Chapter 18 Drug excretion
Drug excretion can occur by several means:
Kidneys
The factors affecting the rate at which the drug or remedy is excreted by the kidneys are:
Kidney Disease
Useful complexes such as plasma proteins (see Chapter 16 ‘How do drugs get into cells?’, p. 126) can be lost, increasing the amount of unbound remedy in the body, with resulting toxicity. Other compounds may be lost in the urine as the reuptake is reduced.
pH of Urine
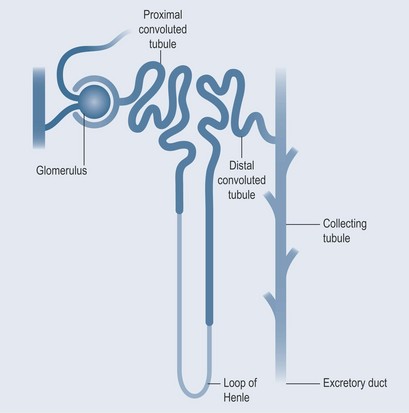
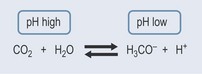
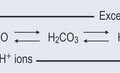
The pHs of the urine and blood are interrelated. Tubular secretion, which takes place in the kidneys, is an active process whereby certain molecules and ions are removed from the blood and actively secreted into the tubules. From the buffering equation (Figure 18.2), it is possible to see that:
The kidneys are capable of absorbing fluctuations in pH and will adjust the removal or retention of hydrogen ions as necessary. This is why the pH of the urine can vary so widely but the blood pH is maintained within very narrow limits.
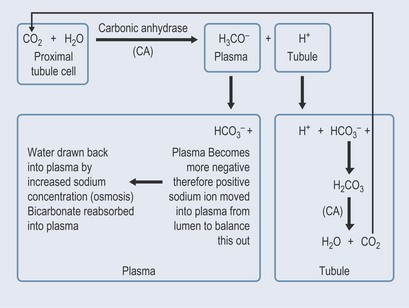
The kidneys act when the blood becomes too acidic (right-hand side of the equation) or too basic (left-hand side of the equation) by altering the amount of water lost through urination. The buffering mechanism is associated with sodium balance, which is also controlled by the kidneys (Figure 18.3) and involves an enzyme called carbonic anhydrase (CA; see Chapter 26 ‘Cardiovascular disorders’, p. 198).
The erythrocytes also contain carbonic anhydrase, which acts as described above to enable them to carry carbon dioxide in the form of the bicarbonate ion (see Chapter 28 ‘Blood disorders’, p. 209).
• How Does pH Affect Drug Excretion?
The filtrate passing into the first part of the renal tubule has the same pH as plasma, roughly neutral. As the pH of urine can be anything from 4.5 to 8.0, this will affect the rate of drug excretion (see Chapter 8 ‘Acids and bases’, p. 55).
Change in Renal Blood Flow
Perfusion rates are as important for removal as the distribution of a drug or remedy (see Chapter 16 ‘How do drugs get into cells?’, p. 125). Any change in renal blood flow due to kidney (or another) disease will increase the time it takes for a substance to leave the body.
Concentration of Drug or Remedy in Plasma
Normally, a high concentration of unbound substance will ensure its removal if it is small and water soluble. Many drugs are bound to plasma proteins (see Chapter 16 ‘How do drugs get into cells?’, p. 126). How tightly they are bound then affects how easily they can be removed when they reach the kidneys. This can be controlled by pH or the amount of free substance in the plasma.
Bile
Drugs can be actively removed into the bile for removal from the body. Because this is done against a concentration gradient, and requires an active process, there might be competition for receptors. The drugs in question will be conjugated to achieve their removal, but might then take part in the enterohepatic cycle (see Chapter 17 ‘Metabolism’, p. 131). However, this process is not 100% effective and some substance is removed from the body in the faeces.