CHAPTER 22 Double-Bundle Anterior Cruciate Ligament Reconstruction
Successful clinical results following single-bundle anterior cruciate ligament (ACL) reconstruction have been reported in 70% to 90% of cases.1,2 Historically, single-bundle ACL reconstruction techniques have been the standard treatment of choice for the ACL-deficient knee. As more and more patients have been helped through advanced surgical techniques and rehabilitation protocols, the expectations of future patients have risen. Today, almost all patients who suffer an acute ACL injury expect to return to full, unlimited sports activity in a rapid fashion. However, residual rotational instability has been reported in approximately 20% of cases after conventional single-bundle reconstruction.3 A recent biomechanical study has shown that single-bundle ACL reconstruction can successfully control anterior tibial translation but could not control a combined rotatory load of internal rotation and valgus torque.4 Similarly, in vivo kinematic studies have shown that ACL reconstruction can restore anteroposterior stability. However, the tibia remains externally rotated relative to the femur during normal kinematics of running.5 The concept of anatomic ACL reconstruction is predicated on a desire to restore native anatomy more closely and in turn, normal kinematics of the knee.
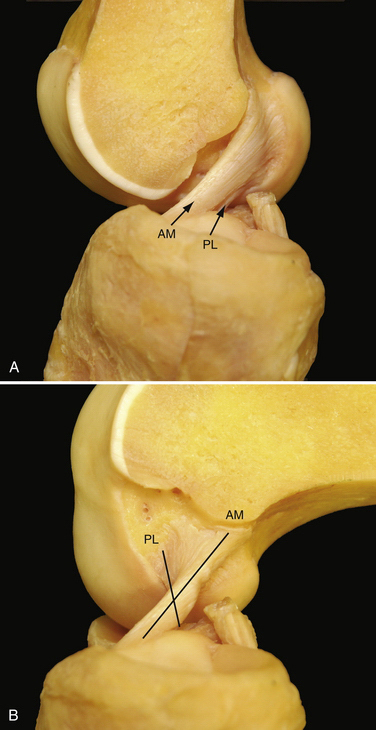
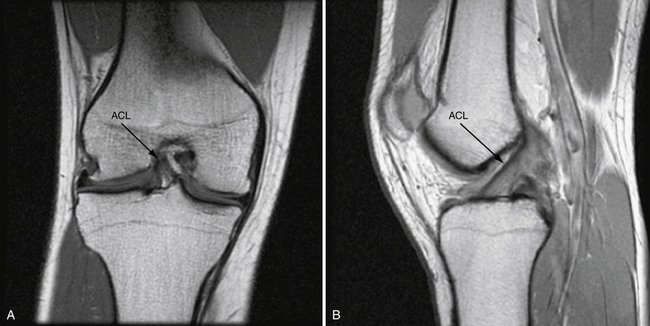
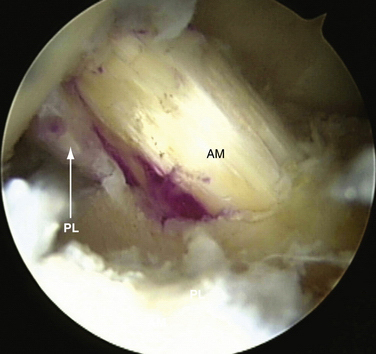
Multiple anatomic studies have used various methods to demonstrate that the ACL is composed of two distinct functional bundles; the anteromedial (AM) and posterolateral (PL) bundles (Fig. 22-1).6–8 Biomechanical studies have demonstrated that in situ forces, in response to an anterior tibial and combined rotatory load, are greatest in the PL bundle near full extension and at 60 degrees flexion in the AM bundle, respectively. Studies have also shown that reconstruction of either of the individual bundles alone cannot reproduce the mechanical properties of the native intact ACL.9,10 Yagi and colleagues11 have measured the in situ forces in response to an anterior tibial load and a combined rotatory load of internal rotation and valgus torque using a robotic universal force moment sensor testing system. When compared with single-bundle ACL reconstruction, the in situ forces in double-bundle reconstructed knees more closely approximated those of the native ACL.
ANATOMY
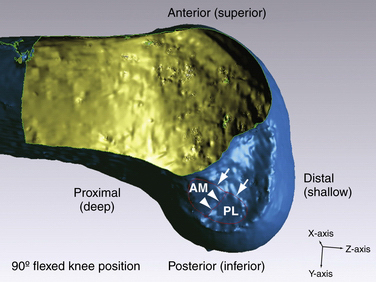
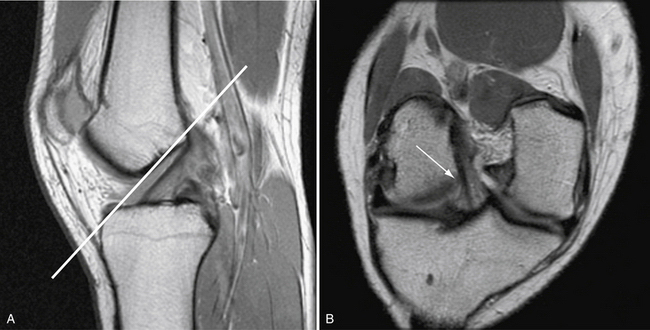
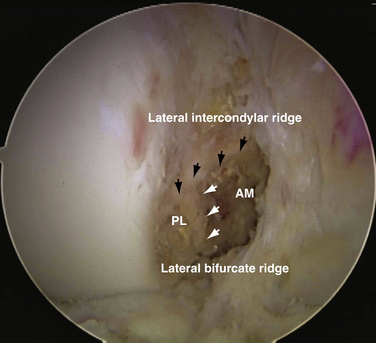
The ACL courses from the medial aspect of the lateral femoral condyle (femoral insertion site) to a position on the tibia between the medial and lateral tibial spines (tibial insertion site). For the sake of consistency, this chapter refers to positional relationships with the knee located in the anatomic position or full knee extension. It is important to realize that arthroscopic evaluation of the ACL typically occurs with the knee flexed to 90 degrees. For this reason, the anatomically anterior border of the ACL will appear arthroscopically as the superior border. Similarly, the anatomically posterior extent of the ACL will appear as the arthroscopic inferior border when the knee is flexed to 90 degrees (Fig. 22-2). The femoral insertion site or ACL origin is located at the anatomically posterior aspect of the lateral surface of the femoral intercondylar notch. The length of the femoral footprint of the ACL is 17.7 ± 1.2 mm and the width is 9.9 ± 0.8 mm. The femoral attachment of the AM bundle forms an angle with the PL bundle of 27.6 ± 8.8 degrees.12 The average length of the femoral AM bundle is 9.8 ± 0.8 mm, and the average length of the femoral PL bundle is 7.3 ± 0.5 mm. The anatomically anterior (arthroscopically superior) border of the femoral insertion of the ACL is defined by an important bony landmark13 referred to as the lateral intercondylar ridge (see Fig. 22-2). In a surgical observational study, no ACL fibers could be seen attaching anterior to the ridge. The lateral bifurcate ridge, another important osseous landmark, delineates the AM and PL insertion areas. Ferretti and associates12 arthroscopically visualized the lateral bifurcate ridge in 49 of 60 patients undergoing ACL reconstruction. Of the 49 patients with a discernible lateral bifurcate ridge, 25 had a prominent ridge with a significant change of slope, whereas the other 24 had a relatively smaller but evident ridge.
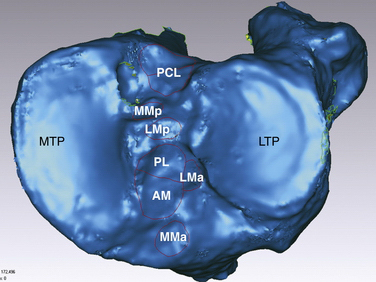
Anatomic ACL reconstruction is based on a comprehensive understanding of normal anatomy. It is important to realize that the course of the AM and PL bundles is distinct, with the bundles lining up in parallel near full extension and then crossing as the knee approaches 90 degrees of flexion (see Fig. 22-1).6,12,14–16 Specifically, the AM bundle originates at the anatomically posterior and proximal (arthroscopically inferior and deep) aspect of the lateral wall of the intercondylar notch. The PL bundle originates at the anatomically posterior and distal (arthroscopically inferior and shallow) aspect of the lateral wall of the notch (see Fig. 22-2). From the femoral origin, the fibers of the ACL course down to the tibial insertion site. In its midportion, the ACL tapers to a thinner diameter, similar to an hourglass shape. The cross-sectional area of the insertion sites are 3 to 3.5 times larger than the cross-sectional area of the ligament’s midsubstance.8,17 The ACL fibers fan out and insert on the center of the tibial plateau, between the two spines. It is for their relative insertion sites on the tibia that the two bundles of the ACL are named, with the AM tibial insertion site slightly anterior and medial to the PL bundle insertion site (Fig. 22-3.) The mean length of the tibial insertion has been reported in multiple studies7,18,19 to range from 14 mm (range, 9 to 18 mm) to 29.3 mm (range, 23 to 38 mm). The total area of the tibial insertion has also been reported with great variability, from 114 to 229 mm2. The area of the AM bundle insertion alone has been reported from 56 to 136 mm2 and the PL bundle from 52 to 93 mm2. The tremendous variability in reported size and area of the tibial insertion sites likely stems from the various methods used for taking measurements and the variable size of the cadaveric specimens used.20
Biomechanics
Recent biomechanical studies have more clearly defined the specific function of the two individual bundles of the ACL. Selective sectioning studies have shown that transection of the AM bundle increases anterior translation of tibia at 60 and 90 degrees of knee flexion. Conversely, transection of the PL bundle increases anterior translation of the tibia at 30 degrees of flexion. Following PL bundle transection, there was also increased rotation at 0 and 30 degrees in response to a combined rotatory load.21
In general, the concept of isometry in ACL reconstruction should be dismissed. The AM and PL bundles do not remain isometric throughout a full functional range of motion. Each bundle of native ACL is exposed to varying degrees of tension at various flexion angles.22 The AM bundle is slightly more isometric than the PL bundle, remaining taut throughout a greater arc of motion. In contrast, the PL bundle is taut with the knee in extension and is lax when the knee is flexed. Logically, the contribution of the PL bundle to knee stability becomes increasingly important under greater degrees of tension, or near full extension.9,10,23
Injury Mechanism and Rupture Pattern
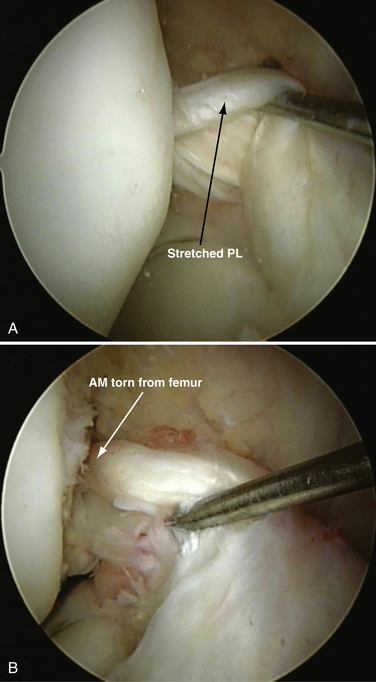
Understanding the mechanism of injury is an important first step in formulating an appropriate treatment strategy. Various injury mechanisms will place differential strains on the AM and PL bundles, thus producing various rupture patterns. Two distinct injury mechanisms of ACL have been classically described by Muller24: (1) hyperextension trauma, with resultant stretch of the ACL over the anterior intercondylar notch roof; and (2) moderate extension trauma, during which the AM bundle is taut and a valgus and/or external rotation force is applied. Based on the variable tension patterns exhibited different positions of knee flexion, we have seen a variety of two bundle injury patterns and isolated AM or isolated PL bundle injuries (Fig. 22-4). Isolated PL bundle injury occurs when stress is applied at or near full extension. In greater degrees of flexion (30 to 60 degrees), isolated AM bundle injury can occur.
In a prospective study, 121 consecutive patients were evaluated arthroscopically. with attention directed to determining the pattern of rupture.25 All patients were evaluated within 120 days of acute injury. There was complete rupture of the AM and PL bundles in 75% of patients. In the remaining 25% of patients, partial tear of the ACL could be seen. In 12% of total patients, no evidence of PL bundle injury could be seen. Therefore, we think that meticulous dissection of the remnant ACL is an important first step prior to proceeding with the reconstructive aspect of ACL surgery. Single-bundle augmentation is performed when appropriate, according to the specific injury pattern.26 Preservation of intact native tissue has distinct advantages, including preservation of the microvasculature and proprioceptive fibers. Isolated reconstruction of the AM or PL bundle alone requires an awareness of such injury patterns and more precise preoperative and intraoperative diagnostic assessment of the injury pattern.
PATIENT EVALUATION
History and Physical Examination
In cases of acute ACL injury, patients typically present with painful swelling, limp, and an obvious history of trauma. Common mechanisms include landing after jumping, a deceleration change in direction seen in pivoting sports (e.g., soccer, basketball, tennis), and direct blow forces on the lateral side of the knee producing valgus and external rotational force on the tibia. Some patients will report an audible pop or the sensation of tearing at the time of injury. A significant effusion typically occurs within a few hours of injury. A flexed posture of the knee joint can be seen caused by the large hemarthrosis, but should also raise concern for the possibility of mechanical obstruction from the remnant ACL or a bucket handle tear of the meniscus. In patients with a history of trauma followed by hemarthrosis, the most common arthroscopic finding is ACL injury (45% in a study of 320 patients).27
A manual assessment of knee laxity includes several tests—the Lachman, anterior drawer, and pivot shift tests. The Lachman test is considered to be the most reliable test for assessment of ACL injury.28 However, false-negative findings can be seen in acute cases when significant hamstring spasm and guarding are present. To avoid a high false-negative rate, effort should be made to have the patient as relaxed as possible. The Lachman test is performed at 20 to 30 degrees of knee flexion. One hand of the examiner stabilizes the femur and the other hand applies an anteriorly directed force to the proximal tibia. The degree of translation and the quality or firmness of the end point should be noted. Classification of anteroposterior translation is categorized as grade I (3 to 5 mm), grade II (6 to 10 mm), or grade III (more than 10 mm).
The pivot shift test is highly specific for the diagnosis of ACL injury, but the sensitivity is low, particularly in the awake patient. Most patients may allow for a single shift to be performed, but will typically guard against allowing the knee to shift thereafter.28,29 The pivot shift maneuver mimics the giving way or buckling phenomenon that patients experience. A pivot shift test is performed with the patient supine and the hip in slight abduction as a valgus force is applied to the extended knee. In the ACL-deficient knee, the tibia rests in an internally rotated and anteriorly subluxated position. As the knee is flexed to 20 degrees, the tibia reduces, suddenly resulting in the pivot shift. The degree of shift is graded as grade I or pivot glide, grade II or distinct shift, and grade III or transiently locked out. This test can be difficult to perform in the awake patient but is highly sensitive and specific during the examination under anesthesia.30
Diagnostic Imaging
In most cases of acute ACL injury, plain radiographs will be negative. Still, radiographs are necessary to rule out combined ligamentous injury, secondary arthritic changes, or physeal injury in the skeletally immature patient. The Segond fracture, or lateral tibial rim avulsion of the meniscotibial ligament, is considered pathognomonic for ACL injury. This lateral capsular avulsion is produced by the stress of knee flexion and internal tibial rotation commonly seen as a mechanism of ACL injury.31
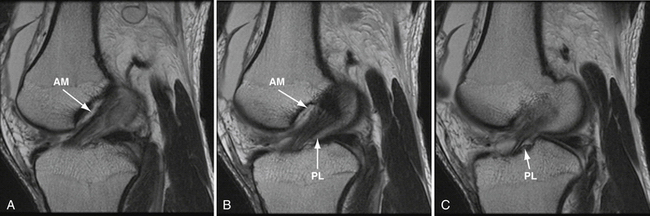
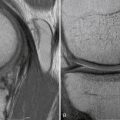
Magnetic resonance imaging (MRI) is highly sensitive and specific for the diagnosis of acute ACL tear.32 However, it is often far more difficult to differentiate partial tears from complete tears or to detect isolated single-bundle tear patterns. There is great variability in clinically available MRI magnets. High-resolution MRI with dedicated ACL sequences greatly increases the sensitivity of MRI. Standard MRI protocols consist of sagittal, coronal, and axial T1- and T2-weighted images (Fig. 22-5). The issue with such sequences is that the ACL does not course through the knee in a perfectly coronal or sagittal plane. Therefore, to improve sensitivity, we use modified oblique coronal and oblique sagittal imaging sequences. When the beam is oriented parallel to the fibers of the ACL, greater detail is appreciated and the AM and PL bundles can be evaluated independently. (Figs. 22-6 and 22-7).
TREATMENT
Arthroscopic Technique
Operating Room Setup and Examination Under Anesthesia
ACL reconstruction can be performed under general or spinal anesthesia based on patient and surgeon preference. The examination under anesthesia can provide additional valuable information and should be performed in all cases. When compared with the office examination, the sensitivity of the pivot shift test is greatly enhanced.29 Once the patient is under anesthesia, we routinely evaluate the range of motion and perform a comprehensive manual assessment of knee laxity including the pivot shift, Lachman, and anterior and posterior drawer tests to evaluate the degree of anteroposterior laxity.
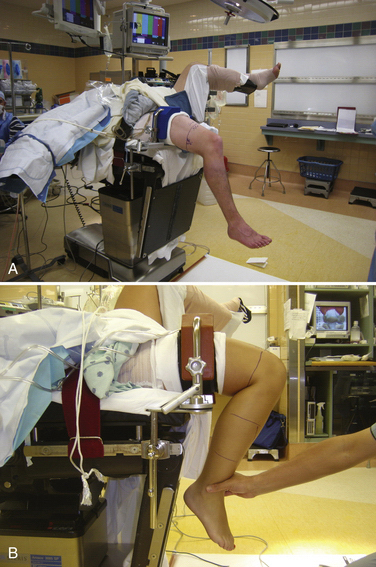
Once the physical examination is complete, the operative limb is placed in an arthroscopic leg holder with a well-padded tourniquet placed high on the thigh. The patient must be positioned far down on the bed and the foot of the bed maximally flexed to allow the knee to be ranged from full extension to at least 120 degrees of flexion during various aspects of the procedure (Fig. 22-8). The operative limb should be comfortably positioned so that the knee assumes a position of 90-degree flexion at rest. The nonoperative leg is placed in a well-leg holder with the hip flexed and abducted to allow for unobstructed approach to the operative knee. The operative leg is then prepped in a sterile fashion and draped.
Portal Placement and Diagnostic Arthroscopy
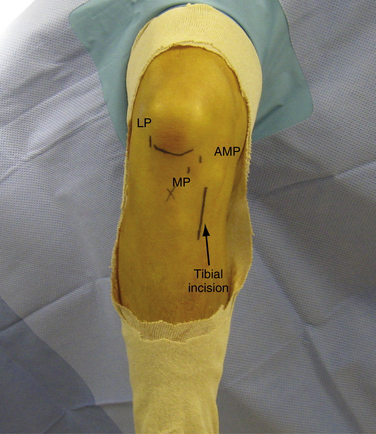
As with all arthroscopic procedures, portal placement is the first and often most important step. Properly placed portals facilitate ease of successful surgery. Conversely, poorly placed portals will only complicate the procedure. In the case of anatomic double-bundle ACL reconstruction, accurately placed portals are necessary to visualize the anatomic landmarks located at the ACL insertion sites properly. With the knee in 90 degrees of flexion, an anterolateral viewing portal is first established just lateral to the patellar tendon (Fig. 22-9). This portal should be placed in a slightly more proximal position, with the distal extent of the portal ending at the level of the inferior pole of the patella. By placing this portal high, less of the patellar fat pad is traversed. Superior visualization of the tibial insertion sites is also achieved. The portal is established with a no. 11 blade and dilated with a straight hemostat to allow easy passage of instruments. The anteromedial portal is established next via 18-gauge needle localization in a similar fashion. This portal will function as both a viewing portal and a working portal. It is positioned at the joint line just above the meniscus and immediately adjacent to the edge of the inferomedial portion of the patellar tendon.
Arthroscopic examination is then performed and associated meniscal or chondral injury is addressed as necessary. The fat pad is débrided to allow clear visualization of the anterior horn of the medial meniscus. Needle localization is again used to establish the accessory medial portal (AMP) at this point. This portal should provide direct access to the most distal and anterior aspects of the lateral intercondylar notch where the PL tunnel will later be drilled (Fig. 22-10). When the needle is introduced, it should be directed toward the PL insertion sited to ensure adequate access for later drilling of the tunnel. The correct position of this portal is significantly more medial than standard medial portals. Therefore, it lies close to the medial condyle and careful attention must be taken to avoid iatrogenic cartilage injury.
Graft Choice and Preparation
Both autograft and allograft options exist for double-bundle ACL reconstruction.33–35 However, there are several important factors that should be appreciated when considering double-bundle ACL reconstruction. Most importantly, with autograft tissue, there are inherent limitations on the size and amount of tissue available. Consistent methods for preoperative prediction of hamstring size do not exist. Therefore, it is very likely that the surgeon could be left with insufficient tissue for double-bundle reconstruction with conventional harvest methods. In contrast, allograft tissue affords a greater quantity of tissue without additional donor site morbidity. Still, allograft tissue has several significant drawbacks, including delayed revascularization, tissue reaction, potential risks of disease transmission, high costs and, in some settings, limited availability. Therefore, graft selection should be individualized for each particular situation.
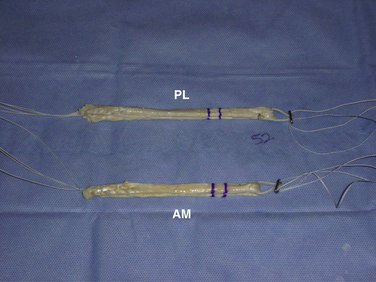
For consistency, we will describe our experience using the EndoButton CL (Smith &Nephew), which is probably our most frequently used form of fixation. Per routine, each graft is looped over an EndoButton CL to serve as the femoral fixation point. The length of the EndoButton loop is tailored to ensure that a minimum of 20 mm of graft is engaged within the tunnel. The length of the EndoButton loop is determined by subtracting 20 mm from the total distance from the aperture of the tunnel to the lateral cortex. The tunnel should be dilated to a distance 7 mm longer than the desired length of graft engagement inside the tunnel to allow for ease of flipping the EndoButton. For example, a 15-mm EndoButton can be used after dilating a 27-mm tunnel when the total length from aperture to the lateral femoral cortex is 35 mm. To ensure that the graft is passed and seated properly, two lines are provisionally marked on the femoral end of the graft. The first mark is placed at 20 mm from the end and a second line at 27 mm. The second line marks the point where the graft must be pulled into the tunnel to flip the EndoButton safely, and the first line marks the appropriate final position of the graft when 20 mm is in the tunnel and the graft is secured (Fig. 22-11). In a similar fashion, a hamstring autograft can be used for double-bundle ACL reconstruction. The semitendinosus tendon is typically prepared as the AM bundle and the gracilis tendon is doubled or tripled over to serve as the PL bundle.33 If this does not provide adequate tissue, the surgeon should be prepared to augment with allograft, as needed.
Identification of the Anterior Cruciate Ligament Insertion Site Footprint
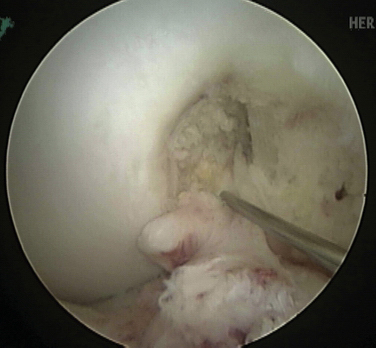
Once the portals have been established and sufficient fat pad has been débrided to visualize the notch clearly, the ACL is closely inspected. The remnant tissue should not be aggressively or quickly débrided, but rather carefully dissected back to the true anatomic insertion sites. In approximately 10% of cases, we have found that one of the bundles is functional and can be preserved. In such cases, the intact bundle is preserved and single-bundle augmentation surgery is performed according to the injury pattern.36 If it has been decided that no functional tissue remains, a radiofrequency thermal device (ArthroCare; Sunnyvale, Calif) is used to define the anatomic insertion sites clearly. Importantly, notchplasty is not routinely performed. Notchplasty would destroy the normal bony landmark and distort normal anatomic structures.
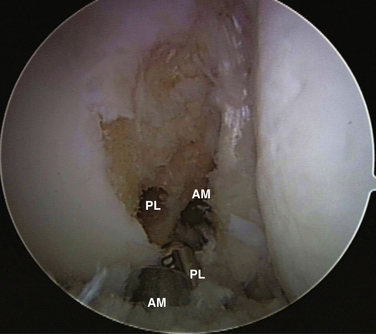
In cases of chronic ACL injury, the remnant ACL is often not clearly preserved. If it is difficult to delineate the anatomic outer margin of the footprint, osseous landmarks at the femoral origin can be used to define the anatomic insertion sites of two bundles.12 The lateral intercondylar ridge forms the anterior (or arthroscopically viewed superior) border of the ACL. The lateral bifurcate ridge divides the AM bundle insertion site from the PL bundle insertion site (Fig. 22-12).
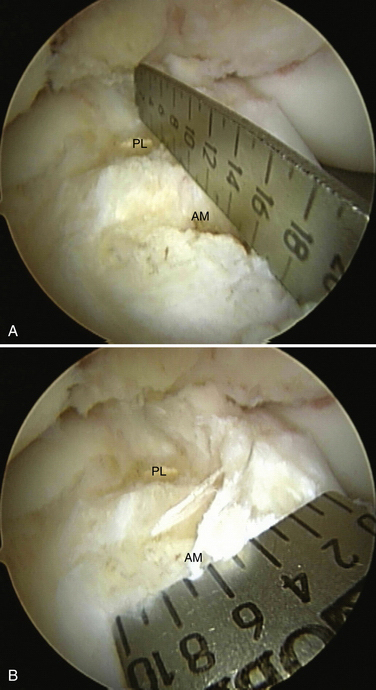
Measurement of Footprint Size
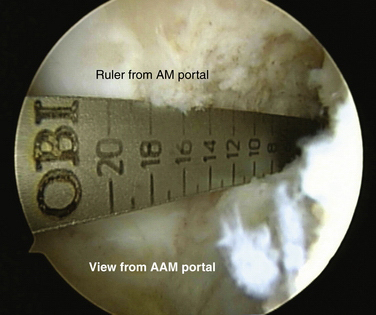
After the femoral and tibial footprints have been defined, measurements are taken. A metallic ruler is inserted through the anteromedial portal, with the arthroscope in the lateral portal. We measure the length and width of each insertion site on the tibia and femur (Fig. 22-13). To measure the femoral insertion sites accurately, the arthroscope must be moved to the accessory medial portal while the ruler is kept in the anteromedial portal (Fig. 22-14).
At this point, based on the total length of the footprint, we determine whether to proceed with double-bundle surgery or to perform anatomic single-bundle surgery. If the total length of the femoral insertion site is 14 mm or less, double-bundle surgery is not technically feasible, because the two tunnels would need to be of extremely small diameter or risk collapsing into one another. It is important to realize that the femoral insertion size is the limiting factor when deciding whether to proceed with double-bundle reconstruction. This occurs for two reasons. First, the total length of the femoral footprint is typically about 2 mm smaller than the tibial footprint. This is because of the manner whereby the ACL fibers fan out at the tibial footprint, with some fibers actually inserting beneath the intermeniscal ligament. Also, as a result of the oblique angle by which the reamer must approach the femur, the size of the drilled tunnel aperture will actually be slightly larger than the true diameter of the reamer.
We individualize the sizes of the AM and PL tunnels based on the size of the native insertion sites in all cases. A recent anatomic study has revealed that the average length of the femoral AM insertion is 9.8 ± 1 mm and that of the femoral PL insertion is 7.3 ± 0.5 mm.12 However, realize that the chosen reamer size should be slightly smaller than the actual size of the insertion site due because of the widening effect of the tunnel created by the oblique approach of the reamer to the bony surface of the notch. Generally, we select a reamer 1 to 2 mm smaller than the size of the insertion site to maintain an adequate bone bridge between tunnels (Fig. 22-15).
Tunnel Placement
The femoral AM tunnel is the last tunnel created. The femoral AM tunnel can be drilled through one of three different approaches: (1) transtibial AM; 2) transtibial PL; or (3) accessory medial portal (Fig. 22-16). The deciding factor is always the ability to re-create the true anatomic location. If a transtibial guide wire can safely reach the previously marked true anatomic location, the transtibial technique is preferred because it creates a slightly longer and divergent tunnel relative to the femoral PL tunnel. If this is not possible, the accessory AM portal should be used to place the femoral AM tunnel. A transtibial approach through the tibial PL tunnel works in about 20% of cases. A correctly positioned accessory medial portal approach will provide excellent access to the true femoral AM tunnel position in all cases. Once the appropriate technique is determined, the tunnel is provisionally drilled to a depth of 25 mm with an acorn bit. The far cortex of the AM femoral tunnel is then breached with a 4.5-mm EndoButton drill (Smith & Nephew Endoscopy), and the depth gauge is used to measure the distance to the far cortex. The tunnel is dilated or hand-reamed up to the final desired diameter with the final determined depth for EndoButton fixation, similar to the PL tunnel.
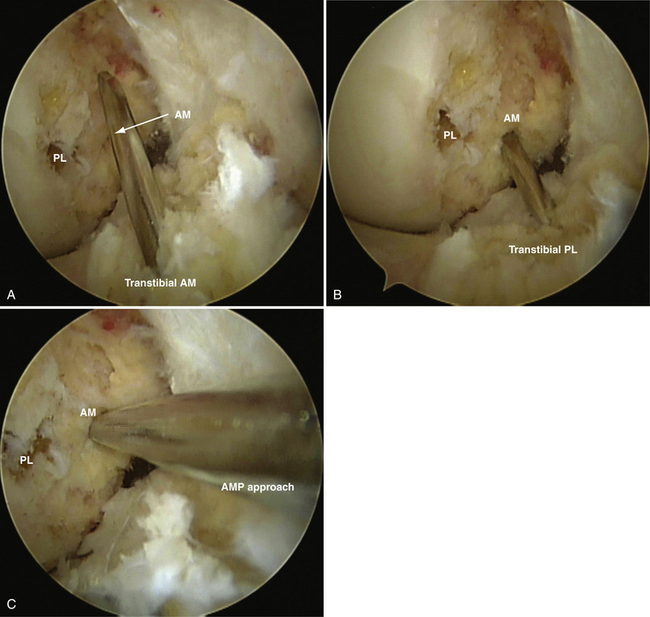
FIGURE 22-16 Arthroscopic images of a right knee viewed from the medial portal. The femoral AM tunnel is created in one of three ways. A, Transtibial AM. Note the high location of the tunnel in this case. B, Transtibial PL, which accesses the center of the AM footprint in this case or via the accessory medial portal (C), which always provides easy access to the center of the AM footprint.
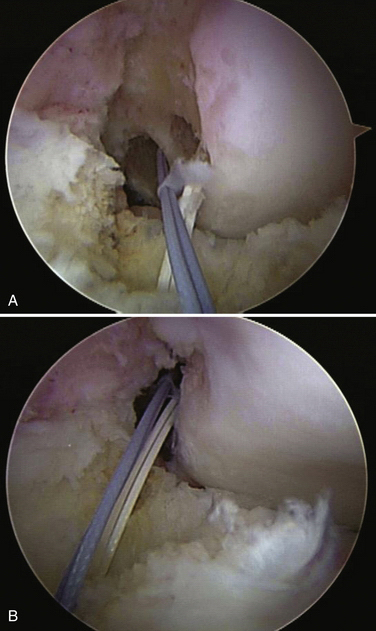
Graft Passage
A Beath pin with a suture loop in its eyelet is first passed through the AAM portal and up through the femoral PL tunnel. The looped suture is retrieved with a suture retriever passed through the PL tibial tunnel. Another Beath pin with a looped suture is similarly passed through the AM femoral tunnel and retrieved through the AM tibial tunnel (Fig. 22-17). The PL graft is passed first, followed by AM graft passage. On the femoral side, an EndoButton CL is used for femoral-sided fixation. In some cases, when the PL femoral tunnel is shorter than 25 mm, an EndoButton Direct is used to maximize the amount of graft in the tunnel. On the tibial side, a bioabsorbable screw that is the same diameter as the drilled tunnel is used for each bundle.
The grafts are pretensioned by flexing and extending the knee through 20 cycles of full motion. Final fixation of the grafts is done at 30 degrees of flexion for the AM bundle and 0 degree for the PL bundle. A final arthroscopic inspection is always performed to confirm correct position and tensioning of the grafts and the absence of graft impingement (Fig. 22-18).
PEARLS& PITFALLS
PEARLS
PITFALL
POSTOPERATIVE REHABILITATION PROTOCOL
Immediately after surgery, the knee is placed into a hinged knee brace locked in full extension for 7 to 10 days. Immediate full weight bearing with crutches is allowed as tolerated based on pain and extent of effusion. The brace is continued for 6 weeks. Continuous passive motion is started immediately after surgery from 0 to 45 degrees of flexion and increased by 5 to 10 degrees daily as tolerated. If a meniscal repair has been performed, flexion beyond 90 degrees is not allowed for 4 weeks postoperatively. Active isometric quadriceps strengthening begins on the first postoperative day. Gradual progression in a structured physical therapy setting continues over the next 3 months. Noncutting, nontwisting activities such as straight line running, cycling, and swimming are generally allowed at 12 weeks after surgery. Functional training begins at 6 months in a supervised setting. Return to unrestricted activity, including contact sports, is allowed at 7 to 9 months. To return to sport, the patient must be pain-free, without effusion, and have a clinically stable knee, full range of motion, and strength grossly equivalent to that of the contralateral side.
CONCLUSIONS
Since the first arthroscopic ACL reconstruction was performed by Dancy in 1980,37 great technical advances have followed. Generally, most published series detailing clinical outcomes after single-bundle surgery have reported satisfactory results.38 Still, significant room for improvement exists. As medical science continues to advance, patient expectations only grow higher. Regardless of the severity of injury, most patients expect to return to a full preinjury level of physical function rapidly. As orthopedic surgeons, we need to seek a higher standard of care to meet the increasing demands of our active patients.39
The concept of the anatomic double-bundle ACL reconstruction surgery is predicated on a very simple principle—restoration of normal ACL anatomy. Through accurate restoration of native anatomy, we hope to restore normal kinematics and function. A detailed understanding of native ACL anatomy, biomechanics, and injury patterns forms the basis for advanced anatomic double-bundle reconstruction techniques. A recent prospective cohort study of 100 consecutive patients who underwent anatomic double-bundle ACL reconstruction has shown promising early clinical results.40 Randomized prospective clinical trials are still needed to determine fully the true clinical benefit of anatomic double-bundle surgery compared with conventional single-bundle ACL reconstruction. At present, results of studies using more sensitive advanced imaging modalities and outcomes measures suggest significant early benefits of anatomic ACL reconstruction methods.6,38
1. Freedman KB, D’Amato MJ, Nedeff DD, et al. Arthroscopic anterior cruciate ligament reconstruction: a meta-analysis comparing patellar tendon and hamstring tendon autografts. Am J Sports Med. 2003;31:2-11.
2. Yunes M, Richmond JC, Engels EA, Pinczewski LA. Patellar versus hamstring tendons in anterior cruciate ligament reconstruction: A meta-analysis. Arthroscopy. 2001;17:248-257.
3. Aglietti P, Giron F, Buzzi R, et al. Anterior cruciate ligament reconstruction: bone-patellar tendon-bone compared with double semitendinosus and gracilis tendon grafts. A prospective, randomized clinical trial. J Bone Joint Surg Am. 2004;86:2143-2155.
4. Woo SL, Kanamori A, Zeminski J, et al. The effectiveness of reconstruction of the anterior cruciate ligament with hamstrings and patellar tendon. A cadaveric study comparing anterior tibial and rotational loads. J Bone Joint Surg Am. 2002;84:907-914.
5. Tashman S, Collon D, Anderson K, et al. Abnormal rotational knee motion during running after anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32:975-983.
6. Ferretti M, Levicoff EA, Macpherson TA, et al. The fetal anterior cruciate ligament: an anatomic and histologic study. Arthroscopy. 2007;23:278-283.
7. Girgis FG, Marshall JL, Monajem A. The cruciate ligaments of the knee joint. Anatomical, functional and experimental analysis. Clin Orthop Relat Res. 1975;(106):16-231.
8. Harner CD, Baek GH, Vogrin TM, et al. Quantitative analysis of human cruciate ligament insertions. Arthroscopy. 1999;15:741-749.
9. Gabriel MT, Wong EK, Woo SL, et al. Distribution of in situ forces in the anterior cruciate ligament in response to rotatory loads. J Orthop Res. 2004;22:85-89.
10. Sakane M, Fox RJ, Woo SL, et al. In situ forces in the anterior cruciate ligament and its bundles in response to anterior tibial loads. J Orthop Res. 1997;15:285-293.
11. Yagi M, Wong EK, Kanamori A, et al. Biomechanical analysis of an anatomic anterior cruciate ligament reconstruction. Am J Sports Med. 2002;30:660-666.
12. Ferretti M, Ekdahl M, Shen W, Fu FH. Osseous landmarks of the femoral attachment of the anterior cruciate ligament: an anatomic study. Arthroscopy. 2007;23:1218-1225.
13. Purnell ML, Larson AI, Clancy W. Anterior cruciate ligament insertions on the tibia and femur and their relationships to critical bony landmarks using high-resolution volume-rendering computed tomography. Am J Sports Med. 2008;36:2083-2090.
14. Steckel H, Starman JS, Baums MH, et al. Anatomy of the anterior cruciate ligament double bundle structure: a macroscopic evaluation. Scand J Med Sci Sports. 2007;17:387-392.
15. Chhabra A, Starman JS, Ferretti M, et al. Anatomic, radiographic, biomechanical, and kinematic evaluation of the anterior cruciate ligament and its two functional bundles. J Bone Joint Surg Am. 2006;88(suppl 4):2-10.
16. Fu FH, Jordan SS. The lateral intercondylar ridge: a key to anatomic anterior cruciate ligament reconstruction. J Bone Joint Surg Am. 2007;89:2103-2104.
17. Odensten M, Gillquist J. Functional anatomy of the anterior cruciate ligament and a rationale for reconstruction. J Bone Joint Surg Am. 1985;67:257-262.
18. Siebold R, Ellert T, Metz S, Metz J. Tibial insertions of the anteromedial and posterolateral bundles of the anterior cruciate ligament: morphometry, arthroscopic landmarks, and orientation model for bone tunnel placement. Arthroscopy. 2008;24:154-161.
19. Luites JW, Wymenga AB, Blankevoort L, Kooloos JG. Description of the attachment geometry of the anteromedial and posterolateral bundles of the ACL from arthroscopic perspective for anatomical tunnel placement. Knee Surg Sports Traumatol Arthrosc. 2007;15:1422-1431.
20. Kopf S, Musahl V, Tashman S, et al. A systematic review of the femoral origin and tibial insertion morphology of the ACL. Knee Surg Sports Traumatol Arthrosc. 2009;17:213-219.
21. Zantop T, Herbort M, Raschke MJ, et al. The role of the anteromedial and posterolateral bundles of the anterior cruciate ligament in anterior tibial translation and internal rotation. Am J Sports Med. 2007;35:223-227.
22. Amis A, Dawkins G. Functional anatomy of the anterior cruciate ligament. J Bone Joint Surg Br. 1991;73:260-267.
23. Buoncristiani AM, Tjoumakaris FP, Starman JS, et al. Anatomic double-bundle anterior cruciate ligament reconstruction. Arthroscopy. 2006;22:1000-1006.
24. Muller W. Form, function, and ligament reconstruction. In: Muller W, editor. The Knee. Berlin: Springer, 1982.
25. Zantop T, Brucker PU, Vidal A, et al. Intraarticular rupture pattern of the ACL. Clin Orthop Relat Res. 2007;(454):48-53.
26. Siebold R, Fu FH. Assessment and augmentation of symptomatic anteromedial or posterolateral bundle tears of the anterior cruciate ligament. Arthroscopy. 2008;24:1289-1298.
27. Sarimo J, Rantanen J, Heikkila J, et al. Acute traumatic hemarthrosis of the knee. Is routine arthroscopic examination necessary? Scand J Surg. 2002;91:361-364. A study of 320 consecutive patients
28. Benjaminse A, Gokeler A, van der Schans CP. Clinical diagnosis of an anterior cruciate ligament rupture: a meta-analysis. J Orthop Sports Physical Ther. 2006;36:267-288.
29. Donaldson WF3rd, Warren RF, Wickiewicz T. A comparison of acute anterior cruciate ligament examinations. Initial versus examination under anesthesia. Am J Sports Med. 1985;13:5-10.
30. Galway HR, MacIntosh DL. The lateral pivot shift: a symptom and sign of anterior cruciate ligament insufficiency. Clin Orthop Relat Res. 1980;(414):5-50.
31. Hess T, Rupp S, Hopf T, et al. Lateral tibial avulsion fractures and disruptions to the anterior cruciate ligament. A clinical study of their incidence and correlation. Clin Orthop Relat Res. 1994;(303):193-197.
32. Vellet AD, Lee DH, Munk PL, et al. Anterior cruciate ligament tear: prospective evaluation of diagnostic accuracy of middle- and high-field-strength MR imaging at 1.5 and 0.5 T. Radiology. 1995;197:826-830.
33. Noh HK, Wang JH, Bada LP, et al. Trantibial anterior cruciate ligament double bundle reconstruction technique: two tibial bundle in one tibial tunnel. Arch Orthop Trauma Surg. 2008;128:1245-1250.
34. Yasuda K, Kondo E, Ichiyama H, et al. Anatomic reconstruction of the anteromedial and posterolateral bundles of the anterior cruciate ligament using hamstring tendon grafts. Arthroscopy. 2004;20:1015-1025.
35. Kim SJ, Jo SB, Kumar P, Oh KS. Comparison of single- and double-bundle anterior cruciate ligament reconstruction using quadriceps tendon-bone autografts. Arthroscopy. 2009;25:70-77.
36. Shen W, Forsythe B, Ingham SM, et al. Application of the anatomic double-bundle reconstruction concept to revision and augmentation anterior cruciate ligament surgeries. J Bone Joint Surg Am. 2008;90(suppl 4):20-34.
37. Strocchi R, de Pasquale V, Gubellini P, et al. The human anterior cruciate ligament: histological and ultrastructural observations. J Anat. 1992;180:515-519.
38. Meredick RB, Vance KJ, Appleby D, Lubowitz JH. Outcome of single-bundle versus double-bundle reconstruction of the anterior cruciate ligament: a meta-analysis. Am J Sports Med. 2008;36:1414-1421.
39. Irrgang JJ, Bost JE, Fu FH. Re: Outcome of single-bundle versus double-bundle reconstruction of the anterior cruciate ligamenta meta-analysis. Am J Sports Med. 2009;37:421-422.
40. Fu FH, Shen W, Starman JS, et al. Primary anatomic double-bundle anterior cruciate ligament reconstruction: a preliminary 2-year prospective study. Am J Sports Med. 2008;36:1263-1274.