Doppler Ultrasound of the Female Pelvis
Doppler Ultrasound of the Female Pelvis
Anatomy
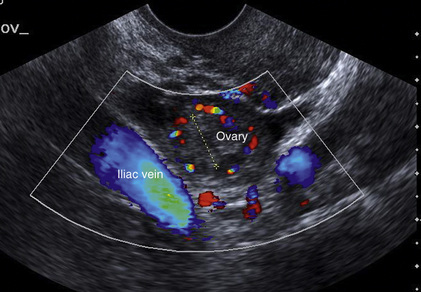
The iliac artery arises at the aortic bifurcation and courses inferiorly and laterally to emerge at the groin as the common femoral artery. The approximate surface markings for this course are given by a line from the umbilicus to the point of maximum pulsation in each groin. The common and external iliac veins run posterior and medial to their accompanying artery. The paired artery and vein often form a lateral boundary to the ovaries (Fig. 14-1). The internal iliac artery arises medially from the common iliac artery approximately 4 cm from the aortic bifurcation. The internal iliac artery divides into anterior and posterior branches; the uterine artery arises from the anterior branch. The uterine artery runs in the base of the broad ligament medially to the cervix, where it gives branches to the upper vagina and cervix. It ascends in the broad ligament giving off branches to the myometrium until it reaches the cornual region. Here it turns laterally to supply the fallopian tube and ovaries and anastomoses with branches from the ovarian artery. The uterine vein mirrors the artery’s course and eventually drains into the internal iliac vein.
Technique
Applications
MENSTRUAL DISORDERS
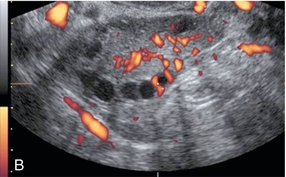
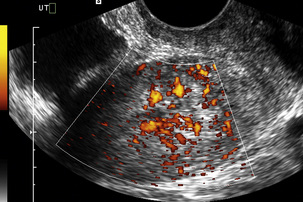
Dysfunctional uterine bleeding is one of the most common gynaecological complaints. Many cases will have an underlying hormonal cause but in up to half of these cases ultrasound may reveal an underlying structural defect – the most frequent being submucosal fibroids, adenomyosis or endometrial polyps. The Doppler characteristics of each of these will be discussed later. However, Doppler also has a more general role to play in these cases as it has been shown that women with pain on menstruation have increased myometrial vascularisation during the early menstrual phase,1 and that those with irregular bleeding are also more likely to show increased perfusion of uterine and sub-endometrial blood vessels (Fig. 14-2).2 The pathophysiology underlying these observations is not yet fully understood. Endometrial ablation is a recognised treatment of dysfunctional bleeding but measurement of the Doppler indices in the uterine arteries does not help determine who will show a good response to ablation; although assessment one year after ablation helps predict the duration of amenorrhoea or eumenorrhoea.3
FERTILITY
Normal Cycle
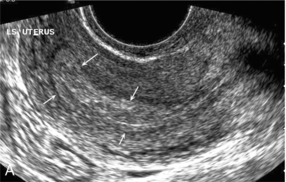
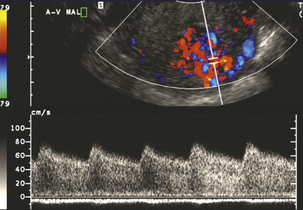
The normal menstrual cycle starts on day one with bleeding heralding the shedding of the endometrium. Ultrasound shows the endometrium reduces to a minimum basal layer and it may occasionally show blood and clot in the cavity. The endometrium then enters the proliferative phase, displaying a distinct trilaminar appearance. As the time of ovulation approaches, the basal endometrium becomes more echogenic; this echogenicity spreading towards the central echogenic line until, in the secretory phase after ovulation, the whole width of the endometrium becomes of equal increased echogenicity (Fig. 14-3). During the proliferative phase, the ovary shows enlargement of a few of the antral follicles, until one becomes dominant (Fig. 14-4). This continues to enlarge until it is about 2.5 cm in diameter at which point the follicle ruptures and ovulation occurs (usually around day 14). The follicle then forms the corpus luteum during the secretory phase. 3D power Doppler has been used to measure the vascular flow index during the normal cycle; the vascular indices in the dominant ovary and the dominant follicle/corpus luteum show an increase in the proliferative phase such that they are 1.7 times greater than in the basal state. After ovulation there is a continued rise in the vascularity with the corpus luteal flow peaking about 7 days after ovulation with an index three times greater than in the basal state. The contralateral, non-dominant ovary in that cycle shows no changes in blood flow.4 Hormonally suppressed ovaries show a low vascular flow index throughout the cycle.5 The resistance index (RI) of the corpus luteum measured by TV colour and spectral Doppler shows that the pre-ovulatory follicle has a high RI, which decreases greatly on ovulation as the corpus luteum is formed. The increased vascularity around the corpus luteum has been likened to a ring of fire (Fig. 14-5). The RI decreases further in the early luteal (secretory) phase but then increases in the late luteal phase. This late increase does not occur if pregnancy has become established, as the RI remains at the low midluteal level until 7 weeks of pregnancy.6 Fertility specialists can use the peri-ovulatory follicular volume and subfollicular vascularisation as predictors of successful pregnancy in intra-uterine insemination techniques – too large a follicle is likely to be anovulatory.7 Furthermore, in oocyte retrieval techniques, it has been suggested pregnancy rates are higher in those whose embryo transfer cohort contains at least one embryo from a highly vascular follicle8 (Box 14-1).
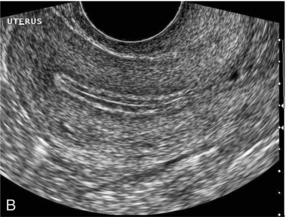
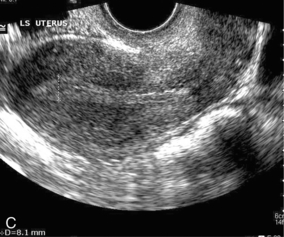
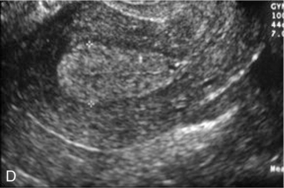
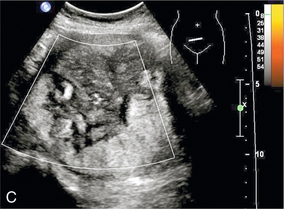
FIGURE 14-3 Normal appearances of cyclical endometrium. (A) Menstrual phase showing the endometrium as a thin line; (B) follicular/proliferative phase showing a trilaminar appearance of the endometrium; (C) periovulatory phase showing the echogenicity of the basal layer of endometrium has extended to the midline echo; (D) luteal/secretory phase showing thickened uniform echogenicity.
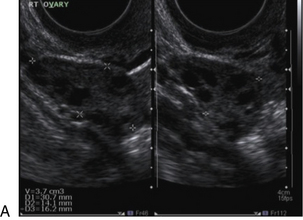
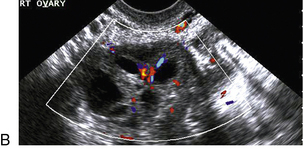
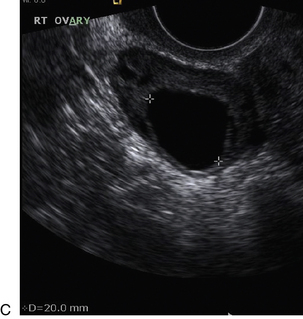
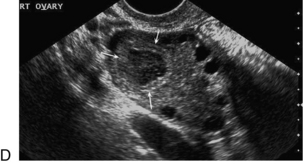
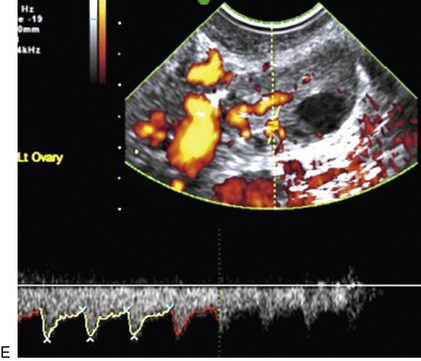
FIGURE 14-4 Normal ovarian cycle. (A) Early phase ovary showing several immature antral follicles of equal size; (B) one follicle has increased flow around it and will start to grow; (C) mid-cycle showing a dominant follicle of 2 cm diameter; (D) post-ovulation ovary with a corpus luteum; (E) spectral trace of ‘active’ ovary showing low-resistance flow with good diastolic flow.
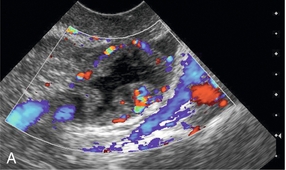
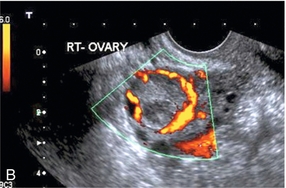
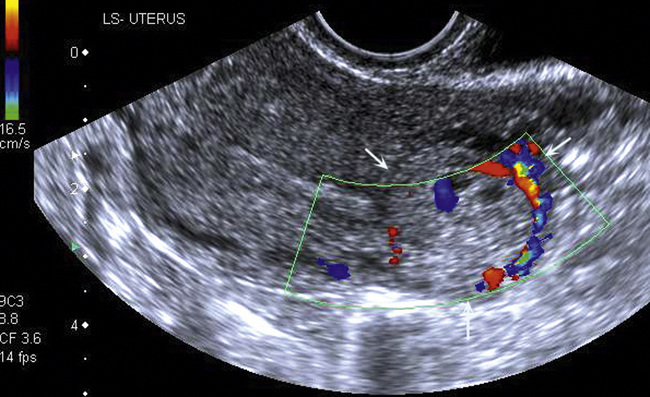
FIGURE 14-5 Corpus luteum showing a ‘ring of fire’ of increased colour Doppler flow around it (A) and another with a ring of power Doppler flow (B).
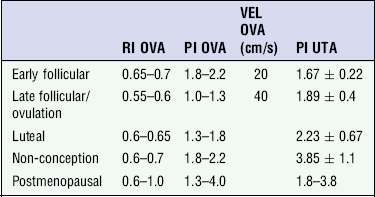
There is also cyclical variation in the supplying ovarian and uterine arteries. An increase in volume flow and a reduction in the RI occur in the luteal (secretory) phase compared with the follicular (proliferative) phase. It is the presence of this variation that appears to correlate with fertility rather than any absolute value. Normal ranges of values are given in Table 14-1. The pre-pubertal uterine artery has a high impedance pattern with absent diastolic flow. The change to a lower impedance pattern and the development of diastolic flow indicates the onset of the menarche.
Ovarian Reserve
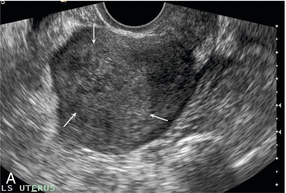
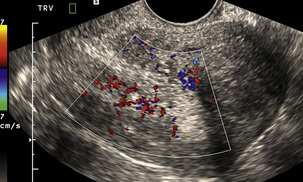
The age at which some women seek to start a family has been increasing. Fertility units in particular, need to determine the ovarian reserve to help evaluate if an in vitro fertilisation (IVF) cycle is likely to be successful. Commonly, an antral follicle count of less than four, or an ovarian volume of < 3 cc measured in the first few days of a menstrual cycle, are associated with non-pregnancy or IVF cycle cancellation.9 More recently, it has been suggested that assessing the basal ovarian stromal blood flow may help.10 Being unable to detect any stromal flow in at least one ovary is not just a technical issue but it is more probably related to low ovarian reserve (Fig. 14-6).
Tubal Patency and Colour Doppler
The use of ultrasound contrast agent instilled directly into the uterine cavity to assess patency of the Fallopian tubes has become an accepted precursor to other tests of tubal patency, such as hysterosalpingography, or laparoscopy and dye injection. Intriguingly, loss of sub-endometrial flow on colour Doppler can also indicate tubal blockage,11 although the mechanism for this is not fully understood.
DISEASES OF THE UTERINE BODY
Fibroids
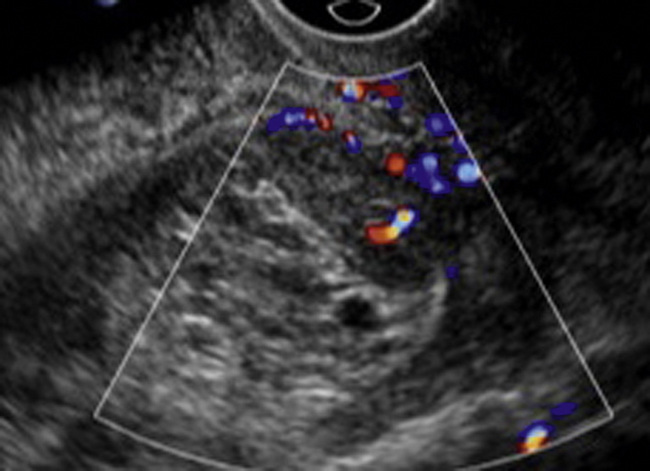
Fibroid vascularity is very variable. The attenuation of sound by some fibroids may make any assessment of vascularity impossible. Detection of flow into a fibroid may help confirm its nature; for instance, demonstrating a vascular connection between the uterus and the fibroid may help distinguish a sub-serosal or pedunculated fibroid from an adnexal mass. Likewise, differentiation of an endometrial polyp from a sub-mucosal fibroid (Fig. 14-7) may be helped by finding more than one feeding vessel as polyps should have only a single vessel (Box 14-2).
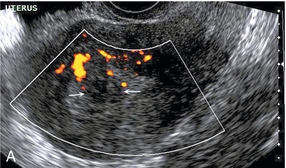
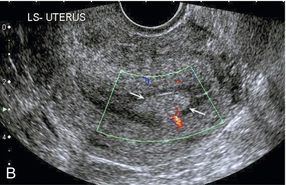
FIGURE 14-7 (A) A submucosal fibroid (arrows) with predominantly reduced echoity and more than one feeding vessel; compare (B) an endometrial polyp (arrows) with a generally increased echoity and only one feeding vessel.
Fibroids are often a cause of dysfunctional uterine bleeding. Fibroids are hormonally driven, being more common during menstrual years and tending to regress after the menopause. Visibly vascular fibroids are thought more likely to respond to hormonal manipulation in the suppression of heavy bleeding. Uterine artery embolisation is an alternative to surgery for fibroid-related bleeding, although magnetic resonance imaging is better than ultrasound at assessing suitability and response. A new technique of transvaginal Doppler ultrasound-guided uterine artery occlusion using a temporary clamp has been proposed as a further alternative with some good results in pilot studies.12
Nearly all sarcomatous fibroids are diagnosed incidentally on histology following fibroid removal for other reasons.13 It has long been a goal to detect some vascular feature that would allow diagnosis of a leiomyosarcoma; factors such as the larger a lesion, the more peripheral and central vascularity that is found and lower impedance values have all been put forward as predictive of sarcoma (Fig. 14-8). None has been found useful in practice. Currently, the use of 3D ultrasound and finding increased vascular density have been linked to higher cellular activity scores14 but the imaging diagnosis of sarcoma remains elusive. The most useful sign remains rapid growth on sequential scans.
Endometrial Cancer
Ultrasound is used to measure the endometrial thickness in women presenting with postmenopausal bleeding. It is used for its negative predictive value as those with a bi-layer thickness of 4 mm or less are extremely unlikely to have cancer and so do not need biopsy or hysteroscopy. However, this still leaves many women with an endometrial thickness of over 4 mm who have unnecessary biopsy as most will still not have cancer. The challenge is to define some combination of endometrial thickness and vascular change that will be more predictive. It has been shown that women with endometrial cancer have lower impedance values in the uterine arteries, as well as in myometrial and endometrial vessels15 but that these changes are not distinct enough to allow prospective diagnosis. Valentin and her group have shown that using power Doppler on ordinary 2D transvaginal ultrasound to define a vascularity index (percentage of the endometrial area on the most vascularised imaging section that contains power Doppler signal) and combining this with the endometrial thickness can improve the positive predictive value for cancer (Fig. 14-9).16 This contradicts some of their earlier work but is more in accord with other researchers using 3D power Doppler. As yet, these models need to be clinically validated (Box 14-3).
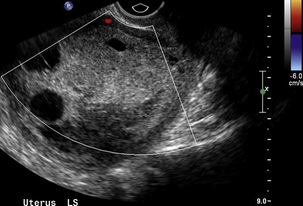
FIGURE 14-9 Endometrial cancer – the image shows a much thickened complex-appearing endometrium that was proven to be cancer. The lack of vascularity clearly does not exclude malignant change.
The depth of invasion of endometrial cancer into the myometrium and the histological grade of the tumour affect prognosis. The more advanced tumours are likely to have a mixed or low echogenicity on greyscale ultrasound and are more likely to have greater vascularity, using either 3D or 2D techniques, together with a higher colour score, lower impedance and a greater number of visible feeding vessels (Fig. 14-10).17,18 In practice, most centres will use postoperative histology to guide therapy.
Endometritis
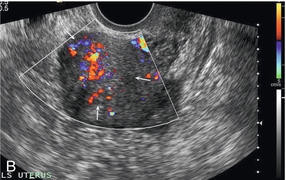
This is usually a clinical diagnosis and there may be no signs on ultrasound. Inflammation of the endometrium may occur post-partum, following instrumentation, or as part of a wider pelvic inflammatory disorder. There are no specific ultrasound signs but finding fluid or debris in the endometrial cavity in someone with appropriate clinical features may help. Doppler features, if any, are of increased endometrial vascularity and reduced impedance (Fig. 14-11). Some authors believe that uterine artery RI showing an increase back to normal higher levels of impedance is a marker of response to treatment.19
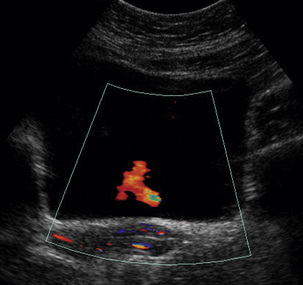
Retained Products of Conception
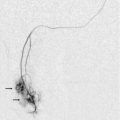
Retained products of conception (RPOC) may occur after normal delivery or after spontaneous or induced miscarriage. It can be very difficult to determine if the material seen in the endometrial cavity is true RPOC, or just blood clot. It has been shown that no clinical or greyscale sonographic feature predicts the disorder when compared against the histology of the removed contents.20 However, there is recent recognition that some RPOC are associated with marked focal hyper-vascularity (Fig. 14-12). This seems to be a much more common finding in women who have had an induced termination of pregnancy.21 It is important to realise that these findings do not represent arterio-venous malformations (AVM) and that the great majority of them will resolve with expectant management.22,23 Ultrasound can be used to monitor the involution of the hyper-vascularity and βHCG should be checked to exclude the possibility of underlying molar change as this may also show hyper-vascularity. True, acquired uterine AVM are usually caused by curettage, show similar focal hyper-vascularity and are likely to require further treatment such as embolisation (Fig. 14-13). Consequently, it is important to be aware of the clinical circumstances when interpreting the Doppler findings. Conversely, women with an incomplete miscarriage have a better rate of spontaneous passage of the products if the suspected products are avascular24 (Box 14-4).
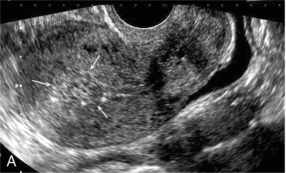
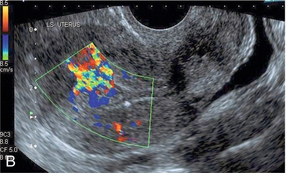
FIGURE 14-12 Longitudinal views of a uterus following miscarriage. (A) Greyscale image showing tissue of increased echogenicity in the endometrial cavity likely to be retained products of conception. (B) There is greatly increased vascularity in the underlying myometrium implying that these are adherent, vascularised products of conception.
Trophoblastic Disease
Contrast-enhanced colour Doppler ultrasound shows that invasive moles and choriocarcinomas show a marked increase in uterine and tumour vascularity following enhancement, whereas non-invasive moles do not show any intra-tumoral blood flow.25 Use of contrast-enhanced ultrasound may permit the detection of small invasive moles within the myometrium (Fig. 14-14).
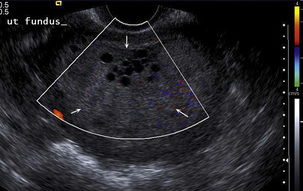
FIGURE 14-14 Molar pregnancy proven on histology. The uterine cavity is distended by uniformly echogenic tissue containing an area of small cysts. Non-invasive moles do not show increased vascularity.
Power Doppler has been used to monitor response to chemotherapy with methotrexate and loss of vascularity in a tumour nodule may be an independent marker that predicts response even when βHCG values appear to have plateaued.26
CERVICAL CANCER
Cervical tumours show vascularity (Fig. 14-15) whereas the normal cervical stroma does not. Prolapsing cervical polyps can be identified on colour Doppler by virtue of their vascularity and their feeding vessels (Fig. 14-16). Both squamous cell carcinomas (SCC) and adenocarcinomas (AC) are vascular and cannot be distinguished on Doppler criteria, though SCC are more likely to be hypo-echoic and AC iso-echoic compared to normal cervical stroma.27 It is postulated that the more abundant the tumour vascularisation, the more likely it is that the tumour will be larger, have deeper invasion, lymphovascular space invasion and nodal metastases.28 Others have not found so strong a correlation, confining themselves to saying that tumour vascularity on 3D power Doppler correlates with cervical volume but not with other markers of worse prognosis.29 This work has not yet found a practical application.
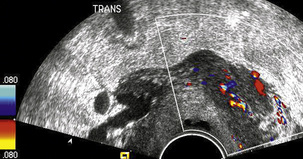
FIGURE 14-15 Cervical cancer. Transvaginal view of tumour on the ectocervix. Note that the tumour is vascular.
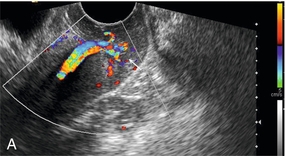
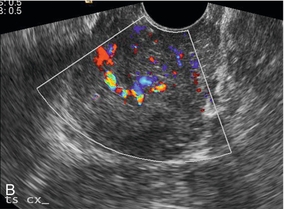
FIGURE 14-16 Prolapsing cervical polyp. (A) Longitudinal view of the cervix showing vessels running through the cervical canal to the polyp (arrow); (B) transverse view through the cervix showing the polyp is vascularised.
The development of thrombo-embolic events, which may be diagnosed on Doppler ultrasound or on computed tomography, is associated with tumour progression in women on follow-up for stage IIIB cervical cancer30 and indicates a poorer prognosis.
OVARIAN DISEASE
Cancer
There has been considerable research effort put into defining ultrasound characteristics that will detect early ovarian cancer and distinguish benign from malignant adnexal masses. Most such programmes use greyscale features such as size, echogenicity, septations, nodularity and solid components, as well as Doppler indices of vascularity, to give a risk of malignancy. When combined with clinical features of age, menopausal status and tumour markers such as CA-125, or tumour-specific growth factor, they can achieve reasonable accuracy.31,32 There are several such indexes or logistic regression programmes to help in the diagnosis but so far, it is known that an experienced observer can out-perform these.33 There will always be a small subset of adnexal masses thought appropriate for surgery that will not be characterisable by ultrasound.
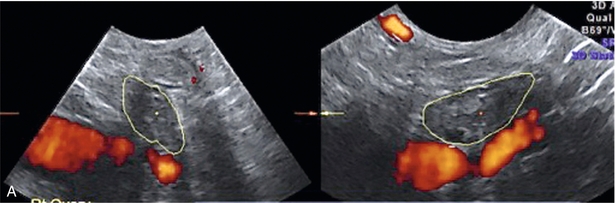
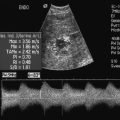
Normal physiological processes in women of menstrual years will produce follicular cysts, neovascularisation and low-impedance flows. The challenge for Doppler ultrasound is to separate these normal events from the abnormal flow patterns seen in pathological lesions. Initially, it was thought that malignant tumours showed lower impedance values on RI and PI than benign processes (Fig. 14-17). However, although there is a trend towards this, the indices are not a useful prospective clinical tool. Interest of late has focused on the pattern of colour/power Doppler spread in the tumour and the amount of vascularity. Not everyone has found 3D power Doppler to be helpful, Ohel et al.34 found no significant differences between benign and malignant ovaries on any of the indices of vascularity and perfusion. Alcazar and his group have been strong advocates for the use of Doppler in ovarian masses. Their work35 shows that finding colour Doppler flow within any solid portion or excrescence in a tumour increases the likelihood of the lesion being malignant and that this increases the diagnostic accuracy over the greyscale findings (Fig. 14-18). The impact of this finding depends on the clinical presentation;36 colour Doppler performs best in those being investigated for symptoms of cancer; it is less effective in those with benign symptoms and is outperformed by greyscale findings in asymptomatic women. Some corroboration is offered by work looking at the development of malignancy within endometriotic cysts. Finding a vascularised solid nodule within a cyst otherwise thought to be due to endometriosis is a marker of malignant change (Fig. 14-19; Box 14-5).37
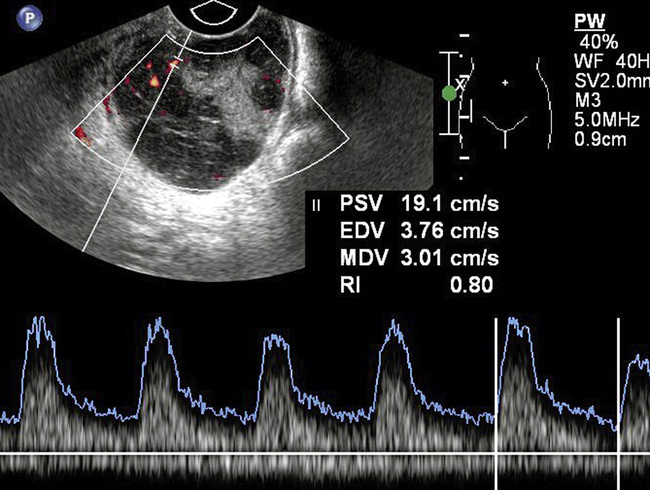
FIGURE 14-17 Ovarian cancer. A typical appearance of an ovarian cancer with a mixed cystic and solid lesion. There is visible colour flow within the tumour and the spectral trace shows diastolic flow. However, the resistance index (RI) of 0.8 falls outside the range that used to be used to predict malignancy. The use of spectral indices has fallen into disuse in the diagnosis of ovarian cancer because of this.
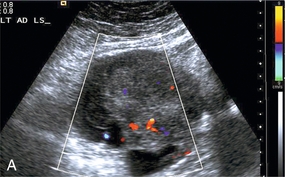
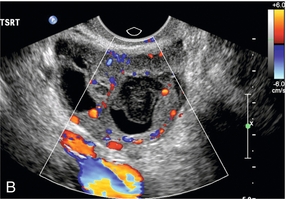
FIGURE 14-18 Ovarian cancer. (A) The solid component of the ovarian lesion contains colour flow indicating that this is a malignant lesion. (B) A large abnormal post-menopausal ovary. The presence of the colour flow increases the predictive value for cancer. Both of these lesions were proven to be cancers.
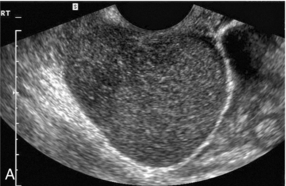
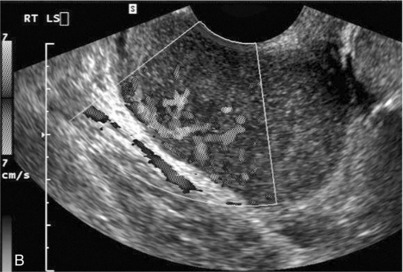
FIGURE 14-19 (A) Initial grey-scale view of an adnexal mass mimicking the features of an endometrioma. However, the use of colour Doppler (B) clearly shows that this is a solid malignant lesion (monochrome image of a colour Doppler study).
The use of Doppler to look for vascularised solid nodules in tumours mirrors the work done on the use of gadolinium enhancement in magnetic resonance imaging; both techniques are assessing vascularity of the lesion. Most centres will use an imaging algorithm that starts with ultrasound. Doppler is used to help solve problems, even though meta-analyses38 indicate that this may not improve sensitivity or specificity. Those masses thought to be benign are followed-up with ultrasound, those thought to be definitely malignant are staged with CT and those which are indeterminate, are further categorised using MRI.
Ovarian Torsion
Ovarian torsion (Box 14-6) is one of the causes of acute pelvic pain. It is more common in children and young adults and may be associated with vomiting. It is often associated with an underlying lesion such as a cyst or dermoid. Diagnosis is made more difficult because the ovary may tort and detort spontaneously; because of this, patterns of Doppler flow can be very variable.
Affected children may present with intermittent abdominal pain and tenderness.39 Some believe all children will show an abnormal flow pattern in the affected ovary, either with reduced overall vascularity and a greater impedance in the supplying vessel or, if recent de-torsion has occurred, with increased vascularity and low impedance when compared to the pain-free side (Fig. 14-20).
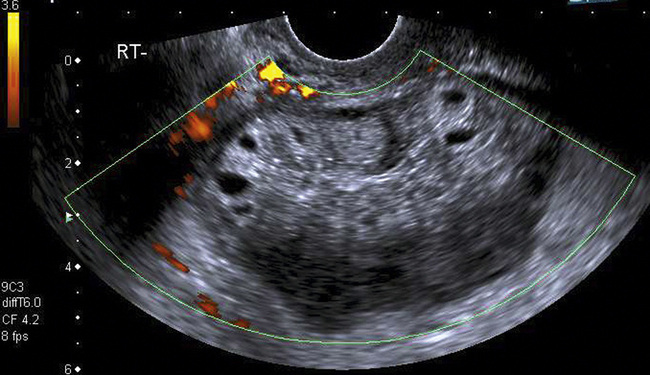
FIGURE 14-20 Ovarian torsion. The ovary is greatly enlarged, the follicles stand out within a small halo and there is absence of detectable blood flow on power Doppler. These are typical features of torsion.
Adults may show similar signs of abnormal blood flow and free fluid in about three quarters of those with torsion. Absence of any signs does not exclude the diagnosis.40 Indeed, some authors find that using Doppler does not add to the accuracy of the pre-operative diagnosis in women with acute pain.41
Additional signs should be sought. The ‘whirlpool’ sign42 of vessels spiralling round each other or the ovary can be seen with greyscale ultrasound, or with Doppler (Fig. 14-21); although if there is torsion, there may be no flow in the vessels. This sign adds specificity to the ultrasound diagnosis of torsion. The flow pattern in the draining ovarian veins43 is also worth interrogating as it has been reported that absent flow, or abnormality in the venous flow may be the only sign of torsion.
OTHER ADNEXAL LESIONS
Sepsis
The majority of inflammatory conditions will be associated with clinical signs of sepsis and it is these clinical signs and symptoms that are essential for diagnosis of these inflammatory disorders. Most inflammatory conditions will show increased blood flow, particularly in circumferential vessels draped around the cystic component (pus) of a tubo-ovarian abscess (Fig. 14-22). Sepsis may provoke thrombosis of the iliac or gonadal veins (Fig. 14-23). More chronic forms of sepsis, such as pelvic actinomycosis associated with long-term intra-uterine device use, do not have any specific Doppler features.
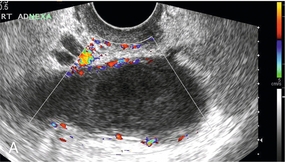
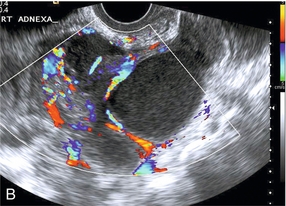
FIGURE 14-22 Pyosalpinx. (A) A dilated fallopian tube filled with pus and with increased vascularity in the walls. (B) A more complex tubo-ovarian abscess with several loculations and increased vascularity. The diagnosis is really made from the clinical features and the increased vascularity is merely confirmatory.
Endometriosis
Endometriomas are classically detected as, ‘an adnexal mass in a pre-menopausal patient with ground-glass echogenicity of the cyst fluid, one to four locules and no solid parts with detectable blood flow’.44 Ground-glass cysts in post-menopausal women are more likely to be malignant, as are any lesions that have vascularised solid parts. However, women who present with pelvic pain from their endometrioma are likely to show increased vascularisation around the endometriotic cyst.45
Ectopic Pregnancy
Ultrasound, particularly transvaginal ultrasound, plays a vital role in the diagnosis of ectopic pregnancy (Box 14-7). Women presenting with pain and bleeding in early pregnancy require ultrasound to establish the site and viability of any gestation. Scans should not be done prior to 6 weeks menstrual age as these have a high rate of being non-diagnostic and may lead to erroneous over-diagnosis of ectopic pregnancy. No-one has suffered tubal rupture from ectopic pregnancy prior to 6 weeks menstrual age. After 6 weeks, failing to find a gestation sac in the presence of a positive pregnancy test leads to the concept of a ‘pregnancy of unknown location’. This includes a pregnancy in the right place but too small to see, a recently miscarried pregnancy and ectopic pregnancy. Transvaginal ultrasound has a high sensitivity for locating an ectopic pregnancy based on the greyscale signs of an adnexal mass, echogenic ring (doughnut) sign (Fig. 14-24), or echogenic free fluid. Some authors believe that the addition of colour or power Doppler to look for the increased flow in the trophoblast improves the detection rate of the ectopic gestation (Fig. 14-25). There is the potential pitfall of mistaking the ‘ring-of-fire’ produced by increased blood flow around a corpus luteum for an ectopic. Uterine artery blood flow indices do not allow distinction of a normally sited pregnancy from an ectopic. Once an ectopic pregnancy has been located, treatment depends on its size and whether or not the pregnancy is dead. Not all will need surgery. Conservative treatment with methotrexate can be guided by Doppler assessment of whether there is active trophoblast or not.
PELVIC CONGESTION SYNDROME
It has long been accepted that testicular vein incompetence is a cause of scrotal varicocele and pain in men. It has been much less accepted that a similar syndrome in women of chronic pelvic pain, dyspareunia and pelvic varices might exist. The finding of dilated pelvic veins on ultrasound is relatively commonplace. The more numerous they are and the greater the diameter, usually over 4 mm, the more likely there is to be evidence of reflux on venous Doppler during Valsalva manoeuvre. Reversed flow velocities of over 2 cm/s are thought to be significant. Confirmation of these findings comes from studies of trans-femoral catheter ovarian vein venography and embolisation46,47 to bring resolution of the symptoms.
NON-GYNAE PATHOLOGY
Iliac and Uterine Artery Aneurysm/Lymphocysts
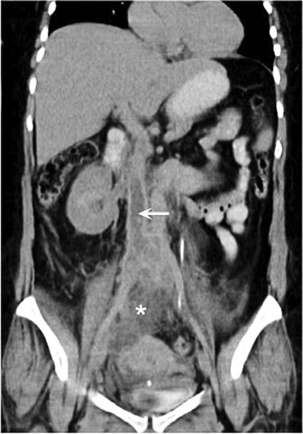
Iliac artery aneurysms should be readily identified by their communication with the main vessels and by the presence of blood flow within them, which is often swirling in nature. If thrombosed, they may present more of a diagnostic challenge in the differentiation from an adnexal cystic mass but mural calcification and the relationship to the iliac vessels should provide the answer (Fig. 14-26).
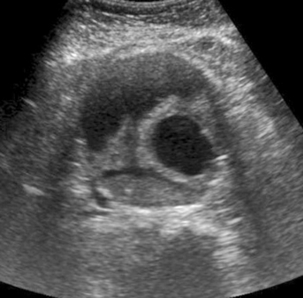
FIGURE 14-26 A large iliac aneurysm demonstrating how the intramural thrombus can produce complex appearances and mimic an adnexal mass for the unwary.
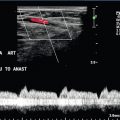
Uterine artery pseudoaneurysms are secondary to operative interventions, usually during delivery (commonly Caesarean section).48 Doppler ultrasound will show an intrauterine cystic mass that has high-speed internal flow. Reported cases have been treated with trans-catheter embolisation.
Lymphocysts are common after pelvic lymphadenectomy for gynaecological cancer.49 They may appear about 2 weeks after operation and can persist for up to a year. Most are asymptomatic. Ultrasound detects them as cystic structures on the pelvic side walls and Doppler is used to confirm that they are not vascular.
Deep Vein Thrombus
Deep vein thrombus (DVT) is usually detected by ultrasound of the lower limb veins. Pelvic and transvaginal sonography may be used to assess the cranial extent of any clot into the iliac veins. Typically, in acute thrombosis, the vein will be swollen by the presence of echo-poor clot and Doppler will either show complete cessation of flow, or flow around the margins of the clot. Chronic thrombosis is more likely to show the vein to be reduced in calibre, with clot of increased echogenicity and flow in re-canalised channels through the clot. DVT remains a leading cause of maternal mortality during pregnancy and the post-partum period.50
Bowel Masses
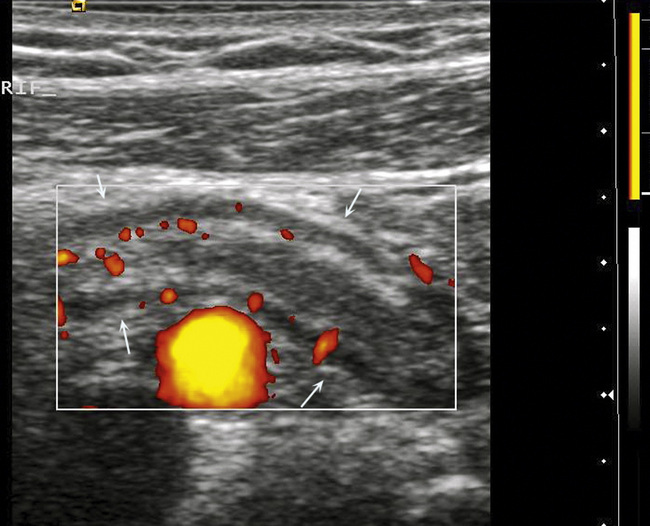
Ultrasound is not the primary imaging modality to detect bowel masses but such masses may be seen serendipitously during pelvic ultrasound for other causes. Bowel wall thickening and mural hyper-vascularity are non-specific markers of inflammatory conditions, including diverticular disease, appendicitis (Fig. 14-27), Crohn’s disease and infective colitis. Indeed, in women, transvaginal ultrasound done for pelvic pain may increase the detection rate of appendicitis, as a proportion of appendixes will lie low in the pelvis.
Ureteric Jets
The passage of urine into the bladder from the ureteric orifices is marked by intermittent jets of urine secondary to ureteric peristalsis, rather than a continuous trickle. These jets can be seen on greyscale ultrasound but are more readily visible using colour Doppler (Fig. 14-28). Injuries to the ureter are a known complication of gynaecological surgery. Finding normal ureteric jets in the postoperative period is a good way of excluding ureteral injury, or occlusion.51
Pelvic organ prolapse is associated with an alteration in ureteric jets. Longer-duration, lower-velocity jets are present when there is associated bladder outlet obstruction and hydronephrosis.52
REFERENCES
1. Royo, P., Alcazar, J. L. Three-dimensional power Doppler assessment of uterine vascularization in women with primary dysmenorrhea. J Ultrasound Med. 2008; 27(7):1003–1010.
2. Hussein, M. Transvaginal Doppler sonography for evaluation of irregular uterine bleeding with DMPA. Arch Gynecol Obstet. 2011; 283(6):1325–1328.
3. Kuzel, D., Toth, D., Fucikoa, Z., et al. Uterine arteries Doppler velocimetry provides 3-years follow up endometrial ablation outcome. Prague Med Rep. 2008; 109(2–3):166–174.
4. Jokubkiene, L., Sladkevicius, P., Rovas, L., et al. Assessment of changes in volume and vascularity of the ovaries during the normal menstrual cycle using three-dimensional power Doppler ultrasound. Hum Reprod. 2006; 21(10):2661–2668.
5. Hope, J. M., Long, K., Kudla, M., et al. Three-dimensional power Doppler angiography of cyclic ovarian blood flow. J Ultrasound Med. 2009; 28(8):1043–1052.
6. Tamura, H., Takasaki, A., Taniguchi, K., et al. Changes in blood-flow impedance of the human corpus luteum throughout the luteal phase and during early pregnancy. Fertil Steril. 2008; 90(6):2334–2339.
7. Engels, V., Sanfrutos, L., Perez-Medina, T., et al. Periovulatory follicular volume and vascularization determined by 3D and power Doppler sonography as pregnancy predictors in intrauterine insemination cycles. J Clin Ultrasound. 2011; 39(5):243–247.
8. Robson, S. J., Barry, M., Norman, R. J. Power Doppler assessment of follicle vascularity at the time of oocyte retrieval in in-vitro fertilization cycles. Fertil Steril. 2008; 90(6):2179–2182.
9. Gibreel, A., Maheshwari, A., Bhattacharya, S., et al. Ultrasound tests of ovarian reserve; a systematic review of accuracy in predicting fertility outcomes. Hum Fertil (Camb). 2009; 12(2):95–106.
10. Younis, J. S., Haddad, S., Matilsky, M., et al. Undetectable basal ovarian stromal blood flow in infertile women is related to low ovarian reserve. Gynecol Endocrinol. 2007; 23(5):284–289.
11. Demir, B., Kocak, M., Beydilli, G., et al. Diagnostic accuracy and efficacy of color Doppler mapping for tubal patency. J Obstet Gynaecol Res. 2011; 37(7):782–786.
12. Vilos, G. A., Vilos, E. C., Abu-Rafea, B., et al. Transvaginal Doppler-guided uterine artery occlusion for the treatment of symptomatic fibroids: summary results from two pilot studies. J Obstet Gynaecol Can. 2010; 32(2):149–154.
13. Wu, T. I., Yen, T. C., Lai, C. H. Clinical presentation and diagnosis of uterine sarcoma, including imaging. Best Pract Res Clin Obstet Gynaecol. 2011; 25(6):681–689.
14. Minsart, A. F., Ntoutoume Sima, F., Vandenhoute, K., et al. Does three-dimensional ultrasound predict histopathologic findings of uterine fibroids? A preliminary study. Ultrasound Obstet Gynecol. 2012; 40(6):714–720.
15. Bezircioglu, I., Baloglu, A., Cetinkaya, B., et al. The diagnostic value of Doppler ultrasonography in distinguishing endometrial malignancies in women with postmenopausal bleeding. Arch Gynecol Obstet. 2012; 285(5):1369–1374.
16. Opolskiene, G., Sladkevicius, P., Valentin, L. Prediction of endometrial malignancy in women with postmenopausal bleeding and sonographic endometrial thickness ≥4. 5 mm. Ultrasound Obstet Gynecol. 2011; 37(2):232–240.
17. Galvan, R., Merce, L., Jurado, M., et al. Three–dimensional power Doppler angiography in endometrial cancer: correlation with tumor characteristics. Ultrasound Obstet Gynecol. 2010; 35(6):723–729.
18. Epstein, E., Van Holsbeke, C., Mascilini, F., et al. Gray-scale and color Doppler ultrasound characteristics of endometrial cancer in relation to stage, grade and tumor size. Ultrasound Obstet Gynecol. 2011; 38(5):586–593.
19. Ozbay, K., Deveci, S. Relationships between transvaginal colour Doppler findings, infectious parameters and visual analogue scale scores in patients with mild acute pelvic inflammatory disease. Eur J Obstet Gynecol Reprod Biol. 2011; 156(1):105–108.
20. Levin, I., Almog, B., Ata, B., et al. Clinical and sonographic findings in suspected retained trophoblast after pregnancy do not predict the disorder. J Minim Invasive Gynecol. 2010; 17(1):66–69.
21. Mungen, E., Dundar, O., Babacan, A. Postabortion Doppler evaluation of the uterus: incidence and causes of myometrial hypervascularity. J Ultrasound Med. 2009; 28(8):1053–1060.
22. Mulic-Lutvica, A., Eurenius, K., Axelsson, O. Uterine artery Doppler ultrasound in postpartum women with retained placental tissue. Acta Obstet Gynecol Scand. 2009; 88(6):724–728.
23. Kitahara, T., Sato, Y., Kakui, K., et al. Management of retained products of conception with marked vascularity. J Obstet Gynaecol Res. 2011; 37(5):458–464.
24. Casikar, I., Lu, C., Oates, J., et al. The use of power Doppler colour scoring to predict successful expectant management in women with an incomplete miscarriage. Hum Reprod. 2012; 27(3):669–675.
25. Emoto, M., Sadamori, R., Hachisuga, T., et al. Clinical usefulness of contrast-enhanced color Doppler ultrasonography in invasive and noninvasive gestational trophoblastic diseases: a preliminary study. J Reprod Med. 2011; 56(5–6):224–234.
26. Cavoretto, P., Gentile, C., Mangili, G., et al. Transvaginal ultrasound predicts delayed response to chemotherapy and drug resistance in stage I low-risk trophoblastic neoplasia. Ultrasound Obstet Gynecol. 2012; 40(1):99–105.
27. Epstein, E., Di Legge, A., Masback, A., et al. Sonographic characteristics of squamous cell cancer and adenocarcinoma of the uterine cervix. Ultrasound Obstet Gynecol. 2010; 36(4):512–516.
28. Jurado, M., Galvan, R., Martinez-Monge, R., et al. Neoangiogenesis in early cervical cancer: correlation between color Doppler findings and risk factors. A prospective observational study. World J Surg Oncol. 2008 Nov 25; 6:126.
29. Belitsos, P., Papoutsis, D., Rodolakis, A., et al. Use of three-dimensional power Doppler ultrasound for the study of cervical cancer and precancerous lesions. Ultrasound Obstet Gynecol. 2012; 40(5):576–581.
30. Renni, M. J., Russomano, F. B., Mathias, L. F., et al. Thromboembolic event as a prognostic factor for the survival of patients with stage IIIB cervical cancer. Int J Gynecol Cancer. 2011; 21(4):706–710.
31. Wang, L. M., Song, H., Song, X., et al. An improved risk of malignancy index in diagnosis of adnexal mass. Chin Med J (Engl). 2012; 125(3):533–535.
32. Rossi, A., Braghin, C., Soldano, F., et al. A proposal for a new scoring system to evaluate pelvic masses: Pelvic Masses Score (PMS). Eur J Obstet Gynecol Reprod Biol. 2011; 157(1):84–88.
33. Valentin, L., Ameye, L., Savelli, L., et al. Adnexal masses difficult to classify as benign or malignant using subjective assessment of gray-scale and Doppler ultrasound findings: logistic regression models do not help. Ultrasound Obstet Gynecol. 2011; 38(4):456–465.
34. Ohel, I., Sheiner, E., Aricha-Tamir, B., et al. Three-dimensional power Doppler ultrasound in ovarian cancer and its correlation with histology. Arch Gynecol Obstet. 2010; 281(5):919–925.
35. Guerriero, S., Alcazar, J. L., Ajossa, S., et al. Transvaginal color Doppler imaging in the detection of ovarian cancer in a large study population. Int J Gynecol Cancer. 2010; 20(5):781–786.
36. Alcazar, J. L., Guerriero, S., Laparte, C., et al. Contribution of power Doppler blood flow mapping to gray-scale ultrasound for predicting malignancy of adnexal masses in symptomatic and asymptomatic women. Eur J Obstet Gynecol Reprod Biol. 2011; 155(1):99–105.
37. Testa, A. C., Timmerman, D., Van Holsbeke, C., et al. Ovarian cancer arising in endometrioid cysts: ultrasound findings. Ultrasound Obstet Gynecol. 2011; 38(1):99–106.
38. Dodge, J. E., Covens, A. L., Lacchetti, C., et al. The Gynecology Cancer Disease Site Group. Preoperative identification of a suspicious adnexal mass: A systematic review and meta-analysis. Gynecol Oncol. 2012; 126(1):157–166.
39. Tsafrir, Z., Azem, F., Hasson, J., et al. Risk factors, symptoms, and treatment of ovarian torsion in children: the twelve-year experience of one center. J Minim Invasive Gynecol. 2012; 19(1):29–33.
40. Mashiach, R., Melamed, N., Gilad, N., et al. Sonographic diagnosis of ovarian torsion: accuracy and predictive factors. J Ultrasound Med. 2011; 30(9):1205–1210.
41. Peled, Y., Ben-Haroush, A., Eitan, R., et al. The accuracy of the preoperative diagnosis in women undergoing emergent gynecological laparoscopy for acute abdominal pain. Arch Gynecol Obstet. 2011; 284(6):1439–1442.
42. Valsky, D. V., Esh-Broder, E., Cohen, S. M., et al. Added value of the gray-scale whirlpool sign in the diagnosis of adnexal torsion. Ultrasound Obstet Gynecol. 2010; 36(5):630–634.
43. Nizar, K., Deutsch, M., Filmer, S., et al. Doppler studies of the ovarian venous blood flow in the diagnosis of adnexal torsion. J Clin Ultrasound. 2009; 37(8):436–439.
44. Van Holsbeke, C., Van Calster, B., Guerriero, S., et al. Endometriomas: their ultrasound characteristics. Ultrasound Obstet Gynecol. 2010; 35(6):730–740.
45. Alcazar, J. L., Garcia-Manero, M. Ovarian endometrioma vascularization in women with pelvic pain. Fertil Steril. 2007; 87(6):1271–1276.
46. Tropeano, G., Di Stasi, C., Amoroso, S., et al. Ovarian vein incompetence: a potential cause of chronic pelvic pain in women. Eur J Obstet Gynecol Reprod Biol. 2008; 139(2):215–221.
47. Tinelli, A., Prudenzano, R., Torsello, M., et al. Suprapubic percutaneous sclero-embolization of symptomatic female pelvic varicocele under local anesthesia. Eur Rev Med Pharmacol Sci. 2012; 16(1):111–117.
48. Isono, W., Tsutsumi, R., Wada-Hiraike, O., et al. Uterine artery pseudoaneurysm after Caesarean section: case report and literature review. J Minim Invasive Gynecol. 2010; 17(6):687–691.
49. Tam, K. F., Lam, K. W., Chan, K. K., et al. Natural history of pelvic lymphocysts as observed by ultrasonography after bilateral pelvic lymphadenectomy. Ultrasound Obstet Gynecol. 2008; 32(1):87–90.
50. Brown, H. L., Hiett, A. K. Deep vein thrombosis and pulmonary embolism in pregnancy: diagnosis, complications, and management. Clin Obstet Gynecol. 2010; 53(2):345–359.
51. Lojindarat, S., Suwikrom, S., Puangsa-art, S. Postoperative color Doppler sonography of the ureteral jets to detect ureteral patency in laparoscopic hysterectomy. J Med Assoc Thai. 2011; 94(10):1169–1174.
52. Lo, T. S., Long, C. Y., Lin, Y. H., et al. Doppler ureteric jet in urogenital prolapse. Int Urogynecol J. 2012; 23(1):49–56.