (mydriasis) |
Tricyclic antidepressants, amfetamines, cocaine, antimuscarinic drugs |
|
Divergent strabismus |
Tricyclic antidepressants |
|
Nystagmus |
Carbamazepine, phenytoin |
|
Loss of vision |
Methanol, quinine |
|
Papilloedema |
Carbon monoxide, methanol |
|
Convulsions |
Tricyclic antidepressants, theophylline, opioids, mefenamic acid, isoniazid, amfetamines |
|
Dystonic reactions |
Metoclopramide, phenothiazines |
|
Delirium and hallucinations |
Amfetamines, antimuscarinic drugs, cannabis, recovery from tricyclic antidepressant poisoning |
|
Hypertonia and hyperreflexia |
Tricyclic antidepressants, antimuscarinic drugs |
|
Tinnitus and deafness |
Salicylates, quinine |
|
Hyperventilation |
Salicylates, phenoxyacetate herbicides, theophylline |
|
Hyperthermia |
Ecstasy (MDMA), salicylates |
|
Blisters |
Usually occur in comatose patients |
MDMA, 3,4-methylenedioxymetamfetamine.
Table 17.14 Common feature clusters in acute poisoning
| Feature clusters | Poisons |
|---|---|
|
Coma, hypertonia, hyperreflexia, extensor plantar responses, myoclonus, strabismus, mydriasis, sinus tachycardia |
Tricyclic antidepressants; less commonly antihistamines, orphenadrine, thioridazine |
|
Coma, hypotonia, hyporeflexia, plantar responses (flexor or non-elicitable), hypotension |
Barbiturates, benzodiazepine and alcohol combinations, tricyclic antidepressants |
|
Coma, miosis, reduced respiratory rate |
Opioid analgesics |
|
Nausea, vomiting, tinnitus, deafness, sweating, hyperventilation, vasodilatation, tachycardia |
Salicylates |
|
Hyperthermia, tachycardia, delirium, agitation, mydriasis |
Ecstasy (MDMA) or other amfetamine |
|
Miosis, hypersalivation, rhinorrhoea, bronchorrhoea |
Organophosphorus and carbamate insecticides, nerve agents |
Principles of management of poisoning (table 17.15)
Care of the unconscious patient (see also p. 1135)
Ventilatory support
If respiratory depression is present, as determined by pulse oximetry or preferably by arterial blood gas analysis, an oropharyngeal airway should be inserted, and supplementaloxygen should be administered. Pulse oximetry alone will not detect hypercapnia. Loss of the cough or gag reflex is the prime indication for intubation. The gag reflex can be assessed by positioning the patient on one side and making him or her gag using a suction tube. In many severely poisoned patients, the reflexes are depressed sufficiently to allow intubation without the use of sedatives or relaxants. The complications of endotracheal tubes are discussed on page 885 in Chapter 16. If ventilation remains inadequate after intubation, as shown by hypoxaemia and hypercapnia, intermittent positive-pressure ventilation (IPPV) should be instituted.
Cardiovascular support
Hypotension and shock may be caused by:
 A direct cardio-depressant action of the poison (e.g. beta-blockers, calcium channel blockers, tricyclic antidepressants)
A direct cardio-depressant action of the poison (e.g. beta-blockers, calcium channel blockers, tricyclic antidepressants)
 Vasodilation and venous pooling in the lower limbs (e.g. ACE inhibitors, phenothiazines)
Vasodilation and venous pooling in the lower limbs (e.g. ACE inhibitors, phenothiazines)
 A decrease in circulating blood volume because of gastrointestinal losses (e.g. profuse vomiting in theophylline poisoning), increased insensible losses (e.g. salicylate poisoning), increased renal losses (e.g. poisoning due to diuretics) and increased capillary permeability.
A decrease in circulating blood volume because of gastrointestinal losses (e.g. profuse vomiting in theophylline poisoning), increased insensible losses (e.g. salicylate poisoning), increased renal losses (e.g. poisoning due to diuretics) and increased capillary permeability.
Hypotension may be exacerbated by co-existing hypoxia, acidosis and dysrhythmias. In people with marked hypotension, volume expansion with crystalloids should be used, guided by monitoring of central venous pressure (CVP). Urine output (aiming for 35–50 mL/h) is also a useful guide to the adequacy of the circulation. If a patient fails to respond to the above measures, more intensive therapy is required. In such patients, it is helpful to undertake invasive haemodynamic monitoring to confirm that adequate volume replacement has been administered. Volume replacement and the use of inotropes are discussed on page 874. All patients with cardiogenic shock should have ECG monitoring.
Other problems
Rhabdomyolysis can occur from pressure necrosis in drug-induced coma, or it may complicate, e.g. ecstasy (MDMA) abuse in the absence of coma. People with rhabdomyolysis are at risk of developing, firstly, acute kidney injury from myoglobinaemia, particularly if they are hypovolaemic and have an acidosis and, secondly, wrist or ankle drop from the development of a compartment syndrome (see p. 509).
Body ‘packers’ and body ‘stuffers’
Packages can be removed most expeditiously in body stuffers by employing whole bowel irrigation (see p. 913). In the past early surgery was advocated in body packers. However, with the development of improved packaging, a more conservative approach (the use of lactulose or whole bowel irrigation) can now be adopted with which there is a complication rate of <5%. Immediate surgery is indicated if acute intestinal obstruction develops, or when packets can be seen radiologically and there is clinical or analytical evidence to suggest leakage, particularly if the drug involved is cocaine.
Specific management
Antidotes
Specific antidotes are available for only a small number of poisons (Table 17.16).
| Poison | Antidotes |
|---|---|
|
Aluminium (aluminum) |
Desferrioxamine (deferoxamine) |
|
Arsenic |
DMSA, dimercaprol |
|
Benzodiazepines |
Flumazenil |
|
β-adrenoceptor blocking drugs |
Atropine, glucagon |
|
Calcium channel blockers |
Atropine |
|
Carbamate insecticides |
Atropine |
|
Carbon monoxide |
Oxygen |
|
Copper |
D-penicillamine, DMPS |
|
Cyanide |
Oxygen, dicobalt edetate, hydroxocobalamin, sodium nitrite, sodium thiosulphate |
|
Diethylene glycol |
Fomepizole, ethanol, |
|
Digoxin and digitoxin |
Digoxin-specific antibody fragments |
|
Ethylene glycol |
Fomepizole, ethanol |
|
Hydrogen sulphide |
Oxygen |
|
Iron salts |
Desferrioxamine |
|
Lead (inorganic) |
DMSA (succimer), sodium calcium edentate |
|
Methaemoglobinaemia |
Methylthioninium chloride (methylene blue) |
|
Methanol |
Fomepizole, ethanol |
|
Mercury (inorganic) |
Unithiol (DMPS) |
|
Nerve agents |
Atropine, HI-6, obidoxime, pralidoxime |
|
Oleander |
Digoxin-specific antibody fragments |
|
Opioids |
Naloxone |
|
Organophosphorus insecticides |
Atropine, HI-6, obidoxime, pralidoxime |
|
Paracetamol |
Acetylcysteine |
|
Thallium |
Berlin (Prussian) blue |
|
Warfarin and similar anticoagulants |
Phytomenadione (vitamin K) |
DMSA, dimercaptosuccinic acid; DMPS, dimercaptopropanesulphonate.
Antidotes may exert a beneficial effect by:
 Forming an inert complex with the poison (e.g. desferrioxamine (deferoxamine), D-penicillamine, dicobalt edetate, digoxin-specific antibody fragments, dimercaprol, HI-6, hydroxocobalamin, obidoxime, pralidoxime, protamine, Prussian (Berlin) blue, sodium calcium edetate, succimer (DMSA), unithiol (DMPS))
Forming an inert complex with the poison (e.g. desferrioxamine (deferoxamine), D-penicillamine, dicobalt edetate, digoxin-specific antibody fragments, dimercaprol, HI-6, hydroxocobalamin, obidoxime, pralidoxime, protamine, Prussian (Berlin) blue, sodium calcium edetate, succimer (DMSA), unithiol (DMPS))
 Accelerating detoxification of the poison (e.g. acetylcysteine, sodium thiosulphate)
Accelerating detoxification of the poison (e.g. acetylcysteine, sodium thiosulphate)
 Reducing the rate of conversion of the poison to a more toxic compound (e.g. ethanol, fomepizole)
Reducing the rate of conversion of the poison to a more toxic compound (e.g. ethanol, fomepizole)
 Competing with the poison for essential receptor sites (e.g. oxygen, naloxone, phytomenadione)
Competing with the poison for essential receptor sites (e.g. oxygen, naloxone, phytomenadione)
 Blocking essential receptors through which the toxic effects are mediated (e.g. atropine)
Blocking essential receptors through which the toxic effects are mediated (e.g. atropine)
 By-passing the effect of the poison (e.g. oxygen, glucagon).
By-passing the effect of the poison (e.g. oxygen, glucagon).
Reducing poison absorption
Cathartics have no role in the management of the poisoned patient.
FURTHER READING
Barceloux D, McGuigan M, Hartigan-Go K et al. Position paper: cathartics. Clin Toxicol 2004; 42:243–253.
Chyka PA, Seger D, Krenzelok EP et al. Position paper: single-dose activated charcoal. Clin Toxicol 2005; 43:61–87.
Krenzelok EP, McGuigan M, Lheureux P et al. Position paper: ipecac syrup. Clin Toxicol 2004; 42:133–143.
Kulig K, Vale JA. Position paper: gastric lavage. Clin Toxicol 2004; 42:933–943.
Tenenbein M, Lheureux P. Position paper: whole bowel irrigation. Clin Toxicol 2004; 42:843–854.
Increasing poison elimination
Non-toxicological investigations (Table 17.17)
Some routine investigations are of value in the differential diagnosis of coma or the detection of poison-induced hypokalaemia, hyperkalaemia, hypoglycaemia, hyperglycaemia, hepatic or renal failure or acid–base disturbances (Table 17.18). Measurement of carboxyhaemoglobin, methaemoglobin and cholinesterase activities are of assistance in the diagnosis and management of cases of poisoning due to carbon monoxide, methaemoglobin-inducing agents such as nitrites and organophosphorus insecticides, respectively.
Table 17.18 Some poisons inducing metabolic acidosis
|
Calcium channel blockers |
Iron |
|
Carbon monoxide |
Metformin |
|
Cocaine |
Methanol |
|
Cyanide |
Paracetamol |
|
Diethylene glycol |
Topiramate |
|
Ethanol |
Tricyclic antidepressants |
|
Ethylene glycol |
|
Routine radiology is of little diagnostic value. It can confirm ingestion of metallic objects (e.g. coins, button batteries) or injection of globules of metallic mercury. Rarely, hydrocarbon solvents (e.g. carbon tetrachloride) may be seen as a slightly opaque layer floating on the top of the gastric contents with the patient upright, or outlining the small bowel. Some enteric-coated or sustained-release drug formulations may be seen on plain abdominal radiographs, but, with the exception of iron salts, ordinary formulations are seldom seen. Ingested packets of illicit substances can sometimes been seen on CT (see p. 912). Radiology can confirm complications of poisoning, e.g. aspiration pneumonia, non-cardiogenic pulmonary oedema (salicylates), acute respiratory distress syndrome (ARDS).
Specific poisons: drugs
Anticonvulsants
Clinical features
The clinical features of poisoning with anticonvulsant drugs are summarized in Table 17.19.
Table 17.19 Clinical features of poisoning with anticonvulsant drugs
| Anticonvulsant drug | Clinical features of poisoning |
|---|---|
|
Carbamazepine |
Dry mouth, coma, convulsions, ataxia, incoordination, hallucinations (particularly in the recovery phase) |
|
Ocular: nystagmus dilated pupils (common), divergent strabismus, complete external ophthalmoplegia (rare) |
|
|
Phenytoin |
Nausea, vomiting, headache, tremor, cerebellar ataxia, nystagmus, loss of consciousness (rare) |
|
Sodium valproate |
Most frequent: drowsiness, impairment of consciousness, respiratory depression |
|
Uncommon complications: liver damage, hyperammonaemia, metabolic acidosis |
|
|
Very severe poisoning: myoclonic jerks and seizures; cerebral oedema has been reported |
|
|
Gabapentin and pregabalin |
Lethargy, ataxia, slurred speech and gastrointestinal symptoms |
|
Lamotrigine |
Lethargy, coma, ataxia, nystagmus, seizures, cardiac conduction abnormalities |
|
Levetiracetam |
Lethargy, coma, respiratory depression |
|
Tiagabine |
Lethargy, facial grimacing, nystagmus, posturing, agitation, coma, hallucinations, seizures |
|
Topiramate |
Lethargy, ataxia, nystagmus, myoclonus, coma, seizures, non-anion gap metabolic acidosis |
|
Metabolic acidosis can appear within hours of ingestion and persist for days |
Antidepressants: tricyclics and SSRIs
Treatment
FURTHER READING
Bradberry SM, Thanacoody HK, Watt BE et al. Management of the cardiovascular complications of tricyclic antidepressant poisoning: role of sodium bicarbonate. Toxicol Rev 2005; 24:195–204.
Isbister GK, Bowe SJ, Dawson A et al. Relative toxicity of selective serotonin reuptake inhibitors (SSRIs) in overdose. Clin Toxicol 2004; 42:277–285.
Thanacoody HK, Thomas SH. Tricyclic antidepressant poisoning: cardiovascular toxicity. Toxicol Rev 2005; 24:205–214.
Antidiabetic drugs
Antimalarials
Treatment
Multiple-dose oral activated charcoal increases quinine and probably chloroquine clearance. Hypokalaemia should be corrected. Sodium bicarbonate 50–100 mmol i.v. is given if the ECG shows intraventricular block but will exacerbate hypokalaemia, which should be corrected first. Mechanical ventilation, the administration of an inotrope and high doses of diazepam (1 mg/kg as a loading dose and 0.25–0.4 mg/kg per hour maintenance) may reduce the mortality in severe chloroquine poisoning. Overdrive pacing may be required if torsades de pointes (p. 710) occurs in quinine poisoning and does not respond to magnesium sulphate infusion. If clinically significant methaemoglobinaemia (generally above 30%) develops in primaquine poisoning, methylthioninium (methylene blue) 1–2 mg/kg body weight should be administered.
Calcium channel blockers
Treatment
Cardiac pacing has a role if there is evidence of AV conduction delay but failure to capture occurs.
Treat hypotension initially with intravenous crystalloid. If significant hypotension persists despite volume replacement, administer glucagon (see p. 409) as it activates myosin kinase independent of calcium. Give i.v. glucagon 10 mg (150 µg/kg) as a slow bolus and repeat. If there is a favourable response in blood pressure, an infusion of 5–10 mg/h can be commenced; if there is no response after the initial boluses, discontinue.
Acidosis impairs L-type channel function (see p. 708) and is corrected by the administration of sodium bicarbonate, which has been shown experimentally to improve myocardial contractility and cardiac output.
Cannabis (marijuana)
Clinical features
Heavy users suffer impairment of memory and attention and poor academic performance. There is an increased risk of anxiety and depression. Regular users are at risk of dependence. Cannabis use results in an overall increase in the relative risk for later schizophrenia and psychotic episodes (see p. 1185). Cannabis smoke is probably carcinogenic.
Digoxin
Toxicity occurring during chronic administration is common, though acute poisoning is infrequent.
Paracetamol (acetaminophen)
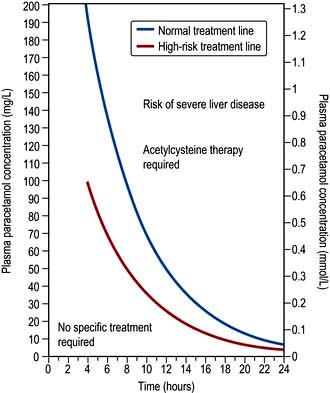
The severity of paracetamol poisoning is dose related. There is, however, some variation in individual susceptibility to paracetamol-induced hepatotoxicity. People with pre-existing liver disease, those suffering from acute or chronic starvation (patients not eating for a few days for example due to a recent febrile illness or dental pain), those suffering from anorexia nervosa and other eating disorders, those receiving enzyme-inducing drugs, and those with HIV infection should be considered to be at greater risk and given treatment at plasma paracetamol concentrations lower than those normally used for interpretation (Fig. 17.2).
Treatment
The treatment protocol is dependent on the time of presentation and this is summarized in Table 17.20. Acetylcysteine has emerged as an effective protective agent provided that it is administered within 8–10 hours of ingestion of the overdose. It acts by replenishing cellular glutathione stores, though it may also repair oxidation damage caused by NAPQI. The treatment regimen is shown in Table 17.21. If a staggered overdose has been taken (multiple ingestions over several hours), acetylcysteine should be given when the paracetamol dose exceeds 150 mg/kg body weight in any one 24-hour period or 75 mg/kg body weight in those at high risk (see above).
8–15 h after ingestion
 Urgent action is required because the efficacy of treatment declines progressively from 8 h after overdose. If >150 mg/kg paracetamol has been ingested, start treatment with acetylcysteine immediately
Urgent action is required because the efficacy of treatment declines progressively from 8 h after overdose. If >150 mg/kg paracetamol has been ingested, start treatment with acetylcysteine immediately
 Take blood for urgent estimation of the plasma paracetamol concentration, INR, plasma creatinine and ALT activity
Take blood for urgent estimation of the plasma paracetamol concentration, INR, plasma creatinine and ALT activity
 Assess whether the patient is at risk of liver damage
Assess whether the patient is at risk of liver damage
 The need for treatment is determined by using Fig. 17.2
The need for treatment is determined by using Fig. 17.2
 In patients already receiving acetylcysteine, only discontinue if the plasma paracetamol concentration is below the relevant treatment line (Fig. 17.2) and there is no abnormality of the INR, plasma creatinine or ALT activity and the patient is asymptomatic. Do not discontinue the infusion if there is any doubt as to the timing of the overdose
In patients already receiving acetylcysteine, only discontinue if the plasma paracetamol concentration is below the relevant treatment line (Fig. 17.2) and there is no abnormality of the INR, plasma creatinine or ALT activity and the patient is asymptomatic. Do not discontinue the infusion if there is any doubt as to the timing of the overdose
 At the end of treatment, measure INR, plasma creatinine and ALT activity. If any test is abnormal or the patient is symptomatic, further monitoring is required and expert advice should be sought
At the end of treatment, measure INR, plasma creatinine and ALT activity. If any test is abnormal or the patient is symptomatic, further monitoring is required and expert advice should be sought
 People with normal INR, plasma creatinine and ALT activity and who are asymptomatic may be discharged
People with normal INR, plasma creatinine and ALT activity and who are asymptomatic may be discharged
15–24 h after ingestion
 Urgent action is required because the efficacy of treatment is limited more than 15 h after overdose. Start treatment with acetylcysteine immediately if >150 mg/kg paracetamol has been ingested
Urgent action is required because the efficacy of treatment is limited more than 15 h after overdose. Start treatment with acetylcysteine immediately if >150 mg/kg paracetamol has been ingested
 In all patients, take blood for urgent estimation of the plasma paracetamol concentration, INR, plasma creatinine and ALT activity
In all patients, take blood for urgent estimation of the plasma paracetamol concentration, INR, plasma creatinine and ALT activity
 Assess whether the patient is at risk of liver damage
Assess whether the patient is at risk of liver damage
 Give treatment if needed (Fig. 17.2). The prognostic accuracy of the ‘200 mg/L line’ after 15 h is uncertain but a plasma paracetamol concentration above the extended treatment line should be regarded as carrying serious risk of severe liver damage
Give treatment if needed (Fig. 17.2). The prognostic accuracy of the ‘200 mg/L line’ after 15 h is uncertain but a plasma paracetamol concentration above the extended treatment line should be regarded as carrying serious risk of severe liver damage
 At the end of treatment, check INR, plasma creatinine and ALT activity. If any test is abnormal or the patient is symptomatic, further monitoring is required and expert advice should be sought
At the end of treatment, check INR, plasma creatinine and ALT activity. If any test is abnormal or the patient is symptomatic, further monitoring is required and expert advice should be sought
Table 17.21 Regimen for acetylcysteine
If liver or renal failure ensues, this should be treated conventionally though there is evidence that a continuing infusion of acetylcysteine (continue 16-h infusion until recovery) will improve the morbidity and mortality. Liver transplantation has been performed successfully in patients who have paracetamol-induced fulminant hepatic failure (see p. 316).
Salicylates
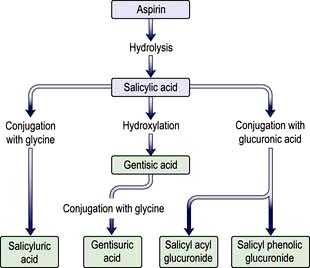
Aspirin is metabolized to salicylic acid (salicylate) by esterases present in many tissues, especially the liver, and subsequently to salicyluric acid and salicyl phenolic glucuronide (Fig. 17.3); these two pathways become saturated with the consequence that the renal excretion of salicylic acid increases after overdose; this excretion pathway is extremely sensitive to changes in urinary pH.
Treatment
Fluid and electrolyte replacement is required and special attention should be paid to potassium supplementation. Severe metabolic acidosis requires at least partial correction with the administration of sodium bicarbonate intravenously. Mild cases of salicylate poisoning are managed with parenteral fluid and electrolyte replacement only. Patients whose plasma salicylate concentrations are in excess of 500 mg/L (3.62 mmol/L) should receive urine alkalinization (see p. 913). Haemodialysis is the treatment of choice for severely poisoned patients (plasma salicylate concentration >700 mg/L; >5.07 mmol/L), particularly those with coma and metabolic acidosis.
Specific poisons: chemicals
Carbamate insecticides
Carbamate insecticides inhibit acetylcholinesterase, as do organophosphorus insecticides, but the duration of this inhibition is comparatively short-lived in comparison since the carbamate–enzyme complex tends to dissociate spontaneously. The clinical features and treatment are similar except that an oxime such as pralidoxime is not usually required because the enzyme complex dissociates spontaneously; recovery invariably occurs within 24 hours (see p. 923).
Ethanol
Ethanol is commonly ingested in beverages and deliberately with other substances in overdose. It is also present in many cosmetic and antiseptic preparations. Following absorption, ethanol is oxidized to acetaldehyde and then to acetate. Ethanol is a CNS depressant and the features of ethanol intoxication are generally related to blood concentrations (Table 17.22).
| Blood (ethanol) | Clinical features | |
|---|---|---|
| mg/L | mmol/L | |
|
500–1500 |
11.0–32.5 |
|
1500–3000
32.5–65.0
3000–5000
65.0–108.5
>5000
>108.5
Ethylene and diethylene glycol
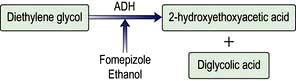
Ethylene and diethylene glycol are found in a variety of common household products including antifreeze, windshield washer fluid, brake fluid and lubricants. The features observed are due to metabolites predominantly, not the parent chemical. Ethylene glycol (Fig. 17.4) is metabolized to glycolate, the cause of the acidosis. A small proportion of glyoxylate is metabolized to oxalate. Calcium ions chelate oxalate to form insoluble calcium oxalate, which is responsible for renal toxicity. Diethylene glycol is metabolized to 2-hydroxyethoxyacetate (Fig. 17.5), which is the cause of metabolic acidosis, and diglycolic acid (the cause of renal failure).
Lead
Clinical features
The characteristic haematological features include:
 Normochromic normocytic anaemia, due to inhibition by lead of several enzymes involved in haem synthesis, including ALA synthetase
Normochromic normocytic anaemia, due to inhibition by lead of several enzymes involved in haem synthesis, including ALA synthetase
 Haemolysis, which is usually mild, resulting from damage to the red cell membrane
Haemolysis, which is usually mild, resulting from damage to the red cell membrane
 Punctate basophilia (or basophilic stippling: the blood film shows red cells with small, round, blue particles), due to aggregates of RNA in immature red cells owing to inhibition by lead of pyrimidine-5-nucleotidase, which normally disperses residual RNA to produce a diffuse blue staining seen in reticulocytes on blood films (polychromasia).
Punctate basophilia (or basophilic stippling: the blood film shows red cells with small, round, blue particles), due to aggregates of RNA in immature red cells owing to inhibition by lead of pyrimidine-5-nucleotidase, which normally disperses residual RNA to produce a diffuse blue staining seen in reticulocytes on blood films (polychromasia).
Methanol
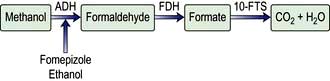
Methanol is used widely as a solvent and is found in antifreeze solutions. Methanol is metabolized to formaldehyde and formate (Fig. 17.6). The concentration of formate increases greatly and is accompanied by accumulation of hydrogen ions causing metabolic acidosis.
Specific poisons: marine animals
Specific poisons: venomous animals
Insect stings and bites
Insect stings from wasps and bees, and bites from ants, produce pain and swelling at the puncture site. Following the sting or bite, patients should be observed for 2 hours for any signs of evolving urticaria, pruritus, bronchospasm or oropharyngeal oedema. The onset of anaphylaxis requires urgent treatment (see p. 69).
Venomous snakes
Approximately 15% of the 3000 species of snake found worldwide are considered to be dangerous to humans. Snake bite is common in some tropical countries (Table 17.23).
|
Sri Lanka |
6 bites per 100 000 population and 900 deaths per year |
|
Nigeria |
500 bites per 100 000 population with a 12% mortality |
|
Myanmar |
15 deaths per 100 000 population |
|
USA |
45 000 bites per year (in a population of 301 million), 8000 by venomous species, with 6 deaths annually |
|
UK |
Approximately 100 people admitted to hospital annually (population 60 million) but only one death since 1970 |
|
Australia |
2 or 3 deaths annually (population 20 million) |
 Viperidae (with two subgroups: Viperinae: European adders and Russell’s vipers; and Crotalinae: American rattlesnakes, moccasins, lance-headed vipers and Asian pit vipers)
Viperidae (with two subgroups: Viperinae: European adders and Russell’s vipers; and Crotalinae: American rattlesnakes, moccasins, lance-headed vipers and Asian pit vipers)
 Elapidae (cobras, kraits, mambas, coral snakes, Australian venomous snakes)
Elapidae (cobras, kraits, mambas, coral snakes, Australian venomous snakes)
In addition, some members of the family Colubridae are mildly venomous (mongoose snake).
Clinical features
Viperidae (Viperinae and Crotalinae)
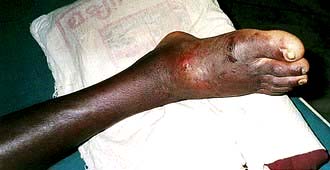
Russell’s viper causes most of the snake-bite mortality in India, Pakistan and Myanmar. There is local swelling at the site of the bite (Fig. 17.7) which may become massive. Local tissue necrosis may occur. Evidence of systemic involvement (envenomation) occurs within 30 minutes, including vomiting, evidence of shock and hypotension. Haemorrhage due to incoagulable blood can be fatal.
Specific poisons: plants
Digitalis purpurea, Nerium oleander, Thevetia peruviana
Ingestion of Digitalis purpurea, or the common (Nerium oleander) or yellow (Thevetia peruviana) oleander can produce a syndrome similar to digoxin poisoning (see p. 917). A randomized controlled trial has shown that digoxin-specific antibody fragments rapidly and safely reverse yellow oleander-induced arrhythmias, restore sinus rhythm, and rapidly reverse bradycardia and hyperkalaemia. The administration of multiple doses of activated charcoal is used, but the effect on survival is debated.