Doppler Ultrasound of the Penis
Introduction
The evaluation of erectile dysfunction (ED) has been the dominant application for penile Doppler, especially in the older population since the prevalence and severity of the disease increases with advancing age.1 While impotence may be the result of psychogenic, neurogenic or hormonal factors, vascular disease is the most common cause of ED.2 With the introduction of phosphodiesterase inhibitors [specifically Sildenafil citrate (Viagra©), Verdenafil HCl (Levitra©), and Tadalafil (Cialis©)], the frequency of Doppler studies for ED has significantly decreased.3 Most centres now prescribe a trial of the phosphodiesterase inhibitors as the initial diagnostic/therapeutic test of ED, with only non-responders being sent on for imaging.
Penile Anatomy and Physiology
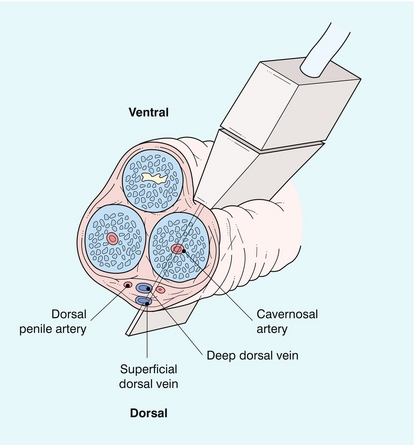
The penis contains three longitudinal, cylindrical erectile bodies. Two corpora cavernosa are located in the dorsal two-thirds of the penile shaft, and a single corpora spongiosum is located in the ventral one-third of the shaft. The corpora cavernosa are enclosed by the tunica albuginea, a tough, non-distensible fascial layer. The septum that divides the corpora cavernosa contains fenestrations that create multiple connecting anastomotic channels between the sinusoidal spaces, allowing for free communication across the midline. The dorsal arteries, veins and nerves are situated centrally along the penile dorsum, superficial to the tunica albuginea and deep to Buck’s fascia. The urethra is contained within the corpus spongiosium.4
On ultrasound, the corpora cavernosa are of uniform hypoechoic echotexture. The tunica can be seen as an echogenic envelope around the corpora. The echogenic walls of the cavernosal arteries can be seen centrally within the corpora. The corpus spongiosum is of higher echogenicity (Fig. 12-1).
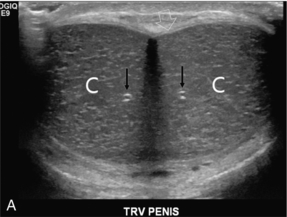
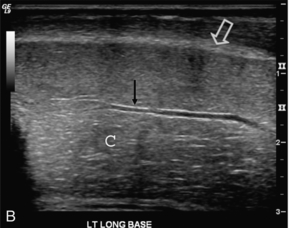
FIGURE 12-1 (A) Transverse and (B) longitudinal ultrasound images of a normal penis. The corpora cavernosa (C) have uniform echogenicity throughout. The echogenic tunica (open arrow) and echogenic walls of the cavernosal artery (arrows) are easily identified.
ARTERIAL ANATOMY
The internal pudendal artery and its branches are the primary source of arterial supply to the penis. The first three branches are the superficial perineal artery, the bulbar artery and a small urethral artery. The perineal artery is a large and constant branch that, in 80% of cases, has an internal and external branch. The bulbar artery, which supplies the proximal penile shaft, is usually easily identified during angiography because it is associated with a bulbar parenchymal blush in the early arterial phase. The urethral artery, which is of small diameter, arises anterior to the bulbar artery. After these branches, the internal pudendal artery continues as the common penile artery. It then divides into left and right penile arteries, which enter the base of the penis and branch into a dorsal artery and a cavernosal artery. The dorsal artery extends along the dorsal aspect of the penile shaft towards the glans and terminates at the level of the arterial corona of the glans; it supplies blood primarily to the skin, subcutaneous tissues and glans. Collateral vessels from the dorsal artery often communicate with the cavernosal artery. The cavernosal, or deep penile artery, enters the tunica albuginea proximally and extends the length of the corpus cavernosum. The cavernosal arteries and their helicine branches are the primary source of blood flow to the erectile tissue of the penis. Just as the cavernosal artery supplies blood to the corpus cavernosum, the spongiosal artery supplies the corpus spongiosum4 (Fig. 12-2).
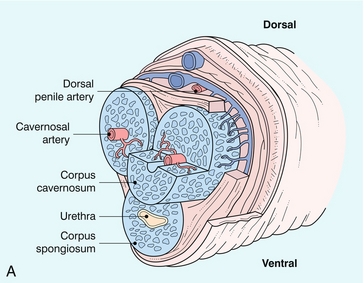
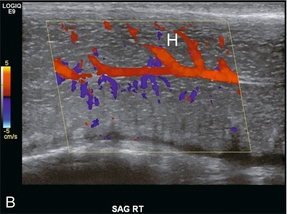
FIGURE 12-2 Normal anatomy. (A) The cavernosal arteries are centrally located in each corpus cavernosus. The urethra courses through the corpus spongiosum. The dorsal penile artery supplies the glands and does not play a direct role in erectile function. (B) Colour Doppler reveals flow in the cavernosal artery and its helicine branches (H).
VENOUS ANATOMY
Venous drainage of the penile erectile tissue (i.e. the sinusoidal spaces) primarily occurs through emissary (efferent) veins which drain the corpus cavernosum, penetrate the tunica albuginea and empty into the circumflex veins; these then drain into the deep dorsal venous system of the penis. The emissary veins may also drain directly into the deep dorsal vein. The superficial dorsal vein drains the distal portion of the corpora cavernosa, as well as the skin and glans. The deep and superficial dorsal veins can be routinely visualised by colour Doppler imaging in the midline of the penile shaft (Figs 12-3 and 12-4). The most proximal portions of the corpora cavernosa are drained by the cavernosal veins directly into the periprostatic venous plexus.4
ERECTILE PHYSIOLOGY
When the penis is flaccid, its smooth muscle is in a tonic state, the cavernous sinusoids are collapsed, and the cavernous venules are open.2 The emissary veins drain the sinusoidal spaces and blood circulates into the dorsal veins. During this state, there is relatively high resistance to blood flow into the penis. Erection starts when an autonomic neurogenic impulse relaxes the cavernosal arterioles and sinusoidal spaces. As erection occurs, there is a marked increase in the volume of arterial inflow into the penis as the cavernous arteries dilate. This is accompanied by relaxation of the smooth muscle of the corpora cavernosa with expansion and elongation of the cavernous sinusoids as they fill with blood. Compression of the cavernous venules between the dilated cavernous sinusoids and the unyielding peripheral tunica albuginea decreases venous outflow. This veno-occlusive mechanism (which depends on neurological stimuli, a sufficient supply of arterial blood, and normal function of the tunica albuginea) maintains sinusoidal distension and rigid erection.
Five stages of erectile physiology have been defined: latent, tumescent, full erection, rigid erection and detumescent. During the latent phase, the diameters of the cavernosal arteries are at their greatest and there is maximum inflow of blood with minimal resistance. During tumescence, the sinusoidal cavities of the corpora cavernosa distend with blood. With full erection, blood flow decreases, as do the diameters of the cavernosal arteries. With a rigid erection, blood inflow (and outflow) ceases and the diameters of the cavernosal arteries are at their narrowest. Detumescence occurs when the trabeculae and arteries contract in response to a release of norepinephrine. During the five stages of erection, different arterial diameters and waveform patterns are normally present on Doppler examination.5
Ultrasound Technique
The evaluation should be performed in a quiet, private setting with the room comfortably warm and darkened, so that the patient is relaxed and not uncomfortable. The scan is performed with the patient lying supine and the penis in the anatomical position (lying superiorly against the anterior abdominal wall). During the examination, the patient may be asked to help keep the penis immobilised by gently holding the corona just under the glans penis and then stretching the shaft along the anterior abdominal wall. Scanning is usually performed on the ventral surface of the penis, but the probe can be placed on the dorsal or lateral surfaces if necessary (Fig. 12-4). Imaging is performed in both the longitudinal and transverse planes from the base of the penis to the glans to visualise anatomical details of the corpora cavernosa, cavernosal arteries and surrounding structures, and also to demonstrate any abnormalities such as fibrosis, scarring, plaques, calcification, haematoma or tumour. The transducer should be applied gently with minimal penile compression. Firm pressure causing vascular compression can resist inflow and affect accuracy of velocity measurement, especially during diastole. The diameter of the arteries and blood flow velocities are measured. Colour Doppler enhances the accuracy of angle correction, which is mandatory for flow velocity determination. In addition, with colour Doppler, blood flow direction can be assessed and the presence of any communications between the cavernosal, dorsal and spongiosal arteries can be detected.6
Erectile Dysfunction
The initial report of Doppler sonography combined with pharmacological induction of erection to evaluate vasculogenic impotence was by Lue and associates7 in the mid-1980s. The use of this study peaked before the introduction of phosphodiesterase inhibitors and has now fallen off significantly. Intra-cavernosal ejection of prostaglandin E1 at a dose of 10 μg into the base of the corpus cavernosum with a 27-gauge needle will result in an erection, even without sexual stimulation. If the response is sub-optimal the initial injection can be supplemented by a further 10 μg after 15 min. If the patient responds with an appropriate erection, then it can be presumed that neurogenic, psychogenic or hormonal issues are the cause of the patient’s erectile dysfunction.
The Doppler examination of the cavernosal artery flow profile should be initiated within two minutes of injection. Reassessment is then performed every 2 to 5 minutes depending on the temporal response to the drug, which can vary considerably from patient to patient. Most examinations are finished by 20 minutes. Assessment up to 30 minutes is occasionally required to ensure the maximal pharmacologic effect has been attained. Doppler measurements are most reliable and most easily reproduced when taken at the base of the penis where the penile vessels angle posteriorly toward the perineum. The arterial diameter and waveform of each cavernosal artery is individually assessed. Peak systolic and end-diastolic velocities are measured and recorded. An asymmetric response of the cavernosal arteries during erection or a lack of arterial dilation may suggest the presence of a significant vascular inflow obstruction. The examiner should also carefully search for anatomical penile arterial variants as they may also contribute to vasculogenic impotence.8
NORMAL DOPPLER FINDINGS
Prior to injection, during the flaccid state, the systolic waveform is damped and has a monophasic flow profile with a relatively small diastolic component of flow (Fig. 12-5A). Following pharmacological induction of erection, the normal progression of haemodynamic events and the associated Doppler waveform patterns of the cavernosal artery can be classified into several haemodynamic stages. The appearance of the spectral waveform must be correlated with the status of the erection (i.e. flaccid, latent, tumescent, full and rigid).
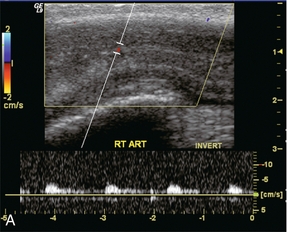
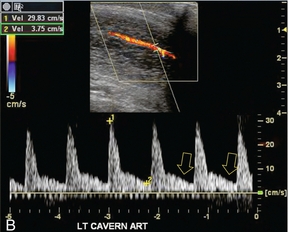
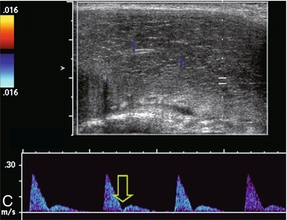
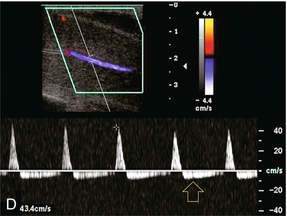
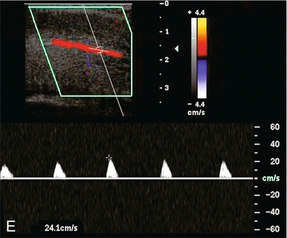
FIGURE 12-5 A sequence of Spectral Doppler tracings illustrating changes in arterial flow dynamics before and after pharmacological induction of erection. (A) Spectral Doppler tracing of the penis flaccid, prior to injection. Flow is weak; velocities are damped. (B) During the initial latent phase of the erectile process brisk flow has developed with systolic velocity approaching 30 cm/s. At this relatively early time after pharmacological enhancement, diastolic flow is still present in the antegrade direction (open arrows). (C) A few minutes later, in the early tumescent phase, systolic flow remains brisk but diastolic velocity has decreased. Note a dicrotic notch is now present in early diastole (open arrow). (D) Spectral Doppler tracing approaching rigid erection. The systolic peak has narrowed but velocity remains brisk. Diastolic flow has now reversed as the intracavernosal pressure now exceeds diastolic pressure (open arrow). (E) Spectral Doppler tracing at full rigid erection. Systolic inflow velocities have now decreased as intracavernosal pressure now approximates systolic pressure. No flow is seen during diastole (compared to D).
During the initial latent state, there is a brisk increase in both systolic and diastolic blood flow in the cavernosal artery, and a rounded systolic peak is observed. The flow of blood is antigrade during both systole and diastole, with a pronounced forward diastolic component (Fig. 12-5B). This spectral waveform reflects the low resistance to flow within the sinusoidal spaces. Minimal tumescence normally accompanies this stage. With the increase in blood flow to the corpora cavernosa, intracavernosal pressure becomes increased. As intracavernosal pressure rises, a dichrotic notch appears at end systole and diastolic flow diminishes (Fig. 12-5C). When intracavernosal and diastolic pressures are the same, diastolic flow ceases and there is only systolic blood flow. The systolic envelope narrows and systolic velocity may fluctuate. Increasing tumescence normally occurs during this stage.
During full erection, intracavernosal pressure becomes greater than the arterial pressure during diastole because of the veno-occlusive mechanism. Flow reversal is usually seen during diastole and the systolic waveform narrows. During this stage, maximal systolic velocity occurs in the normal patient and strong pulsations are normally seen (Fig. 12-5D). In the rigid phase of erection, intracavernosal pressure approximates arterial systolic pressure, with further narrowing of the systolic envelope, and a decrease in peak systolic velocity (Fig. 12-5E). Both systolic and diastolic flow may cease completely as the pressure in the corporal bodies approaches systolic blood pressure.5,9–11
CAVERNOSAL ARTERY DIAMETER
Cavernosal artery diameter should increase as a response to the pharmacologic stimulus, accommodating increased flow. Identification of such dilatation after injection is considered an indicator of appropriate vessel compliance. Various authors suggest a minimal increase of arterial diameter should range between at least 60 to 75%.7,12 However, obtaining cavernosal arterial diameters is time-consuming and accuracy of measurement is dependent on examiner skill. Poor correlation between the degree of cavernosal artery diameter dilatation and arteriographic confirmation of arterial integrity has been reported therefore, cavernosal artery diameter changes are no longer considered indicative or diagnostic of arterial disease.13
ARTERIAL VARIANTS
Variant penile arterial anatomy can be seen in a high percentage of patients suspected of having an arteriogenic cause for their impotence. Communications among the cavernosal, dorsal, and spongiosal arteries are often found along the shaft of the penis (Fig. 12-6). There may be duplication of the cavernosal artery or cross-communication between left and right cavernosal arteries. Rarely, collaterals from the urethral arteries may also be seen. The incidence of communication between dorsal and cavernosal arteries (dorsal–cavernosal perforators) has been reported in as many as 90% of men. ‘Spongiosal–cavernosal communications’, or ‘shunt’ vessels, which course from the corpus spongiosum into the corpus cavernosum are another common variant. Any of these collateral pathways may significantly affect arterial Doppler flow profiles during erection. Although these anatomical variations do not necessarily result in arterial insufficiency, they may cause inaccurate interpretation if they are not appreciated. For example, systolic peak velocities of the cavernosal artery may be significantly lower in men with a full erectile response if arterial collateral communications are present. Careful scanning of the entire penis from the crura to the glans with colour Doppler is essential in identifying these anomalies.13,14
Vasculogenic Impotence
DOPPLER FINDINGS OF ARTERIAL INSUFFICIENCY
Peak systolic velocity in the cavernosal arteries after pharmacological injection is considered one of the most important parameters in identifying cases of impotence due to arterial inflow compromise, focal stenosis, occlusion, or collateral steal between arteries (Fig. 12-7). Peak cavernosal artery systolic velocity less than 25 cm/s is considered abnormal. Asymmetric velocities in the side-to-side comparison of peak systolic velocity between cavernosal arteries are considered abnormal if the difference between right and left cavernosal arteries is greater than 10 cm/s.
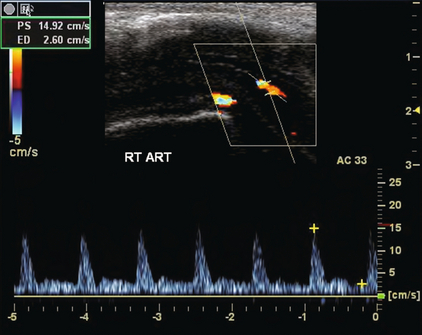
FIGURE 12-7 Spectral Doppler tracing in a patient with arterial insufficiency. Despite appropriate pharmaceutical dose, erectile response was suboptimal. Peak systolic velocity in the cavernosal artery only approximates 15 cm/s. This is well below the accepted normal response.
Underlying arterial disease is assumed in the artery with the lower peak systolic velocity.15
Other indicators used to increase the sensitivity of detecting potential arterial inflow compromise include reversal of blood flow during systole, a penile blood flow index and blood flow (or cavernosal artery) acceleration. During rigid erection, reversal of diastolic blood flow is a normal finding; however, reversal of arterial blood flow direction during systole is always considered abnormal and may indicate an underlying vascular abnormality. Systolic flow reversal after pharmacological induction of erection has been observed in patients with significant proximal penile or cavernosal artery stenosis or occlusion with filling of the distal cavernosal artery by collateral flow.15,16
VENOUS INCOMPETENCE
In some men, venous incompetence, or failure of the veno-occlusive mechanism, may be the primary cause of vasculogenic impotence. Patients with normal arterial inflow parameters (e.g. peak systolic velocity > 25 cm/s) but weak erections will very likely have some degree of venous leakage.2 Because primary venous leakage is a potentially treatable cause of erectile dysfunction, Doppler examination of the penile venous system may be helpful in identifying these patients who may benefit from additional invasive studies. If surgical or endovascular therapy is considered for the patient, then cavernosography is generally still required.
Correlation has been shown between end-diastolic blood flow velocity within the cavernosal arteries and the presence of venous leakage. With a normal erectile response, there should be minimal, if any, flow detected with the cavernosal arteries during the diastolic phase 15–20 min after injection. As previously noted, there should be a decrease and eventual absence or reversal of diastolic flow in the normal spectral Doppler waveform during rigid erection. If there is veno-occlusive dysfunction (venous leak), then this decrease or reversal of diastolic flow will not occur. Persistently high diastolic flow in the cavernosal arteries indicates venous leakage out of the corporal tissue even after maximum peak systolic velocity has been attained (Fig. 12-8). However, just as there are differences of opinion regarding normal and abnormal peak systolic velocity values, various criteria exist as to what constitutes abnormal diastolic velocity. In association with a normal arterial inflow, a persistently high end diastolic velocity of > 5 cm/s is considered indicative of a venous leak.17
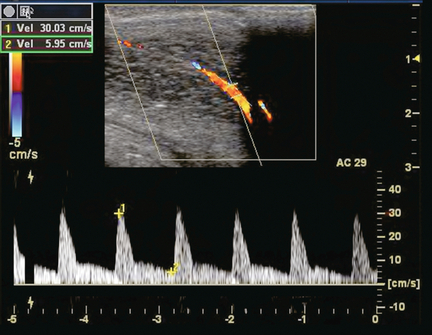
FIGURE 12-8 Spectral Doppler tracing of venous insufficiency. Relatively high-velocity persistent forward flow is seen during diastole (approximating 6 cm/s). This is despite appropriate arterial inflow velocities.
The potential for a false-positive diagnosis of venous leak does exist, especially in young men. The anxiety during a penile ultrasound examination increases sympathetic drive which results in inadequate relaxation of sinusoidal smooth muscle and consequent failure of veno-occlusion. Additional intracavernosal administration of an alpha-adrenergic antagonist such as phentolamine 2 mg, should be considered. Phentolamine blocks the increased sympathetic drive and helps avoid the false-positive diagnosis of venous leak.18
Priapism
Priapism is defined as a persistent erection, unrelated to sexual stimulation, lasting longer than four hours. It is classified as either ischaemic or non-ischaemic. Ischaemic priapism can be drug-induced by antihypertensives, cocaine, or psychotropic medications; especially when combined with phosphodiesterase inhibitors. Ischaemic priapism is painful, requiring prompt medical attention. Initially, corporal needle aspiration is performed to decompress the sinusoids and hopefully relieve the venous outflow obstruction. If that fails then surgery is performed for corporal decompression by cavernosal shunting (Fig. 12-9). If not properly treated, the ischaemic corpora become severely acidotic. Eventually there is degeneration of the tissue, cellular damage and even widespread necrosis. If untreated it will result in irreversible erectile dysfunction and scarring.19 The Doppler sonographic evaluation of priapism is used to identify the presence of blood flow along the length of the corpora cavernosa. Patients with ischaemic priapism have no blood flow in their cavernosal arteries, whereas those with non-ischaemic priapism have abnormally high-velocity flow. Non-ischaemic priapism is rare and most often due to trauma resulting in a high flow state.20
Trauma
Injury to the penis may be the result of blunt or penetrating trauma; or from acute bending of the erect shaft, usually during intercourse. Depending on severity of the insult, subcutaneous or intracorporal haematoma, tunical disruption or urethral tear may occur. The main role of sonography is to exclude albugineal tear because extratunical and cavernosal haematoma can be treated conservatively. Surgery is required, however, when rupture of the tunica albuginea is suspected, especially if the patient reports immediate detumescence associated with a popping sound. Surgical repair of an albugineal tear reduces the risk of post-traumatic curvature, and lowers the incidence of erectile dysfunction. Colour Doppler sonography can be used to help localise small albugineal tears. By squeezing the penile shaft, a flush of blood can be forced from the cavernosal bodies through the tear.21
Following pelvic trauma, Doppler ultrasound is useful for identifying the presence of injury to the penile vasculature.15 Most non-organic causes of erectile dysfunction in young men are secondary to arterial injury with pelvic fracture. Penile arterial inflow may be compromised by development of a post-traumatic stenosis. Doppler ultrasound can be used to look for a tardus parvus waveform of the affected artery. For patients who experience post-traumatic impotence, Broderick et al.22 found that a systolic velocity less than 25 cm/s and asymmetric velocities > 10 cm/s were helpful parameters in establishing a compromise of penile arterial inflow. In some centres, vascular microsurgery is being performed on the internal pudendal artery to bypass a focal stenosis.
With severe trauma, the cavernosal artery may rupture. This results in unrestricted blood flow into the cavernosal space, creating an arteriosinusoidal, arteriolacunar, or arteriovenous fistula. Since venous outflow is maintained from the cavernosal space, the patient develops only a partial erection that is not acutely painful. Persistent venous outflow prevents complete erection, stasis and hypoxia. This condition is known as high-flow priapism and colour Doppler sonography is very effective in its identification (Fig. 12-10). It shows a characteristic arterial colour blush consistent with extravasation of blood from the lacerated artery. Spectral Doppler displays turbulent high-velocity flow.15,19,23 In long-standing priapism, this area may appear more circumscribed, mimicking a pseudoaneurysm. Angiographic embolisation of the lacerated artery is currently considered the treatment of choice. It can be performed with an autologous blood clot.24 Colour Doppler US allows confirmation of successful embolisation by demonstrating disappearance or size reduction of the fistula. If unsuccessful, persistent high-velocity flow in the feeding vessels would be seen.25
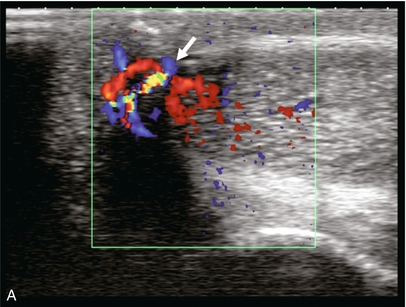
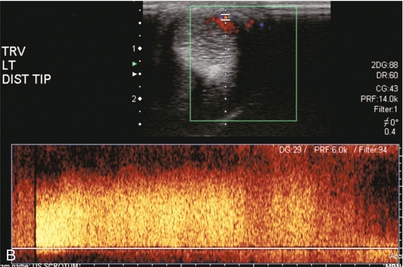
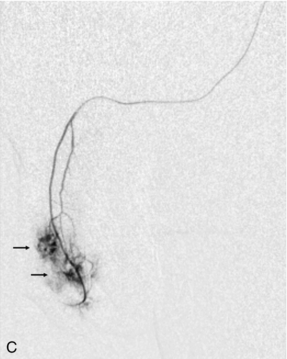
FIGURE 12-10 This patient sustained a penile injury while bull riding 15 days prior to admission. The patient presented with a persistent painless erection. (A) Longitudinal colour Doppler image of the mid-penis. An area of colour flow aliasing within the left corpus cavernosa is the focus of the arterial lacunar fistula (arrow). (B) Corresponding spectral Doppler tracings. There is marked turbulent flow at the site as would be expected with an arterial–lacunar fistula. (C) Pre-embolisation arteriography. An earlyblush (arrows) corresponds to the site of the fistula. This was subsequently successfully embolised. This case is courtesy of Dr Vikram Dogra, University of Rochester Medical Center.
Peyronie’s Disease
Peyronie’s disease (induratio penis plastica) is an idiopathic disorder of the connective tissue in which fibrous plaques form in the tunica albuginea with induration of the corpora cavernosa of the penis or a fibrous cavernositis. The incidence increases with age, being less than 2% at 40 years and increasing to over 6% at 70 years. The disease causes penile deviation and affected patients typically complain of pain during erection.15 Ultrasound imaging can estimate the extent and depth of the plaques, which may be hypo- or hyperechoic and, if longstanding, often contain calcification.
Doppler examination of the penile vasculature is of value to determine if any vascular abnormalities are caused by the fibrous plaques.26,27 Doppler can differentiate between veno-occlusive dysfunction (considered the primary vascular cause of impotence associated with Peyronie’s disease) and arterial insufficiency.28 By doing so, Doppler helps establish the correct treatment option for the patient. If Doppler reveals appropriate arterial inflow and an appropriate response to injection, then plaque removal with grafting of bovine pericardium is preferred. However, if Doppler shows abnormal flow profiles or an inadequate response to injection, in addition to the disfigurement, then implantation of a prosthesis should be considered.29 After surgical intervention Doppler can readily by used for confirmation of success or identification of vascular complications.30
REFERENCES
1. Corona, G., Mannucci, E., Mansani, R., et al. Aging and pathogenesis of erectile dysfunction. Int J Impot Res. 2004; 16(5):395–402.
2. Connolly, J. A., Borirakchanyavat, S., Lue, T. F. Ultrasound evaluation of the penis for assessment of impotence. J Clin Ultrasound. 1996; 24(8):481–486.
3. Weiske, W. H. Diagnosis of erectile dysfunction – what is still needed today? Urologe A. 2003; 42(10):1317–1321.
4. Gray’s Anatomy. 2005 Online. Available:. http://education.yahoo.com/reference/gray/subjects/subject?id=262
5. Halls, J., Bydawell, G., Patel, U., Erectile dysfunction: the role of penile Doppler ultrasound in diagnosis. Abdom Imaging. 2009; 34(6):712–725, doi: 10. 1007/s00261-008-9463-x. [Epub 2008 Oct 15].
6. Wilkins, C. J., Sriprasad, S., Sidhu, P. S. Colour Doppler ultrasound of the penis. Clin Radiol. 2003; 58(7):514–523.
7. Lue, T. F., Hricak, H., Marich, K. W., et al. Vasculogenic impotence evaluated by high-resolution ultrasonography and pulsed Doppler spectrum analysis. Radiology. 1985; 155(3):777–781.
8. Aversa, A., Sarteschi, L. M. The role of penile colour-duplex ultrasound for the evaluation of erectile dysfunction. J Sex Med. 2007; 4(5):1437–1447. [Epub 2007 Jul 21].
9. LeRoy, T. J., Broderick, G. A. Doppler blood flow analysis of erectile function: who, when, and how. Urol Clin North Am. 2011; 38(2):147–154. [Epub 2011/05/31].
10. Ghanem, H., Shamloul, R. An evidence-based perspective to commonly performed erectile dysfunction investigations. J Sex Med. 2008; 5(7):1582–1589.
11. Mihmanli, I., Kantarci, F. Erectile Dysfunction. Semin Ultrasound CT MRI. 2007; 28(4):274–286.
12. Krysiewicz, S., Mellinger, B. C. The role of imaging in the diagnostic evaluation of impotence. AJR Am J Roentgenol. 1989; 153(6):1133–1139.
13. Jarow, J. P., Pugh, V. W., Routh, W. D., et al. Comparison of penile duplex ultrasonography to pudendal arteriography Variant penile arterial anatomy affects interpretation of duplex ultrasonography. Invest Radiol. 1993; 28(9):806–810.
14. Mancini, M., Bartolini, M., Maggi, M., et al. The presence of arterial anatomical variations can affect the results of duplex sonographic evaluation of penile vessels in impotent patients. J Urol. 1996; 155(6):1919–1923.
15. Herbener, T. E., Seftel, A. D., Nehra, A., et al. Penile ultrasound. Semin Urol. 1994; 12(4):320–332.
16. Altinkilic, B., Hauck, E. W., Weidner, W. Evaluation of penile perfusion by colour-coded duplex sonography in the management of erectile dysfunction. World. J Urol. 2004; 22(5):361–364. [Epub 2004 Jul 20].
17. Bassiouny, H. S., Levine, L. A. Penile duplex sonography in the diagnosis of venogenic impotence. J Vasc Surg. 1991; 13(1):75–82. [discussion 83].
18. Gontero, P., Sriprasad, S., Wilkins, C. J., et al. Phentolamine re-dosing during penile dynamic colour Doppler ultrasound: a practical method to abolish a false diagnosis of venous leakage in patients with erectile dysfunction. Br J Radiol. 2004; 77(923):922–926.
19. Sadeghi-Nejad, H., Dogra, V., Seftel, A. D., et al. Priapism. Radiol Clin North Am. 2004; 42(2):427–443. [Epub 2004/05/12].
20. Huang, Y. C., Harraz, A. M., Shindel, A. W., et al, Evaluation and management of priapism: 2009 update. Nat Rev Urol. 2009; 6(5):262–271, doi: 10. 1038/nrurol. 2009. 50.
21. Bertolotto, M., Mucelli, R. P. Nonpenetrating penile traumas: sonographic and Doppler features. AJR Am J Roentgenol. 2004; 183(4):1085–1089.
22. Broderick, G., McGahan, J. P., White, R. D., et al. Colour Doppler US: assessment of post-traumatic impotence. Radiology. 1990; 177(suppl):130–134.
23. Hakim, L. S., Kulaksizoglu, H., Mulligan, R., et al. Evolving concepts in the diagnosis and treatment of arterial high flow priapism. J Urol. 1996; 155(2):541–548.
24. Cantasdemir, M., Gulsen, F., Solak, S., et al, Posttraumatic high-flow priapism in children treated with autologous blood clot embolization: long-term results and review of the literature. Pediatr Radiol. 2011; 41(5):627–632, doi: 10. 1007/s00247-010-1912-3. [Epub 2010 Dec 3].
25. Bertolotto, M., Quaia, E., Mucelli, F. P., et al. Colour Doppler imaging of posttraumatic priapism before and after selective embolization. Radiographics. 2003; 23(2):495–503.
26. Amin, Z., Patel, U., Friedman, E. P., et al. Colour Doppler and duplex ultrasound assessment of Peyronie’s disease in impotent men. Br J Radiol. 1993; 66(785):398–402.
27. Bertolotto, M., de Stefani, S., Martinoli, C., et al. Colour Doppler appearance of penile cavernosal-spongiosal communications in patients with severe Peyronie’s disease. Eur Radiol. 2002; 12(10):2525–2531. [Epub 2002 Apr 30].
28. Levine, L. A., Coogan, C. L. Penile vascular assessment using colour duplex sonography in men with Peyronie’s disease. J Urol. 1996; 155(4):1270–1273.
29. Fornara, P., Gerbershagen, H. P. Ultrasound in patients affected with Peyronie’s disease. World. J Urol. 2004; 22(5):365–367. [Epub 2004 Jul 28].
30. Bertolotto, M., Serafini, G., Savoca, G., et al. Colour Doppler US of the postoperative penis: anatomy and surgical complications. Radiographics. 2005; 25(3):731–748.