Doppler Imaging of the Scrotum
Testicular Anatomy
The normal adult testis is an egg-shaped gland which is approximately 3–5 cm in length and 2–4 cm in width and thickness with a volume of 4 cm3. Testicular size varies with age and stage of sexual development. The surface of the testicle is covered by the tunica albuginea, a thin, dense, inelastic fibrous capsule. Just within the tunica albuginea is the tunica vasculosa, through which the branches of the testicular artery course before entering into the gland (Fig. 13-1). Numerous thin septations (septula) arise from the tunica albuginea and create 250–400 cone-shaped lobules containing the seminiferous tubules (Fig. 13-2). These tortuous tubules course towards the mediastinum of the testis (Fig. 13-3) and progressively merge to form larger ducts known as tubuli recti. These, in turn, join with each other to form a network of epithelium-lined spaces embedded in the fibrostroma of the mediastinum called the rete testis. These continue as 10–15 efferent ductules which pass into the head of the epididymis.
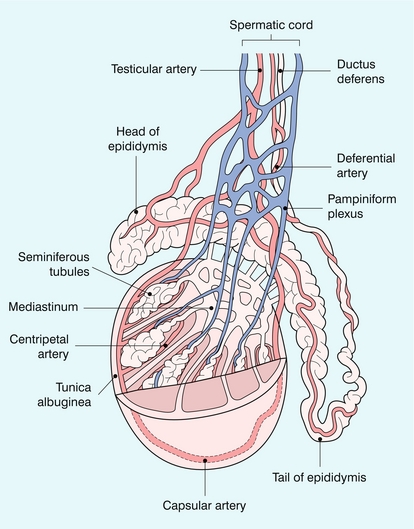
FIGURE 13-1 The testicular artery supplies flow to the epididymis and the testicle. As the testicular artery courses through the spermatic cord, it is surrounded by the pampiniform plexus of veins. The capsular artery, a branch of the testicular artery, courses just beneath the tunica albuginea. The centripetal arteries are branches of the capsular artery and course between the septa supplying the testicular parenchyma.
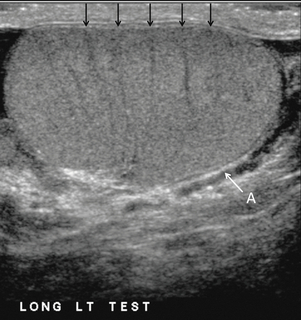
FIGURE 13-2 Longitudinal view of a normal testicle. The testicle is of relatively uniform echogenicity. On this high-resolution image, the testicular septa can be perceived as fine lines dividing the parenchyma into the individual lobules (black arrows). Note the echogenic tunica albuginea surrounding the testicle (A).
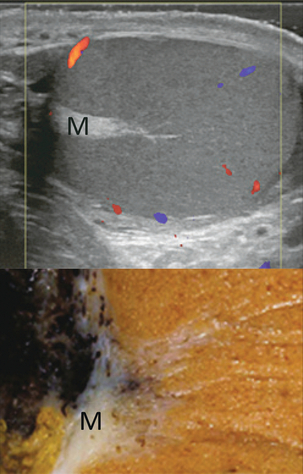
FIGURE 13-3 Transverse view of the left testicle compared to a specimen in the region of the mediastinum testis. The mediastinum testis is an echogenic fibrous structure. It is the point of confluence of the efferent ductules and the septa. Note its triangular echogenic appearance on the ultrasound (M) and how well it correlates with the dense white fibrous stroma on the specimen.
The epididymis is a comma-shaped structure that runs along the posterolateral aspect of the testis (Fig. 13-4). The head of the epididymis is located next to the upper pole of the testis and receives the efferent ductules. The ductules eventually converge through the body and tail and form the vas deferens which continues on in the spermatic cord. Along with the vas, the cord also contains the testicular artery, cremasteric artery, differential artery, the pampiniform plexus of veins, the genitofemoral nerve and lymphatic channels.
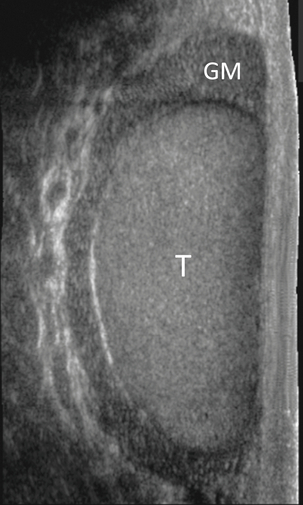
FIGURE 13-4 Longitudinal view of a normal right scrotum. The slightly hypoechoic epididymis wraps as a comma shape around the testis (T). The larger more prominent area is known as the globus major or the head of the epididymis (GM).
TESTICULAR ARTERIAL ANATOMY
The right and left testicular arteries originate from the aorta just below the renal arteries. They course through the deep inguinal ring to enter the spermatic cord, accompanied by the cremasteric and deferential arteries, which supply the soft tissues of the scrotum, epididymis and vas deferens. The testicular artery penetrates the tunica albuginea along the posterior aspect of the testis and gives off capsular branches which course through the tunica vasculosa. These capsular branches then give rise to the centripetal arteries which carry blood from the capsular surface, centrally towards the mediastinum along the septula (Figs 13-5 and 13-6). Branches of the centripetal arteries then course backward towards the capsular surface; these are known as recurrent rami. In about 50% of testes, a more robust artery can be seen passing directly from the testicular artery at the mediastinum into the parenchyma, known as the transtesticular artery (Fig. 13-7). It is occasionally accompanied by a vein. Small anastomoses do exist between the testicular artery, cremasteric, and differential arteries. Branches of the pudendal artery may also supply the scrotal wall.
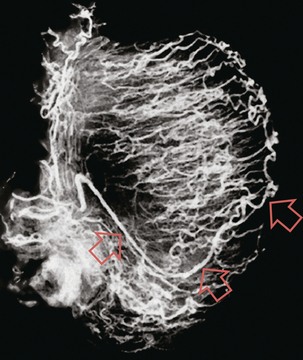
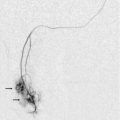
FIGURE 13-5 Microangiogram of the testicle. A prominent capsular branch (open arrows) courses from the testicular artery under the capsular surface. Centripetal arterial branches emanate from it and pass into the lobules of the testicle. Image courtesy of Dr. Thomas Winter.
TESTICULAR VENOUS ANATOMY
Testicular venous outflow courses mostly through the mediastinum testis, into the spermatic cord, and eventually up through the inguinal canal. As the veins exit the scrotum through the spermatic cord, they form a web-like network that surrounds the testicular artery, known as the pampiniform plexus (Fig. 13-8). This plexus functions as a heat exchange mechanism, pulling warmth away from the testicular arterial inflow, thereby helping to maintain spermatogenesis at a lower, more optimal temperature.
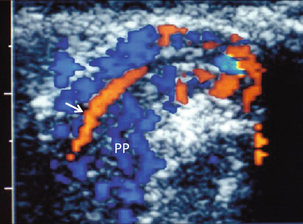
FIGURE 13-8 The pampiniform plexus of veins (PP) is a web-like collection of vessels surround the testicular artery (arrow) as it passes through the spermatic cord.
On the left side, the testicular vein usually drains into the left renal vein; on the right side, drainage is directly into the inferior vena cava just below the right renal vein. The testicular veins normally have valves that prevent retrograde flow of venous blood to the scrotum, but if they are absent or become incompetent; this predisposes to development of a varicocele. This results in compromise of the heat exchange mechanism and, therefore, is a frequent cause of infertility.1
Technique
DOPPLER TECHNIQUE
Ultrasound examination of the scrotum is not complete without the application of colour Doppler. The procedure is a mandatory part of the imaging evaluation to confirm the presence (or absence) of uniform, symmetric vascular perfusion of the testes and epididymides (Fig. 13-9). The settings for colour Doppler scanning must be optimised for low-volume and low-velocity flow. If colour noise is excessive at low-flow settings and interferes with the examination, colour artefact can be decreased by properly adjusting gain and pulse repetition frequency settings. Temporal resolution can be improved by minimising the overall image size and zooming in to the area of interest. Additionally decreasing depth, limiting the number of focal zones and limiting the size of the colour box will also improve temporal resolution. Use of appropriate technical parameters should assure demonstration of intratesticular vascularity in all normal adult patients. In the prepubertal age group, intratesticular flow volume is less and therefore more difficult to identify. The application of power Doppler may be helpful. Spectral Doppler can assess arterial and venous waveforms and quantify velocities (Fig. 13-10), but its application in the scrotum is relatively limited, except for a few conditions such as partial torsion or extreme swelling secondary to inflammation predisposing to ischaemia. In those cases spectral Doppler can best identify the high resistance to arterial inflow caused by venous outflow compromise or parenchymal congestion.
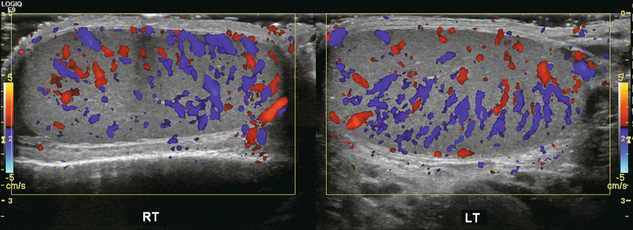
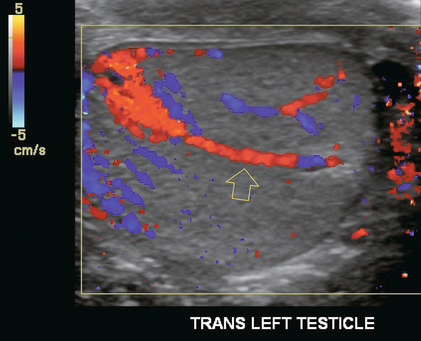
FIGURE 13-9 Transverse colour Doppler ultrasound image of both testicles using a split screen format. Note the symmetry of echotexture and the uniformity and symmetry of colour flow.
NORMAL ULTRASOUND AND DOPPLER FINDINGS
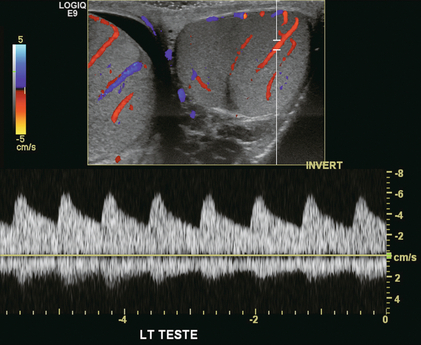
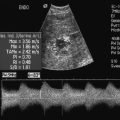
Colour Doppler should reveal bilaterally symmetric and relatively uniform flow through both testes and epididymides (Fig. 13-9). A fan-like array of the centripetal arteries should be present through the testicles (Fig. 13-6). Spectral Doppler tracings of testicular arterial inflow demonstrate relatively low resistance (Fig. 13-10); this is in contrast to the cremasteric and deferential arteries which have relatively high resistance to flow. The normal testicular artery resistive indices in adults range from 0.46 to 0.78, with a mean of 0.64. Similar findings are reported in the intratesticular arteries of postpubescent boys, with resistive indices ranging from 0.48 to 0.75 (mean, 0.62). In prepubertal boys, however, resistance is higher to the point that diastolic arterial flow may not be detectable. Supratesticular arteries to the vas deferens or cremaster muscle, on the other hand, have higher impedance with low-diastolic flow and resistive indices ranging from 0.63 and 1.0, with a mean of 0.84.
Pulsed Doppler is relatively insensitive in detecting arterial flow in prepubescent patients. In contrast, power Doppler has been shown to reveal arterial flow in 92% of testes in pre-pubescent patients, while colour Doppler demonstrates flow in 83% of cases. In postpubescent patients, both Doppler imaging techniques demonstrated flow in 100% of cases.2,3
Acute Scrotal Pain
INFLAMMATORY DISEASE
The cause of the infection varies with age.4 In adult patients less than 35 years of age, Chlamydia trachomatis and Neisseria gonorrhoeae (sexually transmitted organisms) are the most common agents. In prepubertal boys and in men over 35 years of age, the disease is most frequently caused by Escherichia coli and Proteus mirabilis. Cytomegalovirus is the most common agent in the immunocompromised patient. In most normal paediatric patients, a bacterial pathogen is not isolated and the inflammation is presumed to be viral in nature. Those patients who have an underlying urogenital congenital anomaly are prone to infection from Gram-negative bacteria.5
The sonographic appearance of epididymo-orchitis varies depending on the stage of the process. The sensitivity of greyscale sonography for detecting epididymo-orchitis is reported to be about 80%. In the early, acute stage, the epididymis and/or testicle will be enlarged and hypoechoic. With the onset of tissue breakdown and haemorrhage, the echogenicity begins to increase. There may be reactive thickening of the scrotal wall. A hydrocele may be present and it may contain debris. If allowed to progress, microabscesses may develop and the appearance becomes more complex and variable. Further progression can lead to frank intra- or extratesticular abscess, ischaemia and eventually necrosis. Scarring associated with chronic orchitis typically results in a small hyperechoic testis.6 The diagnosis of infection and inflammation typically hinges on the identification of hyperaemia by colour Doppler – an asymmetric appearance with more robust flow (an increased number and prominence of discernible vessels) in association with an enlarged, painful, hypoechoic epididymis and/or testis (Fig. 13-11). Several studies have demonstrated sensitivity and specificity for the diagnosis of scrotal inflammatory disease by colour Doppler sonography approximating 100%. Early in the inflammatory process, vasodilatation and hyperemia results in a low-resistance flow pattern on spectral Doppler (Fig. 13-12).7
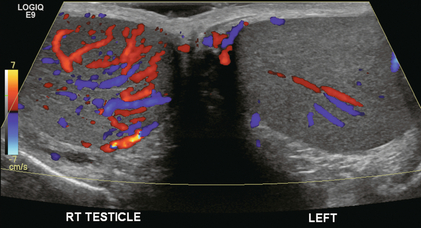
FIGURE 13-11 Transverse colour Doppler image of the scrotum including both testicles. This paediatric patient presented with right scrotal pain. Colour Doppler reveals marked hyperaemia of the right testicle and obvious asymmetry in perfusion. The diagnosis is right orchitis.
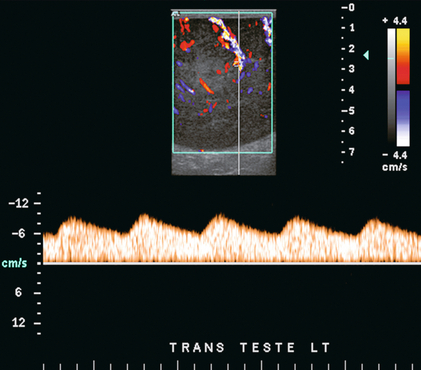
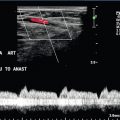
FIGURE 13-12 Spectral Doppler tracing obtained in an area of focal orchitis. Flow during diastole is relatively brisk, resulting in a resistive index of less than 50%. This is seen relatively early in the inflammatory process before the accumulation of significant oedema and/or pus.
In cases of severe epididymitis, periepididymal swelling may obstruct testicular venous outflow, leading to testicular ischaemia or infarction. An enlarged, heterogeneous testicle with reduced or absent colour flow and a contiguous abnormal epididymis may be seen on greyscale imaging (Fig. 13-13). Hyperaemia of the epididymis helps to differentiate testicular ischaemia following inflammation from that caused by torsion. A high-resistance waveform, along with decreased or reversed diastolic flow, may be seen on the spectral Doppler tracing and suggests venous infarction8 (Fig. 13-14).
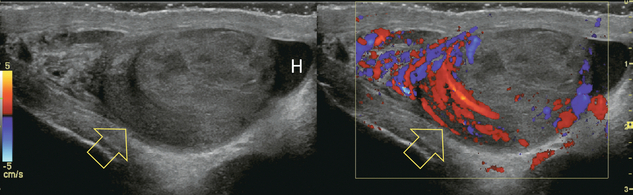
FIGURE 13-13 Longitudinal greyscale and colour Doppler image of the right scrotum. The patient presented with severe pain. The epididymis is oedematous, swollen and markedly hyperaemic (open arrows). Note almost complete absence of flow within the testicle. This is indicative of venous outflow compromise. Note the reactive hydrocele (H) and scrotal wall thickening.
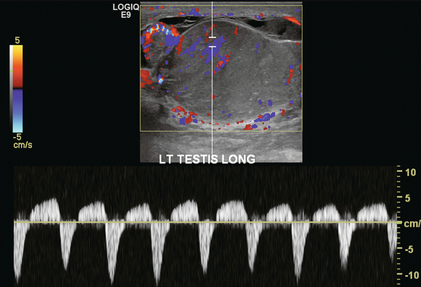
FIGURE 13-14 Colour and spectral Doppler of a severely inflamed left epididymis and testicle. The testicle is markedly enlarged and swollen. The echotexture is irregular. Flow during diastole is reversed resulting in a resistive index of > 100%. These findings indicate a severe inflammatory process with the venous outflow occlusion.
If left untreated, the process may progress to abscess formation (Fig. 13-15), which usually manifests as an enlarged testicle with a complex, septated collection of fluid/debris and tissue of mixed echogenicity. It may be difficult to distinguish from a testicular neoplasm, as both can present as complex cystic/solid masses. Older abscesses may have radiating echogenic septa separating hypoechoic spaces. Increased blood flow around the abscess cavity and no internal flow is present on colour Doppler. Surgical exploration may be necessary to rule out the presence of a tumour and débride the abscess.
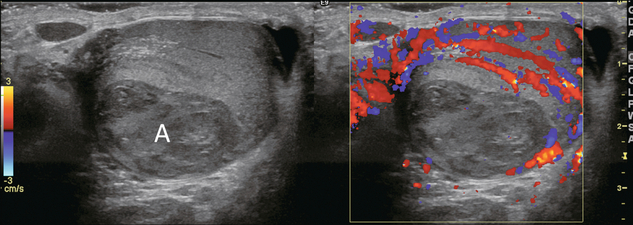
FIGURE 13-15 Greyscale and colour Doppler image of severe epididymoorchitis. The epididymis and outside rim of testicle are markedly hyperaemic. No flow is seen in the central part of the testicle. Note the areas of tissue breakdown and liquification consistent with intratesticular abscess (A).
Occasionally, testicular inflammation may be focal, even rounded, with areas of decreased echogenicity and swelling. Hyperaemia concentrated in the abnormal, tender area may suggest inflammation over neoplasm, but there is overlap in this appearance with neoplasm, especially lymphoma (Fig. 13-16).
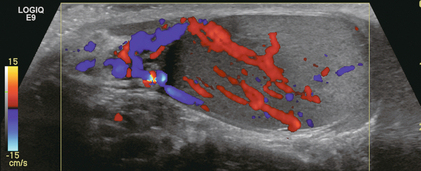
FIGURE 13-16 Longitudinal colour Doppler image of a testicle. This patient complained of severe scrotal tenderness. Note the asymmetric appearance of this testicle. The upper pole is hypoechoic and hypervascular. The lower pole has normal echogenicity and significantly less flow. The diagnosis was focal orchitis with associated epididymitis.
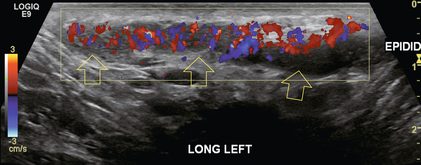
An inflammatory process may be isolated to the spermatic cord. This is most often associated with a recent viral illness. The patient typically presents with a nagging ache that is difficult to localise within the scrotum. Imaging reveals an enlarged and oedematous and hyperaemic spermatic cord. (Fig. 13-17).
TORSION
Torsion is divided into two types – extravaginal which occurs exclusively in newborns and intravaginal. Extravaginal torsion occurs outside the tunica vaginalis when the testes are not yet fixed and are free to rotate.9 The testis is typically necrotic at birth. Ultrasound reveals an enlarged heterogeneous testis, a reactive hydrocele, skin thickening and no colour Doppler flow in the testis or spermatic cord. Intravaginal torsion occurs most frequent in adolescent boys. A bell-clapper deformity, in which the tunica vaginalis completely encircles the epididymis, distal spermatic cord, and testis, is the key predisposing factor. This deformity is usually bilateral and leaves the testis free to swing and rotate within the tunica vaginalis (Fig. 13-18).
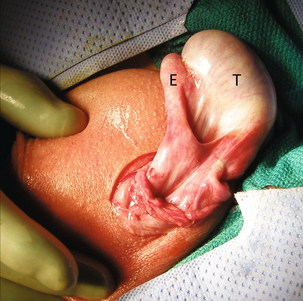
FIGURE 13-18 Intraoperative image during testicular torsion repair. The testicle (T) and epididymis (E) have been delivered through a small incision. Note the great degree of mobility because of the Bell Clapper deformity.
The diagnosis of spermatic cord torsion must be established quickly to allow for prompt surgical intervention, since obstruction of blood flow may result in the loss of testicular viability within a few hours (typically four) of onset of symptoms. Clinical history and physical findings, however, overlap with those of inflammatory disease to such a degree that even an experienced urologist may have difficulty in differentiating the two conditions. Symptoms and signs include sudden pain in the scrotum, lower abdomen or inguinal area (frequently accompanied by nausea, vomiting and low-grade fever), a tender testicle with a transverse orientation, and a swollen, erythematous hemiscrotum. The false-positive rate of nearly 50% for clinical diagnosis of testicular torsion often results in unnecessary surgical exploration.10
The application of colour Doppler to the diagnosis of torsion increases sensitivity in adults to 90–100% with a high specificity. Unlike greyscale imaging, colour and spectral Doppler are almost always abnormal even during the early stages of torsion. Instrument settings should be optimised on the normal side to identify low-velocity flow before ascertaining that there is indeed decreased blood flow on the symptomatic side. If arterial flow cannot be detected in the symptomatic testicle but can in the contralateral testicle, the diagnosis of torsion is effectively established. The characteristic finding of ischaemia is a completely avascular testicle (Fig. 13-19). In the late stages of torsion, colour Doppler may reveal an increase in peritesticular blood flow because of inflammation in the surrounding soft tissues of the scrotum.
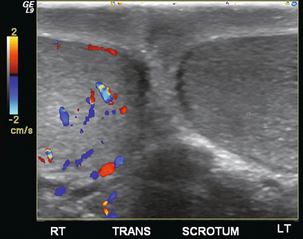
FIGURE 13-19 A five-year-old boy presented with left scrotal pain. This colour Doppler image, optimised for low-flow sensitivity, clearly shows asymmetric perfusion. Some flow is detected on the right whereas there is absolutely no flow seen on the left. The diagnosis was left testicular torsion and the patient was immediately transferred to the operating room.
With full torsion (greater than 360ᵒ) the addition of spectral Doppler does little other than to confirm the obvious fact that there is no flow. It should identify very high resistance in the spermatic artery within the cord above the level of the twist. With partial torsion (between 180ᵒ and 360ᵒ) however, the spermatic cord twists only enough to occlude the venous outflow. Because the artery has a thicker wall, patency is maintained. The resultant spectral Doppler tracing reveals a high-resistance arterial waveform within the cord and the testicle (Fig. 13-20).11,12
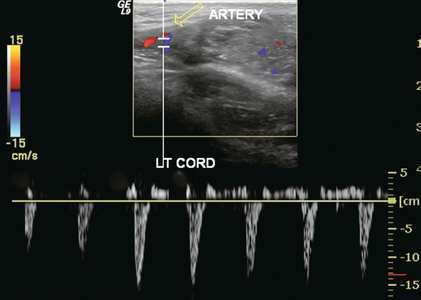
FIGURE 13-20 Longitudinal view of left spermatic cord near the testicle. This young man complained of intermittent left scrotal pain. The colour Doppler image shows only a few small foci of blood flow within the testicle. A spectral Doppler tracing of the epididymal artery (open arrow) shows reversed flow in diastole. These findings support the diagnosis of partial torsion with occluded venous outflow.
Direct evaluation of the spermatic cord on its long axis may reveal a snail-shell-shaped mass at the point of the twist (Fig. 13-21). Running the transducer along the affected cord in the transverse projection may show a vortex-like spin of the cord ending at the point of obstruction.
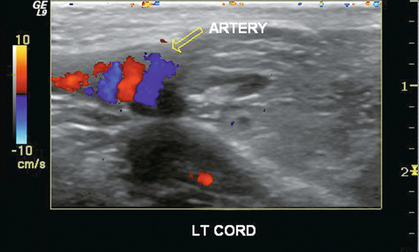
FIGURE 13-21 A longitudinal view of the left scrotum. In the region of the junction of the cord with the epididymis, colour Doppler reveals a “snail shell” appearance of the vasculature (open arrow), the result of a twisting torsion.
The use of echo-enhancing agents has been studied for improving imaging of small testicles with low-velocity and low-volume flow. In an animal study by Brown et al.13 the authors examined induced testicular torsion with greyscale imaging, colour Doppler, power Doppler and spectral Doppler analysis. Injection of contrast media did not enhance greyscale images, but visualisation of all vessels in both normal and rotated testicles was significantly improved with both colour and power Doppler. Asymmetry of blood flow was more obvious. The authors concluded that diagnosis of testicular ischaemia could be made with greater confidence using an intravenous ultrasound contrast agent because of improved demonstration of altered perfusion patterns.
Although testicular torsion may be accurately diagnosed by sonography, this does not guarantee successful surgical salvage. Success depends on a timely diagnosis and the duration of ischaemia. Indeed, the more obvious the ultrasound diagnosis, the less likely the chance of salvage. The testicle devoid of colour flow but with an appropriate ultrasound appearance is much more likely to be salvaged at surgery. The testicle that presents with irregular echotexture is likely necrotic and needs to be removed (Fig. 13-22).14,15
TORSION OF THE APPENDIX TESTIS
The appendix testis is a Müllerian duct remnant that is attached to the upper pole of the testis. Doppler ultrasound is rarely able to identify flow within the structure. Newer, more sensitive equipment can occasionally reveal the presence of flow within a tender appendix testis (Fig. 13-23). Patients who develop torsion of the appendix testis usually present with acute scrotal pain. Physical examination reveals a small firm, palpable, tender nodule. Ultrasound evaluation usually reveals a hyperechoic rounded structure attached to the testicle with a reactive hydrocele. The patient will usually confirm an increase in pain when the transducer is over the appendix testis, and that typically clinches the diagnosis. Colour Doppler may reveal increased flow around the twisted testicular appendage but more importantly it rules out testicular torsion or an acute inflammatory process. After torsion and necrosis the appendix testis may slough and become calcified, in which case it becomes known as a scrotal pearl. Twinkle artifact may be present on colour Doppler and should not be confused for true flow (Fig. 13-24).
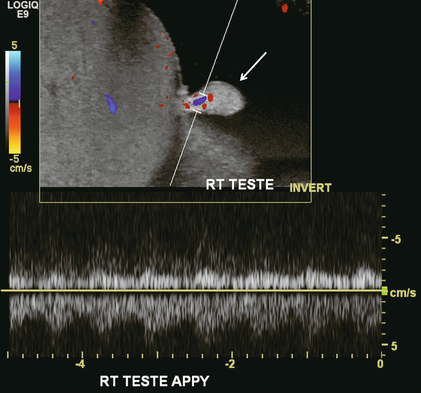
FIGURE 13-23 A longitudinal view of the left testes at the area of maximum tenderness in this patient. The appendix testis (arrow) was conspicuous as the focus of pain. Note the hyperaemia at the base of this structure. It is quite rare to actually identify colour Doppler flow in the appendix testis. Presumably this was torsion/detorsion of this appendix which resulted in focal hyperaemia.
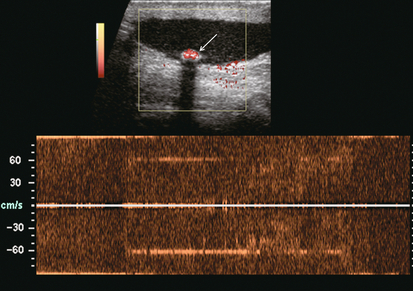
FIGURE 13-24 Power and spectral Doppler image at the base of the right scrotum, distant from the testicle itself. An echogenic structure with acoustic shadowing is layering dependently (arrow). Power Doppler reveals colour emanating from this structure indicating frequency shift, however, the spectral Doppler waveform is completely disorganised. This is consistent with twinkle artifact produced when the Doppler beam interacts with crystalline material, in this case a calcified left scrotal pearl.
TRAUMA
Testicular trauma typically results from an athletic injury, a direct blow or a straddle injury. Clinical examination of a traumatised, tender testicle can be difficult because of pain and swelling; however, ultrasound can visualise and confirm testicular integrity, or identify the presence of haematoma, haematocele, or rupture. Colour Doppler sonography provides excellent delineation of blood flow throughout the testis, differentiating hyperaemic, contused regions from devascularised or ischaemic areas (Fig. 13-25).
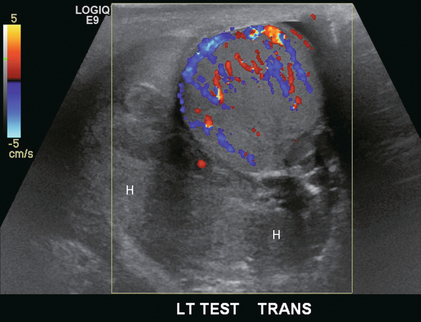
FIGURE 13-25 Transverse view of the left testicle in a patient with acute trauma. Scrotal swelling and tenderness prevented adequate physical examination. Imaging reveals a large echogenic collection within the tunica vaginalis consistent with an acute haematoma (H). The testicle is hyperaemic, however, integrity is maintained and flow is present throughout.
In the case of testicular rupture, sonographic identification is extremely important because prompt diagnosis and quick surgical intervention are required to successfully correct the condition. If surgery is performed within 72 h of the trauma, approximately 80% of ruptured testes can be salvaged. If rupture is present, ultrasound may demonstrate a disrupted tunica albuginea; a heterogeneous testicle with asymmetric, poorly defined margins; thickening of the wall of the scrotum; and/or a large haematocele. Perception of blood flow will be diminished or absent on colour or spectral Doppler examination (Fig. 13-26).
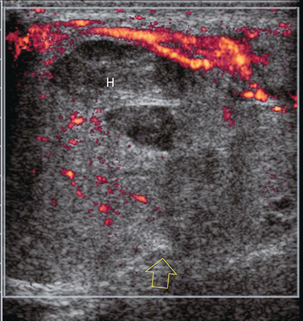
FIGURE 13-26 Longitudinal power Doppler image of a traumatised testicle. There is hyperaemia in the epididymis. Some flow is present in the upper portion of the testicle, but none in the inferior pole. There is a fracture through the mid-testicle with a large haematoma (H). A break of the tunica albuginea is seen in the mid-testicle with disruption of the normal rounded contour (open arrow). This testicular rupture was repaired surgically.
Unlike rupture, intratesticular fracture, small haematomas and haematoceles do not require surgery if the tunica albuginea has not been interrupted and Doppler imaging shows normal blood flow to the testicle. Sonographic findings associated with a testicular fracture include a linear hypoechoic band crossing the parenchyma of the testicle, a smooth well-defined testicular outline, an intact tunica albuginea, and often an associated haematocele. Normal Doppler signals indicate unimpaired blood flow and viable testicular tissue. If Doppler signals are absent, ischaemia is very likely and surgical intervention is called for. Acute haematomas are usually hyperechoic relative to adjacent testicular parenchyma. Older haematomas may have both hyper- and hypoechoic areas, and there may be associated thickening of the scrotal wall. On colour Doppler, the septa of haematomas are avascular. Acute haematoceles are relatively uniform and echogenic. As they mature, proteinaceous septations develop and again are nonvascular on colour Doppler.16,17
Scrotal Mass
TESTICULAR NEOPLASM
Patients with testicular neoplasms usually present with a palpable scrotal mass or a dull aching sensation. For palpable scrotal masses, ultrasound is widely considered the imaging modality of choice. The principal role of ultrasound is to distinguish intra- from extratesticular lesions, because the majority of extratesticular masses are benign, whereas intratesticular masses are considered malignant until proven otherwise.18 Ultrasound can easily differentiate solid from cystic masses and confirm their intra- or extratesticular location. The main role of ultrasound is to identify those masses which require additional assessment and possible surgical intervention.
Seminomas are the most common single-cell-type testicular tumour in adult males (40–50%). The ‘classic’ ultrasound appearance of seminomas has been described as a well-defined, uniformly hypoechoic lesion, with no evidence of calcification, haemorrhage, or cystic areas.19 But the larger the lesion, the less likely it maintains the classic appearance (Fig. 13-27).
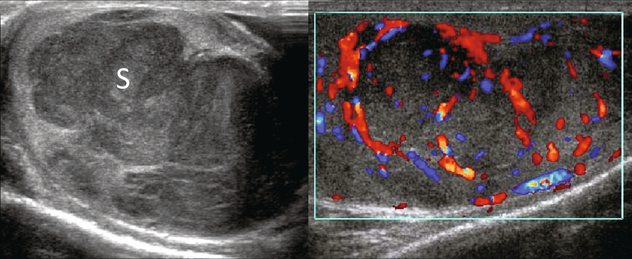
FIGURE 13-27 Ultrasound imaging and colour Doppler reveal a large predominantly hypoechoic multi-lobulated mass occupying the majority of this testicle (S). On colour Doppler, note how the vasculature is displaced around the expansile mass. A seminoma was surgically removed by orchiectomy.
Teratomas generally are very heterogeneous with well-defined margins. Dense echogenic foci caused by calcification, cartilage, immature bone, fibrosis, and non-calcified fibrous tissue are interspersed with areas of haemorrhage, cysts and necrosis.20 Choriocarcinomas and embryonal cell tumours generally appear as small masses with haemorrhage and associated calcification.
The ultrasound appearance of mixed germ cell tumours is that of a heterogeneous, small mass with poorly defined margins, anechoic areas due to cystic necrosis, and echogenic foci of haemorrhage. If the tumour invades the tunica, the normal contour of the testicle may be distorted.8 Testicular neoplasms have mixed histological components in 40–60% of cases, with the most common combination being that of teratoma and embryonal carcinoma (teratocarcinoma). Ultrasound findings of mixed tumours will vary, depending on which cell lines are dominant and if there are no particular ultrasound findings that permit differentiation for possible preoperative planning.
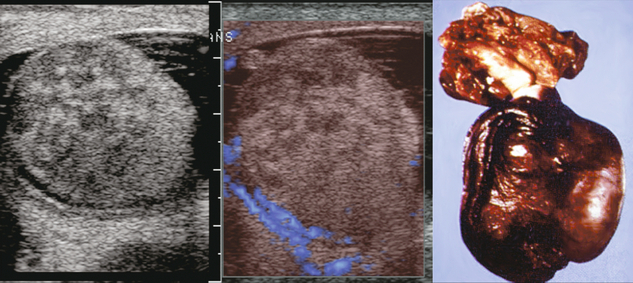
Testicular carcinoid is extremely rare and usually presents as painless testicular enlargement or as a discrete mass. The tumour may be primary, associated with teratoma or metastatic. Primary testicular carcinoid is believed to arise from pluripotential germ cells or from the development of a simplified teratoma without other teratomatous elements. Very few (< 3%) have associated carcinoid syndrome.21 On ultrasound testicular carcinoid appears as a solid well-defined hypoechoic intratesticular mass with or without dense calcification. On Doppler it has increased vascularity similar to seminoma22 (Fig. 13-28).
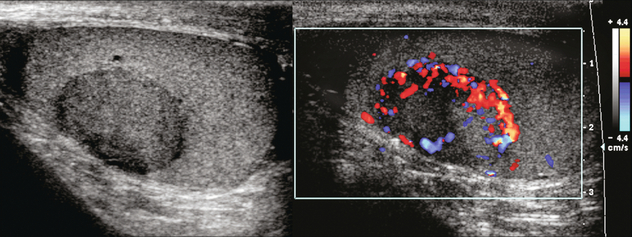
FIGURE 13-28 (A) Right scrotal sonography shows a well-defined solid mass without calcification in the right testis. (B) Colour Doppler shows the hypervascularity of this right testicular mass; a proven carcinoid tumour of the testicle. Case courtesy of Kyoung-Sik Cho, MD, Asan Medical Center, University of Ulsan, College of Medicine.
Colour Doppler and spectral Doppler sonography are considered to be of minimal benefit in the evaluation and characterisation of adult testicular masses and the diagnosis of testicular neoplasm. This is because vascularity of these lesions is extremely variable Small lesions tend to be hypovascular while larger lesions tend toward hypervascular compared with normal testicular parenchyma (Fig. 13-29).
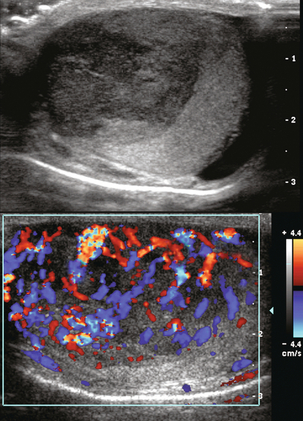
FIGURE 13-29 Greyscale and colour Doppler longitudinal images of the left testis being evaluated for a palpable mass. A large hypoechoic mass occupies the majority of the testicle. Colour Doppler shows vigorous flow throughout this lesion. Note however that the normal fan-shaped distribution has been replaced by neovascularity. This was a surgically proven embryonal carcinoma of the testis.
An infiltrative neoplasm of the testicle, such as leukaemia or lymphoma, typically presents as an enlarged hypoechoic area on greyscale imaging. Colour Doppler often demonstrates hyperaemia in the neoplasm. Since these are infiltrative tumours, the underlying vascular architecture is often preserved (Fig. 13-30), with an appearance quite similar to inflammation. The absence of pain, however, should make one lean towards infiltrative tumour and away from orchitis.
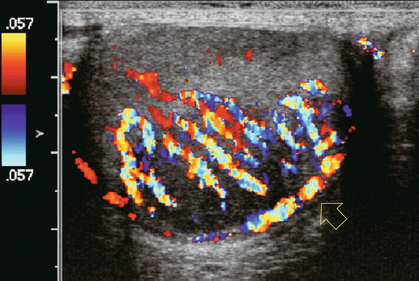
FIGURE 13-30 Longitudinal colour Doppler image of a 59-year-old male with a palpable scrotal mass. Overlying a hypoechoic mass colour Doppler reveals hyperaemia of the capsular artery (open arrow) and centripetal arteries without much anatomical distortion. Colour imaging alone would have suggested an inflammatory process, but the patient is absolutely asymptomatic and therefore lymphoma was entertained and proven at surgery.
BENIGN TESTICULAR LESIONS
Benign intratesticular masses are rare, but recognition is important to avoid unnecessary biopsy, or worse orchiectomy. Almost all intratesticular cystic lesions are benign. The list includes cysts of the tunica albuginea, simple intratesticular cysts, epidermoid cyst (Fig. 13-31), tubular ectasia of the rete testis (Fig. 13-32), and intratesticular spermatocele. Colour Doppler ultrasound helps in confirming the benign nature of these since no alterations in blood flow are demonstrated.23
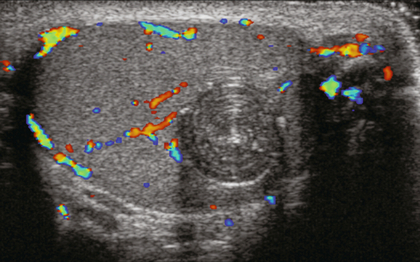
FIGURE 13-31 Colour Doppler image of the testicle reveals a rounded lesion. Note the circumferential rings mimicking an onion. This is a unique feature of epidermoid cysts. Note the absence of any flow within the lesion or at its periphery. Image courtesy of Dr. Jason Wagner.
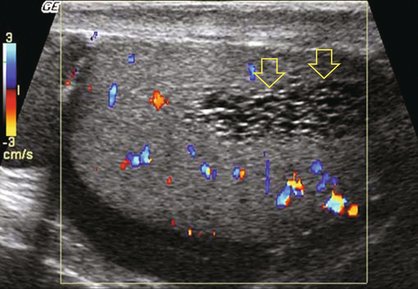
FIGURE 13-32 Colour Doppler image focused on the region of the mediastinum testis. A collection of small tubules is present (open arrows). The absence of colour flow confirms that they are dilated rete testis – prominent efferent ductules commonly associated with a spermatocele.
Bilateral, eccentrically located, intratesticular adrenal rest tumours may be seen in patients with congenital adrenal hyperplasia and primary adrenal insufficiency. In most cases, they are hypoechoic oblong lesions peripherally located close to the mediastinum.24 They are bilateral in 83–100% of cases.25 They may undergo extensive fibrosis and eventually become hyperechoic with acoustic shadowing. Vascularity on colour Doppler is variable relative to the normal testicle. Vessels may be seen entering from the adjacent testis without change in course or calibre (Fig. 13-33). Some lesions may exhibit a spoke-like pattern of converging vessels. There are two theories for the origin of these lesions. One says they originate from hilar pluripotential cells, which proliferate as a result of the elevated level of adrenocorticotropic hormone. The other says they originate from aberrant adrenal cortical tissue that adheres to the testes and descends during prenatal life.26 Whatever the origin, these should be recognised as benign lesions and first treated with adrenal suppression with dexamethasone.
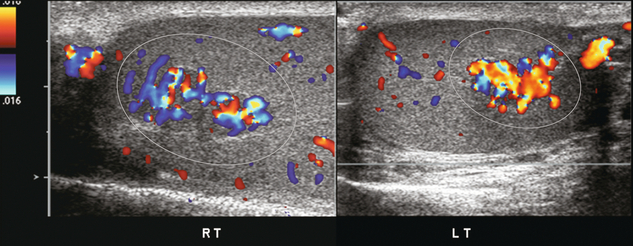
FIGURE 13-33 Colour Doppler images of both right and left testicles show several hyperaemic foci (encircled). Since the patient has a known history of congenital adrenal hyperplasia, these are presumed to represent benign rests of adrenal tissue. Biopsy should not be required. Imaging follow-up can be performed to prove stability.
VARICOCELE
Varicoceles are present in approximately 15% of men. Incompetent or absent valves in the internal testicular veins predispose to stasis or retrograde blood flow, resulting in dilatation of the pampiniform plexus. This is the cause of the majority of varicoceles. Varicoceles occur more commonly on the left; this is attributed to the longer course of the gonadal vein and its direct drainage into the left renal vein. Varicoceles are important clinically because of their association with infertility. Diagnosis of varicoceles is important, because treatment improves sperm quality in over half of cases.27
On ultrasound a varicocele is seen to consist of dilated (> 2 mm diameter), serpiginous channels in the head of the epididymis and spermatic cord. Colour Doppler has been shown to be very accurate in detecting varicocele.1 At rest and with normal respiration, colour will saturate the tubules at intervals related to respiratory pressure fluctuations. With more vigorous respiration, to-and-fro movement of blood may manifest as alternating colour in the same vessel, changing direction between inspiration and expiration. Colour Doppler identification of varicocele is enhanced by having the patient perform a Valsalva manoeuvre or by having the patient stand up. This increases the abdominal pressure and results in the reversal of blood flow into the pampiniform plexus, thereby causing further distension. When the Valsalva manoeuvre is performed, a brief burst of reversed flow is common (Fig. 13-34). This is due to the expulsion of the venous blood in the gonadal vein of the pelvis below the lowest competent valve. As soon as this volume of blood is expressed, then flow usually stops, waiting for the scrotal venous pressure to rise above that generated by the Valsalva. When the pressure is released as the patient relaxes, the direction of blood flow reverts to normal. With valvular incompetence however, when the Valsalva manoeuvre is performed, there is constant reversal of flow. The venous outflow from the left kidney finds the left gonadal vein as the path of least resistance. With the release of Valsalva flow reverts back to the normal direction (Fig. 13-35).
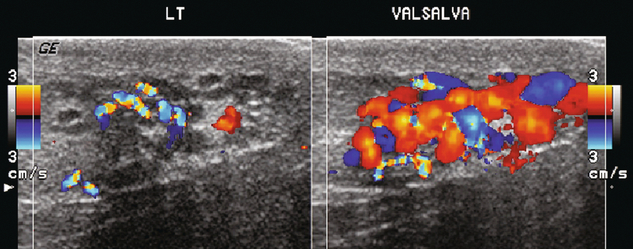
FIGURE 13-34 Longitudinal view of the left spermatic cord in a patient being evaluated for infertility. A serpiginous collection of vessels is present. With Valsalva manoeuvre, note the marked engorgement and increased flow.
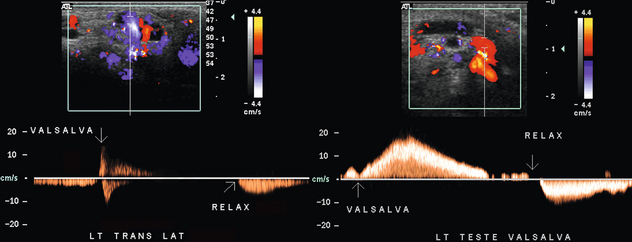
FIGURE 13-35 Spectral Doppler tracings of the left spermatic cord in two patients with a known varicocele. The tracings were obtained as a Valsalva manoeuvre was initiated, maintained, and released. The first patient has a brief reversal of flow at the start of Valsalva as blood below the distal-most valve is forced downward. Then because of valve competency and high central pressure, venous flow becomes stagnant. Upon relaxation of the Valsalva, flow surges forward again. When the second patient initiates Valsalva flow reversal persists for a prolonged time as renal venous outflow is shunted down the gonadal vein because of the high central pressure and incompetent valves of the gonadal vein. As pressure builds within the venous plexus of the scrotum, reverse flow progressively slows until the point of relaxation at which time flow surges forward.
Varicoceles can extend into the testicle. They manifest as dilated intratesticular veins (greater than 2 mm) with a positive response to the Valsalva manoeuvre. Incidence is estimated at less than 1%. Typically these are associated with testicular atrophy (Fig. 13-36).28
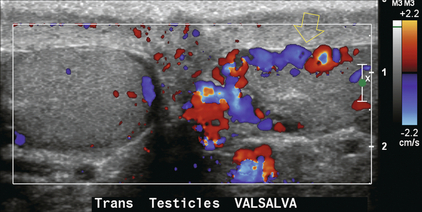
FIGURE 13-36 Transverse colour Doppler image at the level of the mid-testes. A varicocele is present within the left cord. Large dilated vessels are seen extending into the testicle (open arrow). Flow within the veins of the intratesticular varicocele has a similar response to the Valsalva manoeuvre.
After successful surgical varicocelelectomy colour Doppler ultrasound can confirm a decrease in the diameter of the pampiniform plexus of veins.29
Other Imaging Procedures
Computed tomography (CT) is primarily used for staging and follow-up of testicular tumour metastatic to the retroperitoneum or elsewhere. Magnetic resonance imaging (MRI), because of its high cost and limited availability, is reserved for problem solving of difficult cases. MRI has been shown to be diagnostic and cost- effective following equivocal scrotal ultrasound, however, even in an aggressive MRI environment only a small percentage of scrotal sonograms required the addition of MRI. Currently, other than in select paediatric cases and in the evaluation of cryptorchidism, MRI has not been found to hold significant advantage over ultrasound in the evaluation of the scrotum; but the modality continues to evolve.30
REFERENCES
1. Petros, J. A., Andriole, G. L., Middleton, W. D., et al. Correlation of testicular colour Doppler ultrasonography, physical examination and venography in the detection of left varicoceles in men with infertility. J Urol. 1991; 145(4):785–788.
2. Paltiel, H. J., Rupich, R. C., Babcock, D. S. Maturational changes in arterial impedance of the normal testis in boys: Doppler sonographic study. AJR Am J Roentgenol. 1994; 163(5):1189–1193.
3. Luker, G. D., Siegel, M. J. Scrotal US in pediatric patients: comparison of power and standard colour Doppler US. Radiology. 1996; 198(2):381–385.
4. Hermansen, M. C., Chusid, M. J., Sty, J. R. Bacterial epididymo-orchitis in children and adolescents. Clin Pediatr (Phila). 1980; 19(12):812–815.
5. Basekim, C. C., Kizilkaya, E., Pekkafali, Z., et al. Mumps epididymo-orchitis: sonography and colour Doppler sonographic findings. Abdom Imaging. 2000; 25(3):322–325.
6. Cook, J. L., Dewbury, K. The changes seen on high-resolution ultrasound in orchitis. Clin Radiol. 2000; 55(1):13–18.
7. Farriol, V. G., Comella, X. P., Agromayor, E. G., et al. Gray-scale and power Doppler sonographic appearances of acute inflammatory diseases of the scrotum. J Clin Ultrasound. 2000; 28(2):67–72.
8. Lee, J. C., Bhatt, S., Dogra, V. S. Imaging of the epididymis. Ultrasound Q. 2008; 24(1):3–16.
9. Backhouse, K. M. Embryology of testicular descent and maldescent. Urol Clin North Am. 1982; 9(3):315–325.
10. Dubinsky, T. J., Chen, P., Maklad, N. Colour-flow and power Doppler imaging of the testes. World J Urol. 1998; 16(1):35–40.
11. Dogra, V. S., Sessions, A., Mevorach, R. A., et al. Reversal of diastolic plateau in partial testicular torsion. J Clin Ultrasound. 2001; 29(2):105–108.
12. Lin, E. P., Bhatt, S., Rubens, D. J., et al. Testicular torsion: twists and turns. Semin Ultrasound CT MR. 2007; 28(4):317–328. [Epub 2007/09/19].
13. Brown, J. M., Taylor, K. J., Alderman, J. L., et al. Contrast-enhanced ultrasonographic visualization of gonadal torsion. J Ultrasound Med. 1997; 16(5):309–316.
14. Middleton, W. D., Middleton, M. A., Dierks, M., et al. Sonographic prediction of viability in testicular torsion: preliminary observations. J Ultrasound Med. 1997; 16(1):23–27.
15. Prando, D. Torsion of the spermatic cord: the main gray-scale and Doppler sonographic signs. Abdom Imaging. 2009; 34(5):648–661.
16. Deurdulian, C., Mittelstaedt, C. A., Chong, W. K., et al. US of acute scrotal trauma: optimal technique, imaging findings, and management. Radiographics. 2007; 27(2):357–369.
17. Bhatt, S., Dogra, V. S. Role of US in testicular and scrotal trauma. Radiographics. 2008; 28(6):1617–1629.
18. Ulbright, T., Roth, L. Testicular and paratesticular tumours. In: Sternberg S. S., ed. Diagnostic surgical pathology. 3rd ed. Philadelphia: Saunders; 1999:1973–2033.
19. Cotran, R., Kumar, P., Collins, T. The male genital tract. In: Robbins S. L., ed. Pathologic basis of disease. 6th ed. Philadelphia: Saunders; 1999:1011–1034.
20. Woodward, P. J., Sohaey, R., O’Donoghue, M. J., et al. Tumours and tumour-like lesions of the testis: radiologic-pathologic correlation. Radiographics. 2002; 22(1):189–216.
21. Zavala-Pompa, A., Ro, J. Y., el-Naggar, A., et al. Primary carcinoid tumour of testis. Immunohistochemical, ultrastructural, and DNA flow cytometric study of three cases with a review of the literature. Cancer. 1993; 72(5):1726–1732.
22. Kim, B. Scrotum. In: Kim S. H., ed. Radiology Illustrated: Uroradiology. Philadelphia: WB Saunders; 2003:625–664.
23. Dogra, V. S., Gottlieb, R. H., Rubens, D. J., et al. Benign intratesticular cystic lesions: US features. Radiographics. 21, 2001. [Spec No:S273–81].
24. Dogra, V. S., Gottlieb, R. H., Oka, M., et al. Sonography of the scrotum. Radiology. 2003; 227(1):18–36.
25. Proto, G., Di Donna, A., Grimaldi, F., et al. Bilateral testicular adrenal rest tissue in congenital adrenal hyperplasia: US and MR features. J Endocrinol Invest. 2001; 24(7):529–531.
26. Stikkelbroeck, N. M., Otten, B. J., Pasic, A., et al. High prevalence of testicular adrenal rest tumours, impaired spermatogenesis, and Leydig cell failure in adolescent and adult males with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2001; 86(12):5721–5728.
27. Pierik, F. H., Dohle, G. R., van Muiswinkel, J. M., et al. Is routine scrotal ultrasound advantageous in infertile men? J Urol. 1999; 162(5):1618–1620.
28. Tetreau, R., Julian, P., Lyonnet, D., et al. Intratesticular varicocele: an easy diagnosis but unclear physiopathologic characteristics. J Ultrasound Med. 2007; 26(12):1767–1773.
29. El-Haggar, S., Nassef, S., Gadalla, A., et al, Ultrasonographic parameters of the spermatic veins at the inguinal and scrotal levels in varicocele diagnosis and post-operative repair. 3, Andrologia 2012; 44:210–213.
30. Choyke, P. L. Dynamic contrast-enhanced MR imaging of the scrotum: reality check. Radiology. 2000; 217(1):14–15.