Chapter 10. Drug formulary
The Medicines Act allows ambulance paramedics to supply and administer prescription only medications in circumstances specified by local paramedic steering committees or their equivalent.
The Joint Royal Colleges Ambulance Liaison Committee (JRCALC) makes applications for exemption for the drugs listed in the formulary below.
The JRCALC National Clinical Guidelines for use by UK ambulance services provide the most up-to-date list of relevant drugs (see: www.asancep.org.uk/JRCALC/guidelines).
The names of a drug
Each drug has several ‘names’ as the full chemical name is usually unwieldy:
• Chemical name: 4-amino-5-chloro- N-[2-(dimethylamino)ethyl] 1-2-methoxybenzamide
• Generic name: metoclopramide
• Trade name: Maxolon.
Routes of administration
Enteral routes of administration:
• Oral
• Rectal
• Sublingual
• Buccal.
Parenteral routes of administration:
• Dermal patches
• Intradermal
• Intramuscular
• Subcutaneous
• Intravenous
• Inhaled or nebulised
• Intraosseous.
Half-life
This is the time it takes for the plasma concentration of a drug in the body to halve.
After one half-life, only 50% remains, after two half-lives, only 25% remains and so on.
This is important because drugs with a long half-life take a long time to be eliminated compared to those with a short half-life.
Naloxone, the antidote to morphine, has a much shorter half-life than morphine itself.
Prehospital formulary
Amiodarone
Main prehospital use
• Ventricular fibrillation (VF) or pulseless ventricular tachycardia (VT) refractors to three countershocks
• VT with chest pain, heart failure, or heart rate >150 bpm.
Action
An antiarrhythmic drug, which lengthens cardiac action potential and effective refractory period.
Preparations
30 mg/mL, 10 mL ampoule for intravenous injection.
Indications
Replaces lidocaine for:
• VF or pulseless VT
• VT with either chest pain, heart failure, or heart rate >150 bpm provided SBP >90 mmHg.
Cautions
None in cardiac arrest situations.
Contraindications
None in cardiac arrest situations.
For VT, hypotension (BP, 90 mmHg), bradycardia, heart block, thyroid dysfunction, iodine allergy, respiratory failure, congestive heart failure, decompensated cardiomyopathy, pregnancy, breast-feeding.
Side-effects
Not relevant in cardiac arrest situations.
Following treatment for VT: severe bradycardia, vasodilation and hypotension, bronchospasm, arrhythmias ( torsade de pointes).
Dose
• Cardiac arrest: Following persistent VF or VT administer 300 mg IV after 3rd shock. A further 150 mg may also be used
• VT: 150 mg over 10 minutes (3 minutes if life-threatening). May be repeated once after 10 minutes.
Aspirin
Aspirin (acetylsalicylic acid) decreases platelet aggregation and inhibits clot formation on the arterial side of the circulation. Its use can reduce mortality associated with myocardial infarction and unstable angina. When indicated, a 300 mg tablet should be given regardless of any previous aspirin taken that day. In children under 12 years old, aspirin is associated with Reye’s syndrome (acute encephalopathy and liver damage) and is contraindicated.
Main prehospital use
Acute coronary syndromes.
Other uses
• Prevention of thrombotic cardiovascular or cerebrovascular disease
• Simple oral analgesic and mild antiinflammatory.
Action
• Antiplatelet activity prevents or limits formation of clots
• Decreased perception of pain
• Antipyretic (lowers temperature).
Preparations
Dispersible tablet 300 mg.
Indications
Adults with ischaemic chest pain.
Cautions
• Asthma
• Pregnancy
• Kidney or liver failure
• Gastric or duodenal ulcer.
Contraindications
• Known hypersensitivity
• Children under 16 years
• Patients with known clotting disorders (e.g. haemophilia).
Side-effects
• Gastric irritation and bleeding
• Bronchospasm in some asthmatics.
Administration
Place on the tongue and chew or dissolved in water and drink.
Dose
300 mg single dose.
Atropine
Main prehospital use
Management of asystolic cardiac arrest and symptomatic bradycardia (heart rate <60). No longer recommended for routine use in asystole or PEA.
Other uses
Organophosphate poisoning.
Action
• Blocks vagal (parasympathetic) tone – blocks effect of vagus nerve at sinoatrial and atrioventricular nodes, thus increasing sinus automaticity and facilitating AV node conduction
• Reduces likelihood of VF triggered by hypoperfusion associated with extreme bradycardia.
Preparations
• 10 mL disposable syringe with 1 mg (100 μg/mL)
• 5mL disposable syringe with 1 mg (200 μg/mL)
• 10 mL disposable syringe with 3 mg (300 μg/mL)
• 1 mL ampoule with 600 μg/mL.
Indications
1. Symptomatic bradycardia associated with any of:
• Shock
• Syncope
• Myocardial Ischaemia
• Heart failure
2. Heart rate <60 and any indication of high risk of asystole:
• Recent asystole
• Mobility II AV block
• Complete heart block with wide QRS
• Ventricular pauses >3 seconds
3. Organophosphate poisoning.
Cautions
Give cautiously to avoid tachycardia post-myocardial infarction (increases myocardial oxygen demand and worsens ischaemia).
Contraindications
Bradycardia associated with hypothermia.
Side-effects
• Dilation of pupils and blurred vision
• Dry mouth
• Urine retention
• Confusion
• Tachycardia.
Administration
IV
Dose
• 0.5–3 mg IV for symptomatic bradycardia or high risk of asystole
• Children, 20 μg/kg (maximum cumulative dose 0.1 mg, minimum 100 μg)
• Organophosphate poisoning, 2 mg IV repeated as required until skin becomes flushed and dry, pupils dilate and tachycardia develops.
Benzylpenicillin
Benzylpenicillin is one of the penicillin group of drugs. It interferes with bacterial cell wall production and kills a range of bacteria which include those commonly responsible for meningococcal septicaemia and meningitis. Although the most important side-effect of benzylpenicillin is an allergic reaction, very few patients are at risk of anaphylaxis. Many patients think that they may be allergic to penicillin because of transient rashes or an episode of diarrhoea. If a patient is suspected of having meningococcal septicaemia, only a genuine (proven) history of penicillin allergy should stop benzylpenicillin being given.
Main prehospital use
The treatment of meningococcal septicaemia.
Other uses
None prehospital.
Action
Bactericidal by interfering with bacterial cell wall synthesis.
Preparations
Ampoule containing 600 mg of penicillin G (benzylpenicillin) in powder form.
Indications
• Meningococcal septicaemia
• Meningitis.
Cautions
• Previous side-effects after penicillin
• Renal impairment.
Contraindications
Genuine penicillin allergy.
Side-effects
• Rare in context of severe infection
• Hypersensitivity reactions (e.g. urticaria)
• Anaphylaxis (rare)
• Convulsions in high doses
• Hypotension (due to action of drug in releasing toxins. Manage with IV fluid challenges).
Administration
IV or IM.
Dose
Dissolve each 600 mg in 10 mL sterile water for IV use, and 2 mL sterile water for IM use. Give:
• Adult and child older than 9 years – 1200 mg (20 mL IV, 4 mL IM)
• Child 1–9 years – 600 mg (10 mL IV, 2 mL IM)
• Infant – 300 mg (5 mL IV, 1 mL IM).
Chlorpheniramine
Chlorpheniramine is used as a second-line drug in the management of anaphylactic reactions, and as the first-line treatment of less severe allergic reactions, such as severe itching.
Main prehospital use
Management of anaphylactic and allergic reactions.
Other uses
None in the prehospital setting.
Action
Chlorpheniramine blocks the action of histamine released as part of the body’s response to allergens.
Preparation
10 mg/mL, 1 mL ampoule.
Indications
• Severe anaphylactic reactions (after administration of adrenaline)
• Allergic reactions causing distress (e.g. severe itching).
Cautions
• Hypotension
• Epilepsy
• Glaucoma
• Hepatic disease.
Contraindications
• Hypersensitivity
• <1 year of age.
Side-effects
Hypotension if administered rapidly.
Administration
Slow IV injection over 1 minute.
Dosage
• Adult >12 years 10 mg
• Child 6–12 years 5 mg
• Child 1–5 years 2.5 mg.
Compound sodium lactate (hartmann’s/ ringers lactate)
Main prehospital uses
Fluid replacement therapy.
Other uses
None.
Action
As an infusion, transiently increases intravascular volume.
Preparations
• 500 and 1000 mL bags
• 5 and 10 mL ampoules.
Indications
• Status asthmaticus (to limit formation of dry mucous plugs)
• Hypovolaemic shock in the absence of a radial pulse
• Burns
• Anaphylaxis
• Hyperthermia
• Dehydration.
Cautions
None.
Contraindications
• Hyperglycaemic ketoacidosis
• Crush injury.
Side-effects
• Fluid overload in patients with uncontrolled haemorrhage can cause clot disruption and increased bleeding
• Fluid overload causing heart failure (particularly in the elderly)
• Exacerbation of pre-existing acidosis.
Administration
IV infusion or bolus.
Dose
Adults with dehydration, status asthmaticus, hyperthermia:
• Give 500 mL infusion in 20 minutes repeated to effect (maximum dose 2000 mL).
Children with dehydration, status asthmaticus, hyperthermia:
• Give 20 mL/kg bolus repeated once to effect.
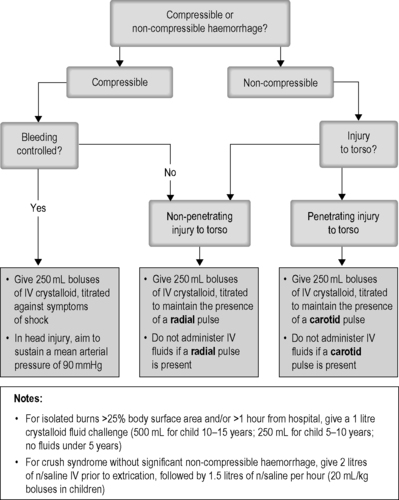
Adults and children with hypovolaemic shock or burns, see Figure 10.1.
Do not give compound sodium lactate to patients with crush syndrome.
Diazepam
Diazepam is the benzodiazepine that has been most commonly used in the management of seizures and status epilepticus. It is ideally given intravenously in someone who is actively fitting at the scene or is having repeated fits. It is given IV as the emulsion Diazemuls to reduce the risk of venous thrombophlebitis. Rectal diazepam is given when IV access cannot be obtained.
Main prehospital use
Management of seizures.
Other uses
Cocaine toxicity.
Action
CNS depressant and anticonvulsant.
Preparations
• Rectal tubes containing 5 mg or 10 mg
• 2 mL ampoule (diazepam emulsion) containing 10 mg (5 mg/mL).
Indications
Prolonged or repeated seizures such as may occur in:
• Status epilepticus
• Convulsions secondary to infections
• Alcohol withdrawal seizures
• Convulsions due to poisoning
• Eclampsia
• Head injury (rule out hypoxia).
Symptomatic cocaine toxicity
• Severe hypertension
• Chest pain
• Fitting.
Cautions
• Respiratory disease/depression
• History of drug or alcohol abuse
• Reduce dose in elderly and debilitated
• Facilities for ventilatory support should be immediately available
• Consider doses previously administered by carers
• Use of CNS depressants.
Contraindications
• Known hypersensitivity
• Respiratory failure.
Side-effects
• Respiratory depression (especially with opioids and alcohol)
• Apnoea
• CNS depression and loss of consciousness
• Cardiovascular depression and postural hypotension
• Amnesia.
Administration
• IV through a large proximal vein at a rate of 3 mg/min
• Rectal via a tube which should be inserted no more than 2 cm in children and 3–4 cm in adults (tubes have markers)
• Rectal tubes should be held in place for a few moments after expelling the contents and the patient’s buttocks held together to reduce seepage from the rectum.
Entonox
Nitrous oxide is an anaesthetic gas, which is rapidly absorbed by inhalation. A mixture of nitrous oxide and oxygen containing 50% of each gas (Entonox) is used in prehospital care to gain rapid control of pain without loss of consciousness. It is administered by the casualty via a demand valve. Slow, deep breaths are required. The casualty must be conscious, cooperative and have sufficient respiratory excursion to operate the demand valve. Nitrous oxide is extremely soluble and will diffuse rapidly into any gas-filled cavity; it may thus increase the size of a pneumothorax. At temperatures below −7°C, nitrous oxide may liquefy and the oxygen and nitrous oxide will separate. The patient may then inhale pure oxygen followed by pure nitrous oxide. It is not adequate to simply shake the cylinder in these situations. Cylinders need to be kept at temperatures above freezing.
Main prehospital use
Rapid control of pain
May be used whilst preparing to give opiates.
Action
Anaesthetic agent.
Preparations
A mixture of 50% nitrous oxide and 50% oxygen in a blue cylinder with a white shoulder.
Indications
Acute pain.
Cautions
• Chest injuries
• Head injuries
• Cold weather
• Alcohol/drug intoxication
• Sickle cell crisis
• >50% oxygen indicated.
Contraindications
• Pneumothorax
• Gastrointestinal obstruction
• Recent diving activity
• Reduced Glasgow coma scale (GCS)
• Disturbed psychiatric patients.
Side-effects
• Decreased level of consciousness
• Nausea and vomiting
• Confusion ± distress.
Administration
Inhalation via demand valve with onset of action within 3–5 minutes.
Dose
As required to relieve pain.
Epinephrine (adrenaline)
Epinephrine is a sympathomimetic drug which stimulates both a and β receptors. a receptor activity increases peripheral vascular resistance without constricting coronary and cerebral vessels. This raises systolic and diastolic pressures during CPR, which makes CPR more effective; β receptor activity increases myocardial contractility in cardiac arrest and relieves bronchospasm in acute severe asthma. Epinephrine also reverses the allergic manifestations of acute anaphylaxis. If epinephrine has already been self-administered by the patient (e.g. EpiPen 0.3 mg for adults or 0.15 mg for children), this should be taken into account when determining the timing and dosage for administration.
Main prehospital use
• Cardiac arrest
• Acute anaphylaxis
• Life threatening asthma with failing ventilation and continued deterioration despite nebuliser therapy.
Other uses
Nebulised in severe croup.
Action
• Increases heart rate
• Increases blood pressure
• Increases myocardial contraction force
• Bronchodilation
• Vasoconstriction.
Preparations
• 10 mL disposable syringe with 0.1 mg/mL (1:10 000)
• 1 mL disposable syringe or ampoule with 1 mg/mL (1:1000).
Indications
• Cardiac arrest
• Acute anaphylaxis
• Severe croup.
Cautions
Hypothermia (give single dose only).
Contraindications
None in cardiac arrest.
Side-effects
• Tachycardia
• Angina and arrhythmias
• Hypertension
• Anxiety
• Headache.
Administration
• IV, IM, intraosseous, nebulised or subcutaneous
• IV administration is far better in cardiac arrest
• IM administration should be used in anaphylaxis.
Dose
• In cardiac arrest, 1 mg (10 mL of 1:10 000) every 3–5 minutes
• In children, initial dose 0.01 mg/kg IV (0.1 mL/kg of 1:10 000) repeated every 3–5 minutes. Use 0.1 mg/kg via ET tube (0.1 mL/kg of 1:1000) in children if IV or IO access cannot be gained quickly. This is the least satisfactory route. In anaphylaxis, if stridor, wheeze, respiratory distress, upper airway or oral swelling or hypotension are present:
• Adults (>12 years of age) 500 micrograms (0.5 mg) IM (0.5 mL of 1:1000) repeated after 5 minutes if necessary (halve dose in prepubertal children)
• Child 6 years – <12 years 250 micrograms IM (0.25 mL of 1:1000) repeat after 5 minutes if necessary
• Child 6 months–<6 years 120 micrograms IM (0.12 mL of 1:1000) repeat after 5 minutes if necessary
• Child <6 months 50 micrograms (0.05 mL of 1:1000) repeat after 5 minutes if necessary.
In severe croup:
• 1 mg (1 mL of 1:1000 diluted to 5 mL with normal saline) via nebuliser.
Frusemide (Furosemide)
Furosemide is a potent loop diuretic with a rapid onset used in pulmonary oedema due to left ventricular failure. IV administration produces rapid relief of breathlessness.
Main prehospital use
Acute left ventricular failure.
Action
• Reduces preload
• Inhibits reabsorption from the ascending limb of the loop of Henle in the kidney.
Preparations
• 5 mL ampoule with 50 mg (10 mg/mL)
• 2 mL ampoule with 40 mg (20 mg/mL)
• Minijet containing 80 mg in 8 mL (10 mg/mL).
Indications
Pulmonary oedema due to left ventricular failure.
Cautions
• Patients with long-standing heart failure
• Pregnancy
• Hypokalaemia.
Contraindications
• Liver failure with pre-comatose state
• Renal failure with anuria.
Side-effects
• Hypotension
• GI disturbance.
Administration
Slow IV injection over 2 minutes.
Dose
• 40 or 50 mg initially
• Repeat to a maximum of 120 mg (3 × 40 mg) or 100 mg (2 × 50 mg).
Glucagon
Glucagon is an alternative to IV glucose in hypoglycaemia. It increases plasma-glucose concentration by mobilising glycogen stored in the liver. It is therefore less effective in hypoglycaemia associated with malnutrition and chronic illness. It can be injected IM in a dose of 1 mg (1 unit) in circumstances when IV glucose would be difficult or impossible to administer. Note that IV glucose is the preferred treatment.
Main prehospital use
Treatment of hypoglycaemia.
Other uses
Poisoning with ß-blockers.
Action
• Breaks down glycogen (a reserve form of glucose found in the liver and other tissues)
• Increases heart rate
• Increases myocardial contractility.
Preparations
Vial with 1 mg powder for reconstitution with sterile water.
Indications
• Blood glucose level is <3.0 mmol/L or
• Suspected hypoglycaemia where oral glucose cannot be administered or
• Unconscious patient, where hypoglycaemia cannot be excluded.
Cautions
• Starvation and malnutrition (ineffective)
• Adrenal insufficiency
• Chronic alcoholism (ineffective).
Contraindications
None.
Side-effects
• Nausea and vomiting
• Hypersensitivity reactions.
Administration
• IM
• The IV route should not be used because it is associated with nausea and vomiting.
Dose
• 1 mg in adult and child over 20 kg, 0.5 mg in children under 20 kg, age <1 month 100 μg
• If not effective in 10 minutes, IV glucose should be given
• Any patient receiving glucagon must be given oral carbohydrates when they are fully conscious. If this is not possible the patient must be hospitalised as blood sugar levels will fall significantly.
Glucose
Main prehospital use
Hypoglycaemic states.
Action
Reverses hypoglycaemia.
Preparations
500 mL bags of 10% (100 mg/mL) glucose solution.
Indications
• Blood glucose level <3.0 mmol/L or
• Suspected hypoglycaemia where oral glucose cannot be administered or
• Unconscious patient, where hypoglycaemia cannot be excluded.
Cautions
Use large-bore cannula in largest available vein to minimise risk of thrombophlebitis.
Contraindications
None.
Side-effects
Tissue necrosis following extravasation.
Administration
IV via a large-bore, free-flowing proximal vein.
Dose
• 5 mL/kg of 10% glucose solution in children (max. 50 mL bolus at once)
• 50 mL of 10% glucose solution (5 g) in adults repeated at 5 minute intervals to effect.
Glyceryl trinitrate
Glyceryl trinitrate may be given as tablets to be dissolved under the tongue (sublingual), modified-release tablets to be dissolved inside the lip (buccal) or as an aerosol spray. The spray has a much longer shelf-life and is therefore more often carried by patients with angina for use when they have symptoms. It is one of the most effective drugs for rapid relief of angina.
Main prehospital uses
Management of ischaemic chest pain associated with acute coronary syndromes
Acute cardiogenic pulmonary oedema.
Other uses
None in the prehospital setting.
Action
Vasodilator which dilates coronary arteries and reduces cardiac preload by dilating systemic veins.
Preparations
• Sublingual tablets, 300 μg
• Aerosol spray, 400 μg/metered dose
• Modified-release buccal tablets 2 mg and 5 mg (Suscard).
Indications
• Cardiac chest pain due to angina and myocardial infarction
• Severe breathing difficulty due to left ventricular failure (acute cardiogenic pulmonary oedema).
Cautions
None.
Contraindications
• Hypotension – do not give if systolic BP <90 mmHg
• Concomitant use of Viagra (sildenafil) or similar drugs. Risk of profound hypotension – do not give within 24 hours
• Hypovolaemia
• Head trauma
• Cerebral haemorrhage.
Side-effects
• Postural hypotension (remove tablet and flush mouth with water)
• Headache
• Flushing
• Dizziness
• Tachycardia.
Administration
Sublingual or buccal.
Dose
• Sublingual tablet, 300 μg repeated as required
• Aerosol, 1–2 sprays under the tongue or into the open mouth, and then close the mouth. Repeat after 5 minutes if required
• Buccal tablet, 2 mg between gum and inner cheek replaced by 5 mg if symptoms not relieved in 3–5 minutes.
HEPARIN (unfractionated heparin)
Main prehospital uses
Given with thrombolytic agents in ST segment elevation myocardial infarction to prevent thrombus reforming (thrombolytic agents are also platelet activators).
Other uses
None in the prehospital setting.
Action
Heparin is a short-acting anticoagulant.
Preparations
1000 units/mL, 5 mL ampoule.
Indications
Immediately following administration of tenecteplase or first bolus of reteplase.
Cautions
• Hepatic and renal impairment
• Pregnancy.
Contraindications
• Haemophilia and other bleeding disorders
• Thrombocytopenia
• Peptic ulcer
• Recent cerebral haemorrhage
• Severe hypertension
• Recent major trauma or surgery
• Recent spinal or epidural anaesthesia
• Hypersensitivity to heparin.
Side-effects
• Haemorrhage
• Skin necrosis
• Thrombocytopenia
• Hyperkalaemia
• Hypersensitivity reactions.
Administration
IV bolus.
Dose
• With Tenecteplase: 4000 units
• With Reteplase: 5000 units.
A Patient Group Directive is required for paramedics to administer heparin.
Hydrocortisone
Hydrocortisone is administered to help prevent the response to secondary mediators in anaphylaxis of severe asthma. Although its onset of action may be delayed by several hours, it should be given as a second-line treatment in prehospital care to avoid unnecessary delays.
Main prehospital use
• Severe/life-threatening asthma
• Anaphylaxis.
Other uses
None in prehospital care.
Action
Limits the effect of secondary mediators (such as kinins) and atopic response.
Preparations
Ampoule with 100 mg in 1 mL.
Indications
• Severe or life-threatening asthma
• Anaphylaxis.
Cautions
None for these indications/doses.
Contraindications
Allergy to the diluent.
Side-effects
• Burning or itching sensation in the groin
• Hypotension if administered quickly.
Administration
Slow IV injection over 2 minutes.
Dose
• Adult/child >12 years 200 mg
• Child 1 month–12 years 4 mg/kg
• Child <1 month 2.5 mg/kg.
Hypostop
Hypostop is a 40% glucose gel containing 23 g of glucose per dose.
Main prehospital use
Management of conscious hypoglycaemic patients able to cooperate and with intact swallow and gag reflexes.
Other uses
None.
Action
Reverses hypoglycaemia.
Preparations
Box containing three single dose tubes of 40% glucose (23 g per tube).
Indications
• Hypoglycaemia in conscious cooperative patients with intact swallow and gag reflexes
• May be used following glucagon administration when GCS is normal.
Cautions
None.
Contraindications
Reduced level of consciousness.
Side-effects
• Aspiration
• Airway obstruction.
Administration
Smear on to gums for most rapid effect.
Dose
Titrated to effect against blood glucose measurements – no upper limit, but if ineffective use 10% glucose IV.
Ipratropium bromide
Main prehospital uses
• Management of acute severe or life-threatening asthma in adults
• Management of acute asthma unresponsive to β2 agonist therapy in children
• Management of acute exacerbations of COPD.
Other uses
Short-term relief of chronic reversible airways obstruction.
Action
Ipratropium is an antimuscarinic (atropine-like) drug that causes bronchodilation.
Preparations
Ipratropium bromide nebuliser solution presented in vials of 250 μg in 1 mL or 500 μg in 2 mL.
Indications
• Life-threatening asthma in adults (mix with first dose of salbutamol)
• Acute severe asthma in adults (mix with first dose of salbutamol)
• Acute exacerbation of COPD in adults that is unresponsive to the first dose of salbutamol (mix with second dose of salbutamol)
• Acute episode of asthma in children that is unresponsive to the first dose of salbutamol (mix with second dose of salbutamol).
Cautions
• Glaucoma (protect patient’s eyes from nebuliser mist)
• Prostatic hyperplasia and bladder outflow obstruction.
Contraindications
None.
Side-effects
• Dry mouth
• Nausea
• Headache
• Acute angle-closure glaucoma (see Cautions, below)
• Constipation
• Tachycardia and atrial fibrillation (rare)
• Paradoxical bronchospasm (rare).
Administration
Via nebuliser.
Dose
• Adults and children >5 years: 500 μg, once only
• Children 1–5 years: 250 μg once only
• Infants (<1 year): 125 μg once only.
Lidocaine (lignocaine)
Lidocaine is a local anaesthetic drug used in the management of wide-complex tachycardia and ventricular fibrillation. It is effective in suppressing ventricular tachycardia and reducing the risk of ventricular fibrillation (especially after myocardial infarction). It has not been shown to reduce mortality when used prophylactically in myocardial infarction.
Main prehospital use
Treatment of refractory VF, pulseless VT, or symptomatic VT (Amiodarone is now the preferred treatment).
Other uses
Local anaesthetic.
Action
• Suppresses ventricular ectopic activity and decreases ventricular automaticity
• Raises threshold for VF (by depressing conduction in ischaemic areas or improving conduction in normal areas)
• Slows conduction of impulses through the Purkinje system
• Raises defibrillation threshold.
Preparations
10 mL disposable syringe containing 100 mg (10 mg/mL).
Indications
• Ventricular fibrillation or pulseless VT refractory to three shocks especially after myocardial infarction, if amiodarone is not available
• Symptomatic ventricular tachycardia.
Cautions
Reduce dose in congestive cardiac and liver failure.
Contraindications
• Patients in heart block
• Premature ventricular contraction with bradycardia
• SBP <90 mmHg (VT with a pulse)
• Known allergy
• If amiodarone has already been administered.
Side-effects
• Central nervous system toxicity with confusion, nausea, vomiting, drowsiness, seizures
• Dizziness and paraesthesia (if injection too rapid)
• Hypotension and bradycardia.
Administration
IV and intraosseous.
Dose
• VF/pulseless VT, 100 mg IV bolus, repeated once if necessary.
Metoclopramide
Metoclopramide is an antiemetic drug which is currently used to reduce the risk of nausea and vomiting following intravenous morphine and to treat severe nausea and vomiting.
Main prehospital use
Reduce risk of nausea and vomiting following administration of morphine sulphate.
Action
• Central effects on chemoreceptor trigger zone
• Peripheral effects on gut.
Preparations
2 mL ampoule with 10 mg (5 mg/mL).
Indications
• Nausea or vomiting in adults
• Concomitant administration with morphine sulphate and other opiates.
Cautions
• Hepatic and renal impairment
• Elderly.
Contraindications
• Avoid in first 12 weeks of pregnancy
• Renal failure
• Patients under age of 20
• Gastrointestinal obstruction
• Phaeochromocytoma.
Side-effects
• Acute dystonic reactions with facial and skeletal muscle spasms. These are more common in the young (especially girls and young women) and the very old.
• Drowsiness
• Rarely, cardiac dysrhythmias.
Administration
• IV over 2 minutes or IM
• Monitor ECG, pulse and BP before, during and after administration.
Dose
10 mg.
Morphine sulphate
Morphine sulphate is the standard opioid analgesic against which all others are judged. It is used for the treatment of severe pain including myocardial infarction, major limb injuries or burns. It produces sedation and euphoria as well as its analgesic effect. Onset of action is within 2–3 minutes if given intravenously with the peak effect in 10–20 minutes. In medical cases, smaller doses may be effective (2.5–5 mg), whereas in injured patients, much larger doses may be needed to achieve effective analgesia.
Prehospital use of opioids has been shown to be safe and effective when used appropriately and titrated to effect according to pain scores. Nevertheless, prehospital practitioners must be able to recognise and deal with the three most important side-effects: respiratory depression, systemic vasodilation and nausea and vomiting. Naloxone should always be available whenever opioids are used.
Morphine is a controlled drug under Schedule 2 of the Misuse of Drugs Regulations 1985. It must be stored and its use documented in accordance with these regulations.
Main prehospital use
Severe pain associated with myocardial infarction, fractures and burns, and other causes.
Other uses
Left ventricular failure.
Action
Acts on μ opioid receptors in the spinal cord and brain.
Preparations
1 mL ampoule with 10 mg (10 mg/mL).
Indications
Severe pain.
Cautions
• Recent alcohol use
• Respiratory depression/disease
• Chest injuries
• Hypotension
• Elderly and debilitated
• Antidepressant use
• Pregnancy.
Contraindications
• Acute respiratory depression (adult <10 bpm)
• Coma or impaired level of consciousness (GCS <12)
• Infants <1 year
• MAOIs
• Phaeochromocytoma
• Hypersensitivity
• Hypotension:SBP
• Adults <90 mmHg
• 5–16 years <80 mmHg
• 1–4 years <70 mmHg.
Side-effects
• Respiratory depression
• Cardiovascular depression
• Nausea and vomiting
• Pupillary constriction.
Administration and dose
• Adults: IV bolus of 2.5–5 mg followed by 1 mg increments titrated against pain score over 10 minutes. Give further 5 mg increments at 5 minute intervals, titrated to effect (max. dose 20 mg)
• Best achieved by diluting 10 mg (1 mL) in 9 mL of water or 0.9% saline to make a 1 mg/mL solution
• Children: use 10 mg in 10 mL solution to administer 0.05 mg/kg (0.05 mL/kg) over 2–3 minutes. Repeat dose at 5–10 minute intervals titrated to effect (max. dose 0.2 mg/kg).
Naloxone
Naloxone is the specific antidote to opioid-induced coma or respiratory depression. Since naloxone has a shorter duration of action than many opioids, close monitoring and repeated injections are necessary according to the respiratory rate and depth of coma.
Main prehospital use
Reversal of respiratory depression associated with opioid excess.
Action
• Competitive antagonist at opioid μ receptors
• In the context of opioid excess, will increase respiratory rate and level of consciousness.
Preparations
1 mL ampoule with 400 μg.
Indications
• Reversal of opioid-induced respiratory, cardiovascular and CNS depression
• Overdose with opioids and opioid-containing medicines
• Unconsciousness associated with respiratory depression or arrest where opiate or opioid overdose is a possibility.
Cautions
• Pain
• Short duration of action
• May precipitate a withdrawal syndrome in those dependent on opiates
• May precipitate fits in patients with epilepsy.
Contraindications
• Known hypersensitivity
• Neonates with opioid-dependent mother.
Side-effects
• Nausea and vomiting
• Tachycardia
• Withdrawal symptoms in opioid dependency.
Administration
• IV or IM
• Where rapid reversal is undesirable (e.g. acute pain or risk of withdrawal), then dilute 2 mL (0.8 mg) in 8 mL of water or 0.9% saline and titrate to effect.
Dose
• 0.4 mg repeated every 2–3 minutes up to a maximum of 10 mg in adults
• 0.01 mg/kg in children followed once by 0.1 mg/kg
• 0.1 mg (0.25 mL) in neonates IM once only.
Patients who are opioid-dependent are at risk of absconding on recovery. Because IV naloxone has shorter half-life than most opioids, respiratory depression may recur. Consider giving a ‘depot’ IM injection of 0.8 mg in this group before any IV dose.
Normal saline
Main prehospital uses
Fluid replacement.
Other uses
• Flush to keep intravenous cannula patent
• Flush to ‘push’ IV drugs into the circulation.
Action
As an infusion, transiently increases intravascular volume.
Preparations
• 500 and 1000 mL bags
• 5 and 10 mL ampoules.
Indications
• Hyperglycaemic ketoacidosis
• Hypovolaemic shock in the absence of a radial pulse
• Burns
• Crush injury
• Anaphylaxis
• Hyperthermia
• Dehydration
• Post-cannulation flush
• Post-IV drug administration flush.
Cautions
None.
Contraindications
None.
Side-effects
• Fluid overload in patients with uncontrolled haemorrhage can cause clot disruption and increased bleeding
• Fluid overload causing heart failure (particularly in the elderly).
Administration
IV infusion or bolus.
Dose
• As a post-drug flush: 10–20 mL (adults and children)
• As a post-cannulation flush: 2 mL (adults and children)
• Adults with dehydration, status asthmaticus, diabetic ketoacidosis, hyperthermia:
Give 500 mL infusion in 20 minutes repeated to effect (maximum dose 2000 mL)
• Children with dehydration, status asthmaticus, diabetic ketoacidosis, hyperthermia:
Give 20 mL/kg bolus repeated once to effect
• Adults and children with hypovolaemic shock, burns, or crush injury.
Oxygen
Regardless of whether a patient is known to have COPD, if they are acutely unwell (pulse oximetry under 90% on room air or low respiratory rate, severe dyspnoea and abnormal respiratory pattern), they should be given high-concentration oxygen via a non-rebreathing mask with a flow rate set at 10–15 L/min.
Main prehospital use
Supplemental oxygen in trauma and acute medical emergencies.
Action
• Essential component of the chemical reaction that occurs in all cells and supports life
• Combines with glucose to liberate energy and carbon dioxide.
Preparations
• Oxygen cylinders are black with a white shoulder
• New light-weight cylinders do not follow this convention.
Indications
• Hypoxia from any cause
• Cardiorespiratory arrest
• Significant trauma
• Pulmonary disease.
Cautions
Patients with COPD.
Contraindications
Paraquat poisoning.
Side-effects
• Respiratory depression in COPD
• Dry mouth.
Administration
Inhalational via non-rebreathing or fixed concentration masks.
Dose
• 100% (in practice approximately 85%) via non-rebreathing reservoir masks usually 10–15 L/min: A sufficient flow rate should be used to keep the reservoir bag inflated in all acutely hypoxic or injured patients
• 24–40% via fixed flow mask in patients with COPD who are not acutely hypoxic (may require more to maintain pulse oximetry in the region of 88–92%).
Paracetamol
Paracetamol is a simple and safe pain-relieving and temperature-reducing drug, which is widely available. In prehospital emergency care, administration of paracetamol in children can control pain and rapidly reduce temperature.
Main prehospital use
Pain relief and temperature control in children.
Action
Simple analgesic and antipyretic (temperature-reducing) drug.
Preparations
Syrup containing 120 mg/5 mL of solution or 250 mg/5 mL of solution.
500 mg tablets.
Indications
• Mild to moderate pain
• Pyrexia.
Cautions
Hepatic and renal impairment.
Contraindications
• Paracetamol overdose
• Previous administration. Within 4 hours or maximum. Cumulative dose given.
Side-effects
Rare.
Administration
Orally as a single dose using a 5 mL syringe without needle or a measuring spoon.
Dose
• 3 months–1 year: 60–125 mg (repeat at 4 hourly intervals to max. 500 mg)
• 1–5 years: 120–250 mg (max. dose in 24 hours = 1 g)
• 6–12 years: 250–500 mg (max. dose in 24 hours = 2 g)
• Adult: 1 g (max. dose in 24 hours = 4 g).
Each dose repeated every 4–6 hours as necessary (max. 4 doses in 24 hours).
Salbutamol
Salbutamol is used in the management of uncontrolled, acute severe and life-threatening asthma and other causes of reversible airways obstruction.
Main prehospital use
Treatment of acute asthma.
Other uses
Bronchospasm from any other cause (COPD, anaphylaxis, LVF).
Action
• Sympathomimetic
• Selective ß2-adrenoreceptor stimulant which reverses bronchospasm.
Preparations
• Nebules containing either 2.5 mg or 5 mg
• Metered dose inhaler (various doses).
Indications
• Acute asthma attack where normal inhaler therapy has failed to relieve symptoms
• Wheezing associated with allergy, anaphylaxis or smoke inhalation
• Exacerbation of COPD
• Second-line treatment for LVF.
Cautions
• Hypertension
• Angina
• Hyperthyroidism
• Late pregnancy.
Contraindications
None.
Side-effects
• Tremor
• Palpitations
• Tachycardia
• Headache
• Peripheral-vasodilation.
Administration
Inhaled as nebulised solution or as an aerosol via a spacer device if the patient has one.
Dose
• Adults and children >5 years: 5 mg via nebuliser repeated to effect or side-effects are intolerable
• 12 months–5 years: 2.5 mg repeated to effect at 15 minute intervals
• <12 months: 2.5 mg once only.
In severe or life-threatening asthma, continuous nebulisation is required.
Syntometrine
Oxytocic (uterus-stimulating) drugs are used to minimise blood loss from the placental site during the routine management of the third stage of labour. The combination of ergometrine 500 μg with oxytocin 5 units is given by IM injection with or after delivery of the shoulders.
Main prehospital use
Management of third stage of labour.
Other uses
• Postpartum haemorrhage within 24 hours of delivery
• Control of bleeding in incomplete miscarriage.
Action
Stimulates contraction of the uterus within 7 minutes of IM injection.
Preparations
1 mL ampoule containing ergometrine 500 μg and oxytocin 5 units.
Indications
• Active management of the third stage of labour
• Following delivery of the placenta to prevent or treat postpartum haemorrhage
• Control of bleeding in incomplete miscarriage.
Cautions
• Cardiac disease
• Hepatic and renal impairment.
Contraindications
• Known hypersensitivity
• First or second stage of labour
• Severe cardiac, liver or kidney disease
• Hypertension
• Eclampsia/pre-eclampsia
• Multiple pregnancy (fetus still in utero).
Side-effects
• Nausea and vomiting
• Abdominal pain
• Headache
• Hypertension
• Cardiac arrhythmias (bradycardia)
• Chest pain
• Anaphylactic reactions (rare).
Administration
IM with or after delivery of the shoulders.
Dose
1 mL.
Tenecteplase and reteplase
Tenecteplase and reteplase are thrombolytic drugs which are indicated for myocardial infarction due to their ability to break up thrombus and permit return of blood supply. The benefits of treatment must be considered to outweigh the risks (hence the specific indications and contraindications given below). Trials have shown that the benefit is greatest in those with ECG changes that include ST segment elevation (especially in those with anterior infarction) and in those with new bundle brand block.
Main prehospital use
Treatment of acute myocardial infarction with pain of >15 minutes and <6 hours duration.
Other uses
None prehospital.
Action
Activates the fibrinolytic system, inducing the breaking up of intravascular thrombi and emboli.
Preparations
Tenecteplase
Powder for reconstitution 40 mg (8000 units), or 50 mg (10 000 units).
Reteplase
Powder for reconstitution 10 units to be dissolved in 10 mL of water for injection
Both with prefilled syringe of water for injection.
Indications
• Acute myocardial infarction, where pain has been present continuously for at least 15 minutes and less than 6 hours
• Patient must fulfill all JRCALC guideline criteria
• JRCALC criteria for paramedic-administered thrombolysis (version 3).
Primary assessment
1. Can you confirm that the patient is conscious, coherent and able to understand that clot-dissolving drugs will be used?
2. Can you confirm that the patient is aged 75 or less?
3. Can you confirm that the patient has had symptoms characteristic of a heart attack (i.e. continuous pain in a typical distribution and of 15 minutes duration or longer)?
4. Can you confirm that the symptoms started less than 6 hours ago?
5. Can you confirm that the pain built up over seconds and minutes rather than starting totally abruptly?
6. Can you confirm that breathing does not influence the severity of the pain?
7. Can you confirm that the heart rate is between 50 and 140 bpm?
8. Can you confirm that the systolic blood pressure is more than 80 mmHg and less than 160 mmHg and that the diastolic blood pressure is below 110 mmHg?
9. Can you confirm that the electrocardiogram shows abnormal ST segment elevation of 2 mm or more in at least two standard leads or in at least two adjacent precordial leads, not including V 1 (ST elevation can sometimes be normal in V 1 and V 2)?
10. Can you confirm that the QRS width is 0.16 mm or less, and that left bundle branch block is absent from the tracing? ( Note: RBBB permitted only with qualifying ST segment elevation.)
11. Can you confirm that there is NO atrioventricular block greater than 1st degree? (If necessary after treatment with IV atropine.)
Secondary assessment
12. Can you confirm that the patient is not likely to be pregnant, nor has delivered within the last 2 weeks?
13. Can you confirm that the patient has not had a peptic ulcer within the last 6 months?
14. Can you confirm that the patient has not had a stroke of any sort within the last 12 months and does not have permanent disability from a previous stroke?
15. Can you confirm the patient has no diagnosed bleeding tendency; has had no recent blood loss (except for normal menstruation); and is not taking warfarin (anticoagulant) therapy?
16. Can you confirm the patient has not had any surgical operation, tooth extractions, significant trauma, or head injury within the last 4 weeks?
17. Can you confirm that the patient has not been treated within the last 3 months for any other serious head or brain condition? (This is intended to exclude patients with cerebral tumours.)
18. Can you confirm that the patient is not being treated for liver failure, renal failure, or any other severe systemic illness?
Contraindications
Does not meet JRCALC guideline criteria.
Side-effects
• Elevation of body temperature
• Nausea and vomiting
• Haemorrhage, including stroke
• Hypotension
• Reperfusion arrhythmias
• Anaphylaxis (rare)
• Allergic responses including urticarial rash and low back pain.
Administration
• Tenecteplase: slow IV bolus
• Reteplase: slow IV bolus repeated after 30 minutes.
Dose
Tenecteplase
Single intravenous dose according to the patient’s weight
• <60 kg 6000 μ
• 60–69 kg 7000 μ
• 70–79 kg 8000 μ
• 80–89 kg 9000 μ
• >90 kg 10 000 μ
Reteplase
10 units repeated exactly 30 minutes later.
Tetracaine 4% gel (AMETOP)
Main prehospital uses
Use to provide local anaesthesia prior to non-urgent venepuncture.
Other uses
None in the prehospital setting.
Action
Local anaesthetic agent designed to penetrate intact skin to provide local numbing prior to venepuncture.
Preparations
1.5 g tubes of white semitransparent gel.
Indications
Patient requiring non-urgent venepuncture after 30–45 minutes and at risk of distress from the procedure.
Cautions
Check for allergy to occlusive dressing of choice.
Contraindications
• Venepuncture required in less than 30–45 minutes
• Do not apply to open wounds, broken skin, lips mouth, eyes, ears, anal or genital region or mucous membranes
• Known allergy to tetracaine, any of its constituents or other local anaesthetic agents. Infants less than 1 month old
• Pregnancy or breast-feeding.
Side-effects
• Inappropriately rapid absorption from mucous membranes, wounds or inflamed tissue
• Hypersensitivity reactions.
Administration
Topical application at proposed venepuncture site(s). Cover with transparent occlusive dressing. Wait for 30–45 minutes before removing dressing and cream and attempting venepuncture.
Dose
• Apply sufficient cream to cover intended venepuncture site(s)
For further information, see Ch. 10 in Emergency Care: A Textbook for Paramedics.