Chapter 10 Drug Effects, Electrolyte Abnormalities, and Metabolic Factors
Drug Effects
Drugs Used to Treat Arrhythmias
Cardiologists often use a shorthand classification system when referring to drugs primarily used to treat arrhythmias (Box 10-1). This system has a number of flaws, but is widely employed—so students and clinicians need to be aware of it.
BOX 10-1 Commonly Used Classification of Drugs Used to Treat Arrhythmias
Class 1: Sodium channel blocking (conduction slowing) effects
Class 2: Beta-blocking effects (examples: atenolol; carvedilol; metoprolol; propranolol)
Class 3: Potassium channel (repolarization) blocking effects (examples: amiodarone; dofetilide; dronedarone; ibutilide; sotalol)
Class 4: Calcium channel blocking effects (examples: diltiazem; verapamil)
Class 5: Other (examples: glycosides, such as digoxin; adenosine)
Class 1 drugs have a sodium channel blocking action, so they may prolong the QRS duration. The class I drugs are subdivided into A, B, and C groups. Class 1A drugs also prolong repolarization via potassium channel blocking effects. Therefore, they may prolong the QT(U) interval, leading to increased risk of torsades de pointes and sudden cardiac arrest (Chapters 16 and 19; Figs. 2-13 and 19-7). Class 1B drugs include lidocaine and mexiletine. Class IC drugs, such as flecainide and propafenone, used to treat atrial fibrillation and other supraventricular tachycardias, are the most likely to produce clinically important widening of the QRS complex (intraventricular conduction delays) due to their prominent sodium channel blocking effects.
All “antiarrhythmic” class 1 (sodium channel blocking) drugs, along with many other pharmaceutical agents, may, paradoxically, induce or promote the occurrence of life-threatening ventricular arrhythmias by altering basic electrical properties of myocardial cells. These so-called proarrhythmic drug effects, which are of major clinical importance, are discussed further in Chapters 16 and 19.
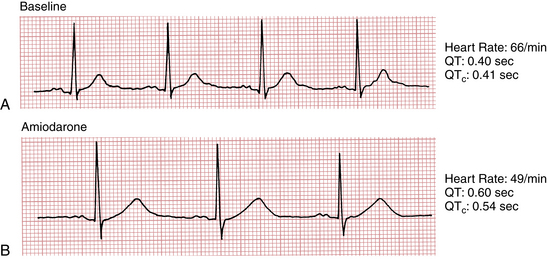
Prolongation of the QT(U) interval with a life-threatening risk of torsades de pointes, another type of ventricular proarrhythmia, can also occur with class 3 drugs, notably ibutilide, dofetilide, sotalol (also has beta-blocking effects), amiodarone (also beta-blocking, among other, effects), and dronedarone (Fig. 10-1). This effect is also related to blocking of potassium channel function with prolongation of myocardial cellular repolarization.
Weaknesses of this classification scheme include its failure to account for drugs with “mixed” effects (like amiodarone and sotalol) and the fact that important drugs, such as adenosine and digoxin, do not fit in. Instead, they are placed under the class 5 or “other” category. The therapeutic and toxic effects of digitalis-related drugs, like digoxin, are discussed separately in Chapter 18.
Psychotropic and Related Drugs
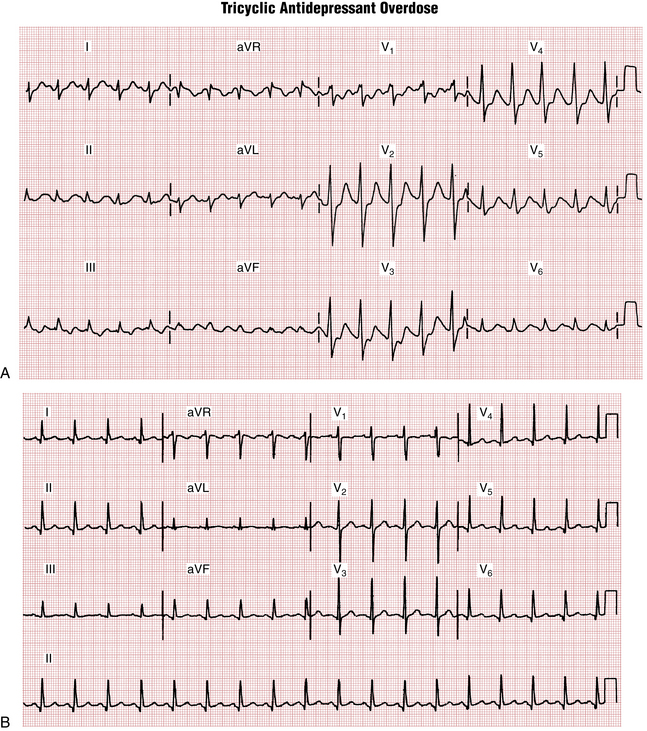
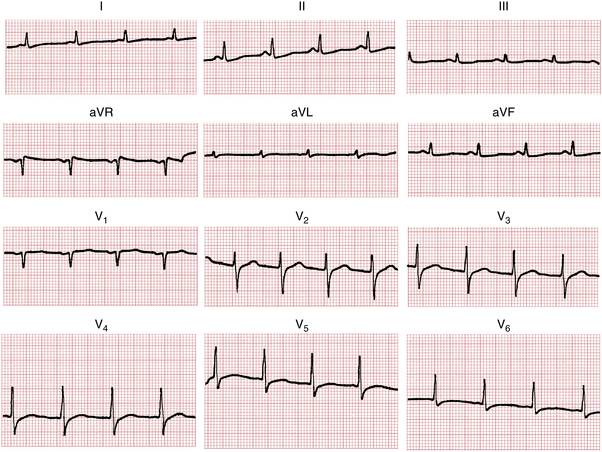
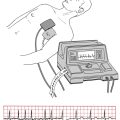
Psychotropic drugs (e.g., phenothiazines and tricyclic antidepressants) can markedly alter the ECG and in toxic doses can induce syncope or cardiac arrest due to a ventricular tachyarrhythmia or asystole. They may also prolong the QRS interval, causing a bundle branch block–like pattern, or they may lengthen repolarization (long QT-U intervals), predisposing patients to develop torsades de pointes. Figure 10-2 presents the classic ECG findings of tricyclic antidepressant overdose, in this case in a young adult. Notice the prolonged QRS and QT intervals, as well as sinus tachycardia.
A variety of drugs used in psychiatric practice can prolong the QT interval, predisposing to torsades de pointes type ventricular tachycardia. These drugs include methadone and the so-called “atypical or second generation antispsychotics” (e.g., risperidone and quetiapine) agents. This topic is discussed further in Chapter 16.
Lithium carbonate, widely used in the treatment of bipolar disease, may cause sinus node pacemaker dysfunction or sinus exit block and severe bradycardia (Chapter 11).
Electrolyte Disturbances
Hyperkalemia
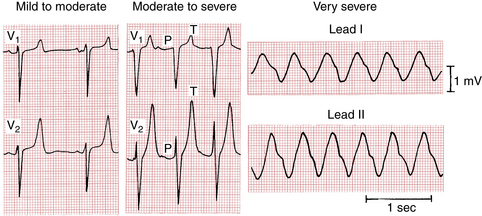
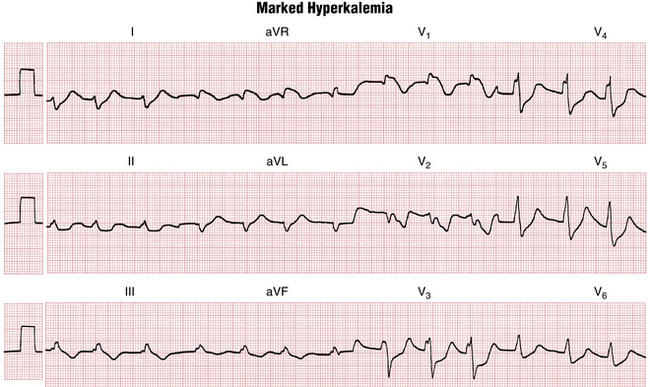
As shown in Figure 10-3, progressive hyperkalemia produces a distinctive sequence of ECG changes affecting both depolarization (QRS complex) and repolarization (ST-T segments). The normal serum potassium concentration is between 3.5 and 5 mEq/L. The first change seen with abnormal elevation of the serum potassium concentration is narrowing and peaking of the T waves. As Figure 10-4 demonstrates, the T waves with hyperkalemia have a characteristic “tented” or “pinched” shape, and they may become quite tall. With further elevation of the serum potassium concentration, the PR intervals become prolonged and the P waves are smaller and may disappear entirely. Continued elevations produce an intraventricular conduction delay, with widening of the QRS complexes (see Figs. 10-3 and 10-4). As the serum potassium concentration rises further, the QRS complexes continue to widen, leading eventually to a large undulating (sine wave) pattern and asystole, with cardiac arrest (Chapter 19).
Hypokalemia
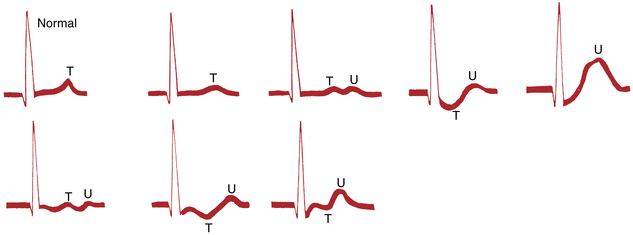
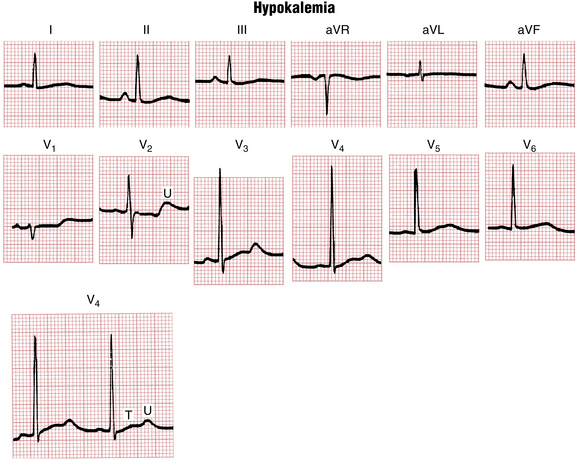
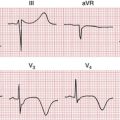
Hypokalemia produces distinctive changes in the ST-T complex. The most common pattern seen is ST depressions with prominent U waves and prolonged repolarization (Figs. 10-5 and 10-6). With hypokalemia the U waves typically become enlarged and may even exceed the height of the T waves. Technically the QT interval with hypokalemia may remain normal whereas repolarization is prolonged (as shown by the prominent U waves). Because the T waves and U waves often merge, the QT intervals cannot always be accurately measured.
Hypercalcemia and Hypocalcemia
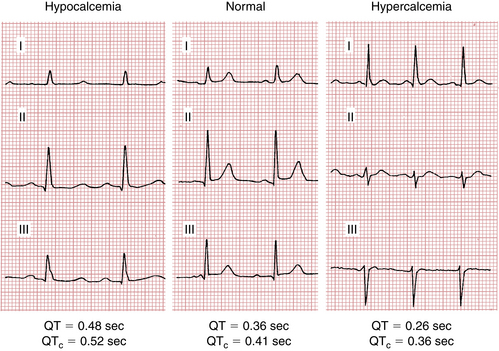
Ventricular repolarization is shortened by hypercalcemia and lengthened by hypocalcemia (Fig. 10-7). In hypercalcemia the shortening of the QT interval is due to shortening of the ST segment. With marked hypercalcemia the T wave appears to take off right from the end of the QRS complex. High serum calcium concentrations may lead to coma and death. A short QT interval in a patient with mental status changes is sometimes the first clue to the diagnosis of hypercalcemia. Hypocalcemia lengthens or prolongs the QT interval, usually by “stretching out” the ST segment. Note, however, that patients may have clinically significant hypocalcemia or hypercalcemia without diagnostic ECG changes.
Magnesium Disturbances
Hypomagnesemia, usually due to gastrointestinal or renal losses (e.g., with certain diuretics) may also play a pathogenetic role in causing or increasing the severity of hypokalemia. The ECG may be dominated by signs of the latter (see preceding discussion) in such cases. Hypomagnesemia has been implicated in ventricular arrhythmogenesis with acute myocardial infarction and also in torsades de pointes. Administration of intravenous magnesium is recommended empiric therapy in cases of torsades and may help suppress the early after-depolarizations that initiate this polymorphic ventricular tachyarrhythmia (Chapter 16). Hypomagnesemia may also potentiate digitalis toxicity (Chapter 18). In addition, hypomagnesemia is important because it may foster hypocalcemia (see preceding discussion) by inhibiting release of parathyroid hormone.
Other Metabolic Factors
Hypothermia
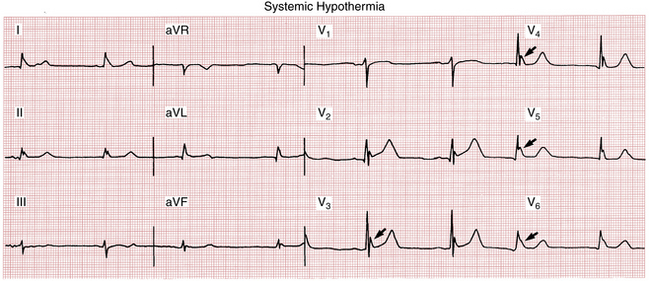
Patients with systemic hypothermia may develop a distinctive ECG pattern in which a humplike elevation is usually localized to the junction of the end of the QRS complex and the beginning of the ST segment (J point) (Fig. 10-8). These pathologic J waves are sometimes called Osborn waves. This pattern disappears with rewarming. The basic mechanism of these prominent J waves appears to be related to the differential effects of systemic cooling on repolarization of different layers of the ventricles.
Endocrine Abnormalities
Most endocrine disorders do not produces specific changes on the ECG. In some instances, however, the ECG may play an important role in the diagnosis and management of hormonal abnormalities. For example, hyperthyroidism (most commonly due to Graves’ disease) is often associated with an inappropriately high resting sinus heart rate. The finding of an unexplained high sinus rate at rest should always lead to suspicion of hyperthyroidism, as should the finding of atrial fibrillation (see Chapter 15).
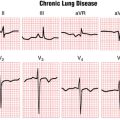
In contrast, hypothyroidism is often associated with a slow resting heart (sinus bradycardia). Severe hypothyroidism (myxedema) may lead to pericardial effusion, thereby causing low voltage QRS complexes. Low QRS voltage is said to be present when the total amplitude of the QRS complexes in each of the six extremity leads is 5 mm or less, or 10 mm or less in the chest leads. Low QRS voltage is not a specific finding but can be related to a variety of mechanisms and causes, including increased insulation of the heart by air (chronic obstructive pulmonary disease) or adipose tissue (obesity); replacement of myocardium, for example, by fibrous tissue (in cardiomyopathy), amyloid, or tumor; or the accumulation of extracellular fluids (as with anasarca, or with pericardial or pleural effusions) (see Chapter 24).
Acidosis and Alkalosis
Metabolic acidosis and alkalosis are, by themselves, not associated with specific ECG findings. Metabolic acidosis is typically associated with hyperkalemia and metabolic alkalosis with hypokalemia. The ECG morphologic appearance will be dominated by these electrolyte perturbations, both of which, when severe, may lead or contribute to cardiac arrest (see Chapter 19).
ST-T Changes: Specific and Nonspecific
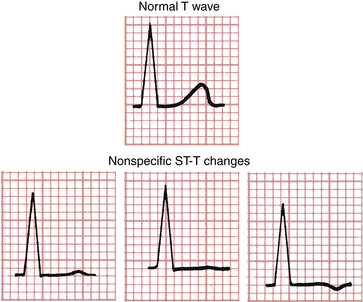
The concluding topic of this chapter is a brief review of the major factors that cause ST-T (repolarization) changes. The term nonspecific ST-T change (defined in Chapter 9) is commonly used in clinical electrocardiography. Many factors (e.g., drugs, ischemia, electrolyte imbalances, infections, and pulmonary disease) can affect the ECG. As already mentioned, the repolarization phase (ST-T complex) is particularly sensitive to such effects and can show a variety of nonspecific changes as a result of multiple factors (Figs. 10-9 and 10-10). These changes include slight ST segment depressions, T wave flattening, and slight T wave inversions (see Fig. 10-9).
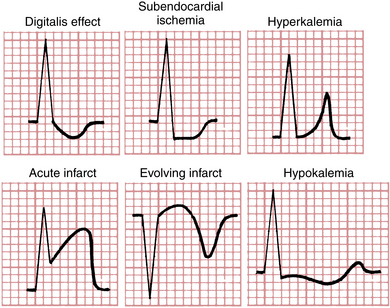
In contrast to these nonspecific ST-T changes, certain fairly specific changes are associated with particular conditions (e.g., the tall, tented T waves of hyperkalemia). Some of these relatively specific ST-T changes are shown in Figure 10-11. However, even such apparently specific changes can be misleading. For example, ST elevations are characteristic of acute transmural ischemia, but they are also seen in ventricular aneurysms, pericarditis, and benign (normal) early repolarization. Similarly, deep T wave inversions are most characteristic of ischemia but may occur in other conditions as well (see Chapters 9 and 24).
In summary, repolarization abnormalities can be grouped into two general categories: (1) Nonspecific ST-T changes include slight ST segment deviation and flattening or inversion of the T wave. These changes are not diagnostic of any particular condition but must always be interpreted in clinical context. (2) Relatively specific ST-T changes are more strongly but not always definitively diagnostic of some particular underlying cause (e.g., hyperkalemia or myocardial ischemia).