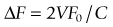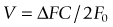Chapter 5 Doppler and Contrast Agents
Introduction
Color Doppler imaging (CDI) or color Doppler ultrasonography (CDUS) is a useful diagnostic tool in evaluating ocular and orbital diseases (Table 5.1). Based on its capacity to combine two-dimensional B-mode image and functional blood flow analysis, this non-invasive method is suitable to study vascular abnormalities involving the central retinal artery, central retinal vein, posterior ciliary arteries, ophthalmic artery, and superior ophthalmic vein. It is also applied to assess hemodynamic changes in orbital vasculature. Furthermore, in comparison to other imaging methods such as computerized tomography (CT) and magnetic resonance imaging (MRI), CDI has the advantage of not using radiation. Ease of use and portability of equipment are also distinct advantages with CDI. Most importantly, CDI offers the capability of visualizing ocular and orbital vessels not frequently assessed by other methods.
| Color Doppler | Velocity information is displayed as a color code and superimposed on top of a B-mode image |
| Continuous Doppler | Continuous generation of ultrasound waves coupled with continuous ultrasound reception performed by two different transducer heads (crystals) without bidimensional (2D) discrimination |
| Pulsed Doppler | Doppler information is sampled from a small sample volume (defined in 2D image), and presented on a timeline. It uses a transducer that alternates transmission and reception |
| Duplex scanning | A common name for the simultaneous presentation of 2D and Doppler information |
| Spectral analysis | Time × frequency diagram representative of blood flow in a cardiac cycle |
| Power Doppler | Improved technology in Doppler sonography that has the advantages of less direction dependence, higher sensitivity, and better contrast of vasculature |
More recently, intravenous contrast agents have been used to enhance the properties of the diagnostic CDI. The contrast agents increase the amount of sound scattering in the blood, thus increasing the amplitude of the back-scattered signal by 20–30 dB, facilitating identification of blood flow within the neoplasms.1–3
CDI: background and physical considerations
The Doppler effect is the property of sound waves or electromagnetic waves to undergo changes in their velocity of propagation by the movement of a given reflector. This is a physical principle first described by Johann Christian Doppler, an Austrian scientist in 1842.4 The Doppler effect, or Doppler shift is applied in several areas of science such as astronomy, radar, and medical imaging.
Doppler ultrasonography is based on ultrasound wave reflection by a reflector (e.g. a blood column), which moves at a given velocity in one direction in reference to a transducer. The frequency of the emitted sound is altered by the velocity of blood particles. This frequency is increased when the blood flow is moving towards the transducer and decreases when blood moves away. Calculation of the magnitude of reflected sound yields an estimate of blood flow; the faster the blood flow (reflector), the greater the difference between the reflected and emitted frequencies (Doppler shift – ΔF), as demonstrated by the formula:5
which can be rearranged to give:
This equation is valid when transducer and reflector are parallel. Although orbital vessels are frequently parallel to the ultrasound beam, angle correction should be used when needed. Newer versions of the equipment automatically adjust for angle correction in the velocity calculations. Improvements in CDI techniques have tried to overcome limitations, such as angle dependence and difficulty in separating background noise from true flow in slow-flow states. Power Doppler sonography is able to evaluate low blood flow and has relative angle independence thereby offering superior depiction of tissue perfusion.6,7 Power Doppler sonography may be particularly valuable in assessing small vasculature of the eye.
Examination technique and device parameters
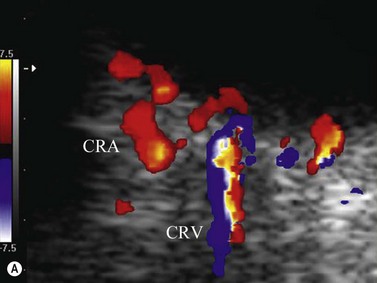
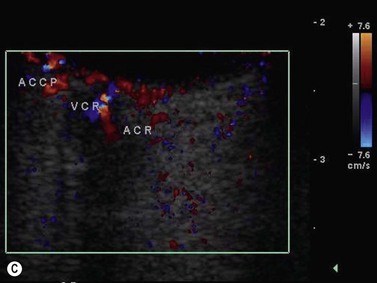
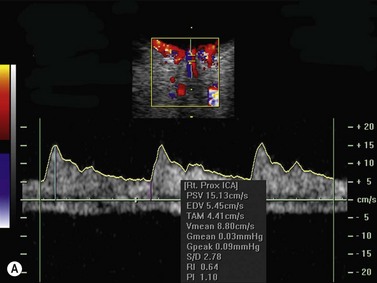
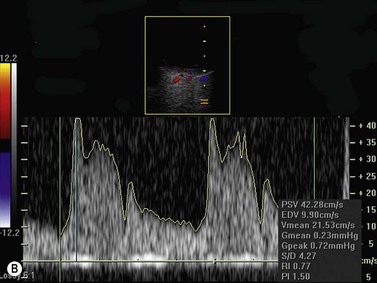
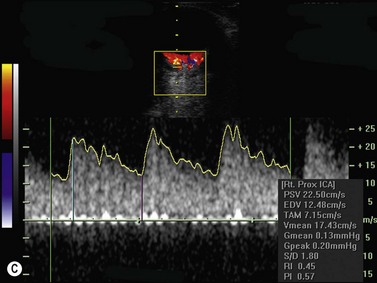
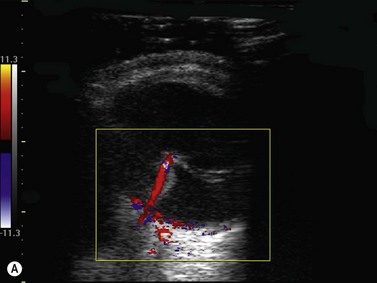
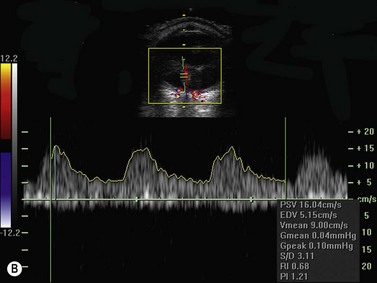
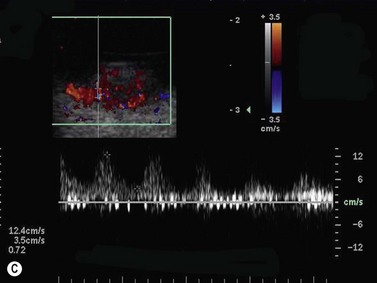
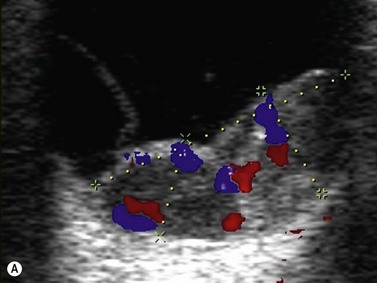
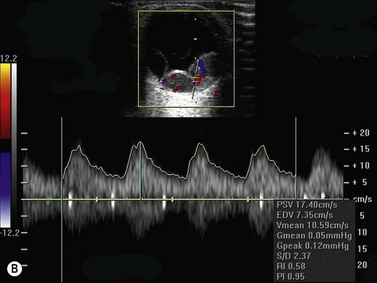
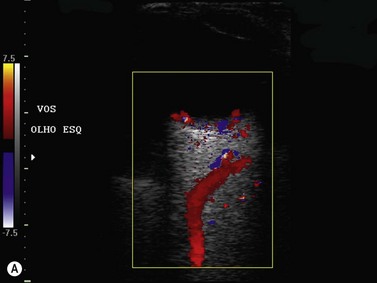
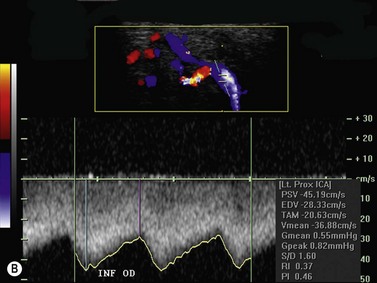
Color flow information is used to depict the major vascular structures in the orbit, which can be displayed in either blue or red. Color parameters can be subjectively assigned regarding the direction of the blood flow with respect to the transducer. In general, the flow moving towards the transducer is displayed in red, and away from it, is showed in blue (Figure 5.1). Orbital vessels are frequently parallel to the sound beam, thus, for the majority of the time arteries are displayed in red and veins in blue. A pulsed Doppler spectral analysis is also obtained to distinguish between arterial and venous flow (Figure 5.2).
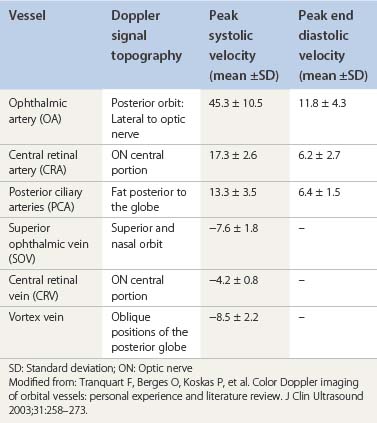
CDI application in ophthalmology dates from the late 1980s. It is applied to evaluate several ocular and orbital diseases (Table 5.2).8–20 Several studies have demonstrated CDI velocities of orbital vessels in normal eyes with relatively good reproducibility (Table 5.3).10,11,15,17,20–23
Table 5.2 Indications for Color Doppler imaging in ophthalmology.
| Intraocular disease |
Clinical applications
Retinal detachment
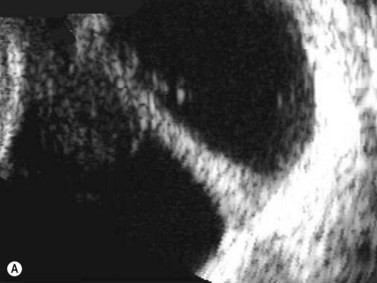
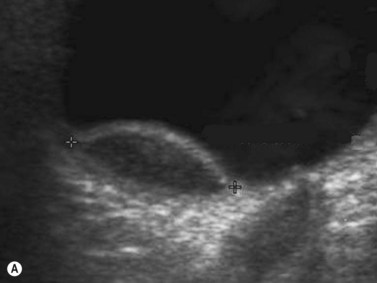
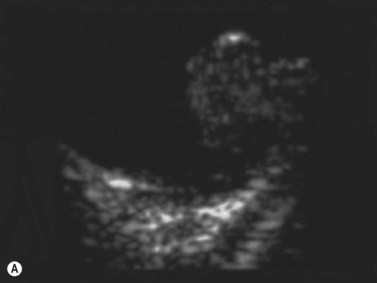
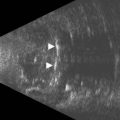
CDI can add useful information in differentiating a detached retina from a dense vitreous band through retinal vasculature depiction in retinal detachment (Figure 5.3).24,25
Persistent fetal vasculature
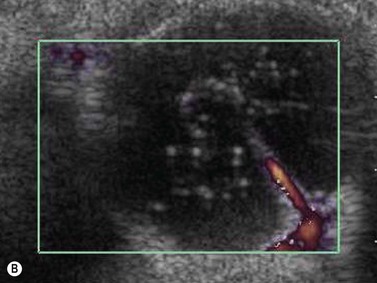
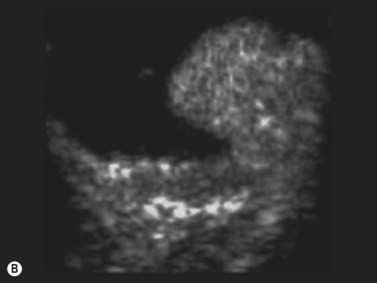
Persistent fetal vasculature or persistent hyperplastic primary vitreous has been studied with CDI, adequately demonstrating the patency of the persistent hyaloid artery.26,27 Besides diagnosis, hyaloid artery flow assessment can guide the surgeon in vitreoretinal surgery planning (Figure 5.4).
Intraocular tumors
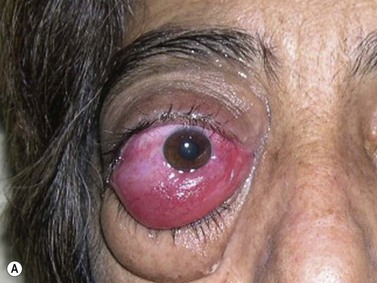
Besides clinical parameters, ancillary examination techniques provide critical information that helps to establish accurate diagnosis, plan radiation treatment, and reliably follow intraocular tumors, such as uveal melanoma, metastasis, and choroidal hemangioma. Conventional B-mode and standardized A-mode ultrasonography are considered the gold-standard for assessing qualitative and quantitative characteristics of intraocular lesions including tumor vascularization (Chapter 11).
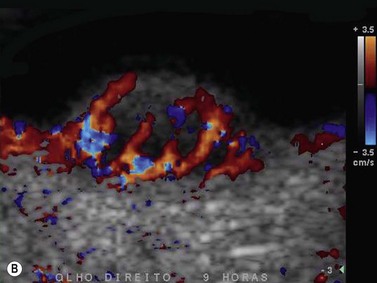
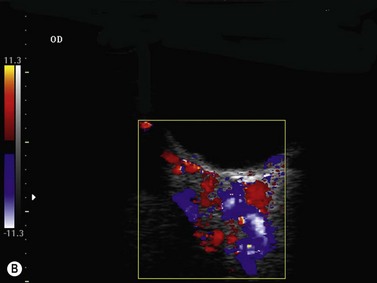
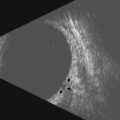
Tumor-associated vasculature is classically assessed by intravenous angiography and indocyanine angiography, which require clear media and no contraindication for intravenous contrast media. CDI has come to play an important role in tumor blood flow assessment, not only in displaying tumor microvasculature (color Doppler signals) but also in allowing functional analysis (spectral analysis) (Figure 5.5).28–33 Differential diagnosis between various intraocular tumors based on their vasculature pattern is still being defined. However a distinction between a non-tumoral process, such as a large hemorrhagic choroidal detachment, and a tumor is facilitated by this method, by identifying internal blood flow (Figure 5.6).
Some investigators have correlated CDI characteristics with tumor response after treatment.20,34,35 Tranquart et al evaluated 165 patients treated by radiation therapy (brachytherapy or proton beam therapy) as conservative treatment for uveal melanoma.20 CDI was performed 12 months after treatment to analyze vascular changes in tumor vascularity. A decrease in the number of Doppler tumor signals was the most frequent feature. Other observations included complete absence of vascularization and stability of tumor vasculature in a small percentage of cases.
Power Doppler mode and contrast agents, such as BR1 (SonoVue®[sulfur hexafluoride], Bracco)36 and SH U 508A (Levovist®, Schering)2,37 have enhanced the CDI ability to detect tumor intrinsic fine vascularity with minimal flow. Contrast agents will be discussed later in this chapter.
Ocular and orbital vascular diseases
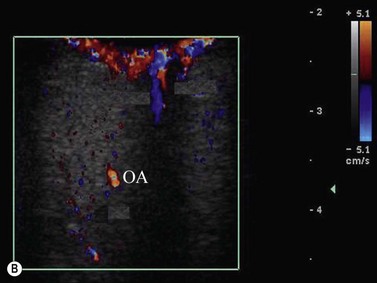
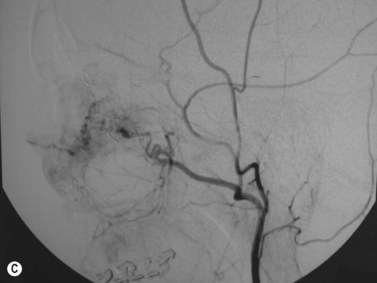
The main studies published in the literature involve the use of CDI in central retinal artery (CRA) occlusion,38–41 central retinal vein (CRV) occlusion,42–45 anterior ischemic optic neuropathy,46–49 thrombosis of the superior ophthalmic vein,50,51 orbital varix,52 carotid–cavernous fistula,53–57 and arteriovenous malformation (Figure 5.7). In addition to this, CDI has been used to identify hemodynamic changes on the orbital vessels induced by drugs,58 systemic illness,59–62 surgical interventions,63 and glaucoma.64–68
Central retinal artery (CRA) and central retinal vein (CRV) occlusion
CDI is a reasonable diagnostic tool in evaluating retinal vascularization when clinical examination is difficult or when the result of clinical examination is doubtful, since the preferred method of examination is fluorescein angiography. The most important signal demonstrated in CRA occlusion is lack or minimum blood flow in the CRA, with both decreased maximum systolic amplitude and diastolic flow.69 In CRV occlusion, the blockage of venous outflow generates a condition of high resistance microvasculature. This is demonstrated by CDI as an absence or decrease in magnitude of both maximum and minimum venous velocity in the CRV, as well as decreasing in peak systolic velocity and decreasing in end-diastolic velocity. The CRA waveform may appear altered.70,71
Anterior ischemic optic neuropathy (AION)
CDI of AION demonstrates decreased flow velocities of the posterior ciliary arteries (PCAs) with preserved flow of the CRA and CRV, although the PCAs can be difficult to evaluate because they can vary in number from individual to individual and not all the PCAs need to be affected for ischemia to develop.72
Orbital varix
Orbital varix demonstrate dilated superior ophthalmic vein with low blood flow velocities. Hemodynamic changes induced by position or by Valsalva’s maneuver can be recorded by CDI.52
Carotid–cavernous fistula
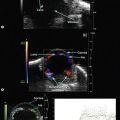
Carotid–cavernous fistula (CCF) is an abnormal communication between the cavernous sinuses and the carotid artery following trauma or occurring spontaneously. The transmission of arterial pressures into the venous structures results in orbital vascular engorgement and superior ophthalmic vein dilation. CDI typically demonstrates extraocular muscle enlargement, arterial pulsations, and an arterialized superior ophthalmic vein. Spectral analysis depicts increased velocity of blood flow with reversed flow direction (Figure 5.8).73–75 Furthermore, CDI has been used to monitor treatment response in serial evaluation.75
Orbital tumors
CDI has been evaluated in several orbital tumors and its applicability has been described basically in the field of tissue characterization9 and identification of vascular supply to help treatment planning76 along with CT and MRI. Some reports show that vascular tumors such as hemangiomas and hemangiopericytomas usually exhibit intrinsic tumor vasculature.77 The pattern of such vasculature is very little, with almost no flow for hemangioma, and prominent arterial and venous vessel vascularity for hemangiopericytomas, lymphoma, and metastasis.5 Meningiomas and gliomas tend not to have a CDI demonstrable vascularity.77
Contrast agents
In routine diagnostic imaging the use of contrast agents enhance the resolution and improve the accuracy of the imaging methods. Similar to contrast enhanced computer tomography and contrast enhanced magnetic resonance imaging, ultrasonography with contrast agent is used to improve the diagnostic power (Chapter 19). These substances alter the echo amplitude by changes in absorption, reflection and/or refraction of the ultrasound waves.78,79
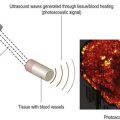
Use of gas bubbles in ultrasonography is not new. In the late 1960s it was observed that fluid injections during cardiac catheterization produced small gas bubbles into the bloodstream that lead to echo scattering.80 However, such bubbles had low stability in the circulation and were also too large to pass the pulmonary vasculature. Bubbles larger than 10 µm may transiently obstruct the capillaries and act as gas emboli. Since then research efforts have led to microbubbles with size comparable to red blood cells (1–10 µm). Owing to their properties, microbubbles are capable of remaining in microcirculation because they are too large to pass though vascular endothelium and their composition confers them stability. Currently, all commercial ultrasound contrast media for human applications are encapsulated microbubbles.78,79 Microbubbles enhance both gray-scale images and flow mediated Doppler signals and may produce up to 25 dB increase in echo strength.
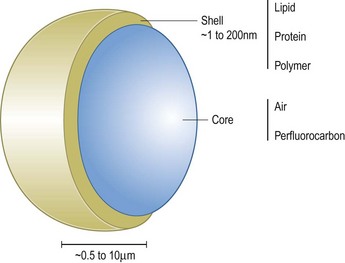
Microbubbles are basically composed of two parts (Figure 5.9). A gas core determines its echogenicity. After injection, ultrasound waves are directed on the area of interest and the bubbles are captured by an ultrasonic frequency window, wherein they compress, oscillate and reflect a unique echo under ultrasound stimulation.81,82 The gas core can be composed of air or various perfluorocarbons. Heavy gases are less water soluble, which increases the enhancement time of the contrast medium as compared to air. An outer shell is made up of stabilizing coatings of albumin, galactose, polyglutaminic acid, and lipophilic monolayer surfactants. They are in part metabolized in the liver and in part taken up by the immune system.83
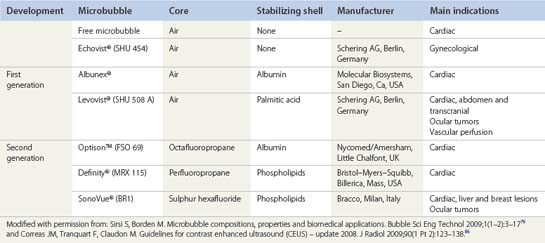
Several generations of the microbubble agents have been developed. The earliest version consisted of agents containing air core and no stabilizing shell: for example free microbubbles and SHU 454 (Echovist®, Schering AG, Berlin, Germany) (Table 5.4). First-generation agents such as Albunex® (Molecular Biosystems, San Diego, CA, USA, distributed by Mallinckrodt, St Louis, MO, USA) and SHU 508 A (Levovist®, Schering AG, Berlin, Germany) are comprised of air core and albumin and palmitic acid respectively as the stabilizing shell. Despite their short-life, such microbubbles are both sufficiently small and stable to pass into the systemic circulation, capable of enhancing Doppler signal in a variety of arteries after injection. The second generation, such as Optison™ (Nycomed/Amersham, Little Chalfont, UK), Definity® (Bristol-Myers-Squibb, Billerica, MA, USA) and SonoVue® (Bracco, Milan, Italy) are more echogenic and stable enabling them to be used in new functional imaging methods.84–86 Currently there are two ultrasound contrast agents being marketed in the USA with Food Drug and Administration approval: Definity® and Optison™ only for echocardiographic application. For general radiology applications, ultrasound contrast agents are approved in Europe and Asia.
Table 5.4 Microbubble contrast agents. Composition, manufacturer, main indications and eventual ocular applications.
Contrast-enhanced ultrasonography can detect flow in the intracranial arteries by transcranial Doppler where the skull strongly attenuates the ultrasound signal.87 Another use is demonstrating flow in smaller vessels (less than 40 µm), even non-detectable by power Doppler ultrasonography, which makes it useful in the study of circulation of malignant tumors.88–90 Microbubbles can also be injected into body cavities to facilitate functional tests, for instance in Fallopian tube patency evaluation after instilling microbubble contrast into the uterine cavity.91
Besides diagnosis, the most promising use of microbubble agents may be in the field of therapy. Several researchers have described the utility of microbubbles as drug carriers for site-specific treatment,92 such as gene therapy, since vector DNA can be conjugated to the microbubbles,93 and as a tool for non-invasive clot lysis.94
There are relatively few reports in the literature utilizing contrast enhanced ultrasound in ophthalmology. The majority of them come from Europe where they report their experience using two classes of microbubble contrast, Levovist® and SonoVue®, not approved for commercial use in the USA. The first report in humans by Cennamo was in 1994.1 They utilized SHU 508 A (Levovist®) to study 10 patients with a variety of malignant orbital and ocular tumors and demonstrated varying degrees of enhancement of the Doppler signal. Ten years later, the same group reported the vascular pattern of choroidal melanoma including treated and untreated cases using second generation contrast SonoVue® (Figure 5.10).90 Other investigators have also reported on usefulness of ultrasound contrasts agents for imaging of ocular tumors.36,95,96 Ophthalmic vascular perfusion with contrast enhanced ultrasound has also been evaluated.97 Barbrand evaluated retrobulbar arteries (ophthalmic, central retinal, nasal, and temporal posterior ciliary arteries) using Levovist® and concluded that the contrast did not add any substantial diagnostic information.37
1 Cennamo G, Rosa N, Vallone GF, et al. First experience with a new echographic contrast agent. Br J Ophthalmol. 1994;78(11):823-826.
2 Grussner S, Klingmuller V, Bohle R. [Increased signal intensity of velocity measurements in duplex sonography by using the contrast agent levovist: a prospective, randomized study in a fetal sheep model]. Rofo. 2004;176(1):91-97.
3 Melany ML, Grant EG. Clinical experience with sonographic contrast agents. Semin Ultrasound CT MR. 1997;18(1):3-12.
4 Taylor KJ, Holland S. Doppler US. Part I. Basic principles, instrumentation, and pitfalls. Radiology. 1990;174(2):297-307.
5 Lieb WE. Color Doppler imaging of the eye and orbit. Radiol Clin North Am. 1998;36(6):1059-1071.
6 Guerriero S, Alcazar JL, Ajossa S, et al. Comparison of conventional color Doppler imaging and power doppler imaging for the diagnosis of ovarian cancer: results of a European study. Gynecol Oncol. 2001;83(2):299-304.
7 Hamper UM, DeJong MR, Caskey CI, et al. Power Doppler imaging: clinical experience and correlation with color Doppler US and other imaging modalities. Radiographics. 1997;17(2):499-513.
8 Lieb WE, Cohen SM, Merton DA, et al. Color Doppler imaging of the eye and orbit. Technique and normal vascular anatomy. Arch Ophthalmol. 1991;109(4):527-531.
9 Lieb WE, Flaharty PM, Ho A, et al. Color Doppler imaging of the eye and orbit. A synopsis of a 400 case experience. Acta Ophthalmol Suppl. 1992;204:50-54.
10 Aburn NS, Sergott RC. Color Doppler imaging of the ocular and orbital blood vessels. Curr Opin Ophthalmol. 1993;4(6):3-6.
11 Giovagnorio F, Quaranta L, Bucci MG. Color Doppler assessment of normal ocular blood flow. J Ultrasound Med. 1993;12(8):473-477.
12 Lieb WE. Color Doppler ultrasonography of the eye and orbit. Curr Opin Ophthalmol. 1993;4(3):68-75.
13 Munk P, Downey D, Nicolle D, et al. The role of colour flow Doppler ultrasonography in the investigation of disease in the eye and orbit. Can J Ophthalmol. 1993;28(4):171-176.
14 Williamson TH, Baxter GM, Dutton GN. Colour Doppler velocimetry of the arterial vasculature of the optic nerve head and orbit. Eye (Lond). 1993;7(Pt 1):74-79.
15 Yang H, Wu Z. [Color Doppler imaging in the study of normal orbital vessels and its hemodynamics]. Yan Ke Xue Bao. 1993;9(4):208-212. 202
16 Giovagnorio F, Quaranta L, Fazio V, et al. [Color Doppler echography of the orbit. Its normal aspects and pathological conditions with vascular involvement]. Radiol Med. 1994;88(5):588-593.
17 Baxter GM, Williamson TH. Color Doppler imaging of the eye: normal ranges, reproducibility, and observer variation. J Ultrasound Med. 1995;14(2):91-96.
18 Venturini M, Zaganelli E, Angeli E, et al. [Ocular color Doppler echography: the examination technic, identification and flowmetry of the orbital vessels]. Radiol Med. 1996;91(1–2):60-65.
19 Ivekovic R, Lovrencic-Huzjan A, Mandic Z, et al. Color Doppler flow imaging of ocular tumors. Croat Med J. 2000;41(1):72-75.
20 Tranquart F, Berges O, Koskas P, et al. Color Doppler imaging of orbital vessels: personal experience and literature review. J Clin Ultrasound. 2003;31(5):258-273.
21 Tolwinski R, Tarasow E, Szulc S, et al. [Use of color Doppler ultrasonography for evaluation of blood flow in orbital vessels]. Klin Oczna. 1997;99(6):359-362.
22 Williamson TH, Harris A. Color Doppler ultrasound imaging of the eye and orbit. Surv Ophthalmol. 1996;40(4):255-267.
23 Stefanczyk L, Mysior M, Gralek M, et al. [Doppler color ultrasonography in the evaluation of orbital and eyeball vessels]. Klin Oczna. 1994;96(10–11):305-308.
24 Stefanczyk L, Orawiec B, Gralek M, et al. [Usefulness of color Doppler ultrasonography in diagnosis of retinal detachment]. Klin Oczna. 1996;98(4):287-290.
25 Wong AD, Cooperberg PL, Ross WH, et al. Differentiation of detached retina and vitreous membrane with color flow Doppler. Radiology. 1991;178(2):429-431.
26 Jain TP. Bilateral persistent hyperplastic primary vitreous. Ind J Ophthalmol. 2009;57(1):53-54.
27 Yang W, Hu S, Wang J, et al. Color Doppler imaging diagnosis of intra–ocular tumor. Chin Med J (Engl). 1997;110(9):664-666.
28 Dudea SM, Seceleanu A, Botar-Jid C, et al. [Doppler ultrasound assessment of intraocular and orbital tumors]. Oftalmologia. 2007;51(2):87-92.
29 Brovkina AF, Amirian AG, Leliuk VG. [Role of high-frequency duplex scanning in the differential diagnosis of uveal melanomas and solitary choroidal hemangiomas]. Vestn Oftalmol. 2005;121(6):3-5.
30 Nemeth J, Tapaszto B, Toth J, et al. [Evaluation of color Doppler imaging of ophthalmic tumors based on histopathologic findings]. Magy Onkol. 2005;49(1):35-41.
31 Wu Z, Yang H, Li X. [Color Doppler ultrasonography in evaluation of intraocular lesions]. Zhonghua Yan Ke Za Zhi. 1997;33(2):88-91.
32 Ozdemir H, Yucel C, Aytekin C, et al. Intraocular tumors. The value of spectral and color Doppler sonography. Clin Imaging. 1997;21(2):77-81.
33 Gulani AC, Morparia H, Bhatti SS, et al. Colour Doppler sonography: a new investigative modality for intraocular space–occupying lesions. Eye (Lond). 1994;8(Pt 3):307-310.
34 Vecsei PV, Kircher K, Nagel G, et al. Ocular arterial blood flow of choroidal melanoma eyes before and after stereotactic radiotherapy using Leksell gamma knife: 2 year follow up. Br J Ophthalmol. 1999;83(12):1324-1328.
35 Proniewska-Skretek E, Zalewska R, Ustymowicz A, et al. [An application of color Doppler ultrasonography in evaluate of brachytherapy in patients with uveal melanomas]. Klin Oczna. 2007;109(4–6):187-190.
36 Schlottmann K, Fuchs-Koelwel B, Demmler-Hackenberg M, et al. High–frequency contrast harmonic imaging of ophthalmic tumor perfusion. AJR Am J Roentgenol. 2005;184(2):574-578.
37 Brabrand K, Kerty E, Jakobsen JA. Contrast-enhanced ultrasound Doppler examination of the retrobulbar arteries. Acta Radiol. 2001;42(2):135-139.
38 Schicke SH, Duncker GI. [Retinal color Doppler scanning of arteria centralis retinae by retinal diseases and healthy people]. Klin Monbl Augenheilkd. 2007;224(10):775-779.
39 Yamamoto T, Mori K, Yasuhara T, et al. Ophthalmic artery blood flow in patients with internal carotid artery occlusion. Br J Ophthalmol. 2004;88(4):505-508.
40 Hwang JF, Chen SN, Chiu SL, et al. Embolic cilioretinal artery occlusion due to carotid artery dissection. Am J Ophthalmol. 2004;138(3):496-498.
41 Foroozan R, Savino PJ, Sergott RC. Embolic central retinal artery occlusion detected by orbital color Doppler imaging. Ophthalmology. 2002;109(4):744-747. discussion 747–8
42 Kiseleva TN, Koshevaia OP, Budzinskaia MV, et al. [Value of color doppler imaging in the diagnosis of occlusive retinal venous lesions]. Vestn Oftalmol. 2006;122(5):4-7.
43 Ozbek Z, Saatci AO, Durak I, et al. Colour Doppler assessment of blood flow in eyes with central retinal vein occlusion. Ophthalmologica. 2002;216(4):231-234.
44 Keyser BJ, Flaharty PM, Sergott RC, et al. Color Doppler imaging of arterial blood flow in central retinal vein occlusion. Ophthalmology. 1994;101(8):1357-1361.
45 Berger RW, Guthoff R, Helmke K, et al. [Color-coded Doppler sonography of orbital blood vessels with special reference to the central retinal artery and vein]. Fortschr Ophthalmol. 1991;88(6):690-693.
46 Schmidt WA, Krause A, Schicke B, et al. Do temporal artery duplex ultrasound findings correlate with ophthalmic complications in giant cell arteritis? Rheumatology (Oxford). 2009;48(4):383-385.
47 Sanjari MS, Falavarjani KG, Mehrabani M, et al. Retrobulbar haemodynamics and carotid wall thickness in patients with non–arteritic anterior ischaemic optic neuropathy. Br J Ophthalmol. 2009;93(5):638-640.
48 Kaup M, Plange N, Arend KO, et al. Retrobulbar haemodynamics in non–arteritic anterior ischaemic optic neuropathy. Br J Ophthalmol. 2006;90(11):1350-1353.
49 Li X, Wang J, He S, et al. [Observation of the anterior ischemic optic neuropathy by color Doppler flow imaging]. Zhonghua Yan Ke Za Zhi. 1999;35(2):122-124.
50 Tacke J, Dick A, Kutschbach P, et al. [Color-coded duplex ultrasonography of the orbit in central vein thrombosis]. Rofo. 1997;166(4):329-334.
51 Flaharty PM, Phillips W, Sergott RC, et al. Color Doppler imaging of superior ophthalmic vein thrombosis. Arch Ophthalmol. 1991;109(4):582-583.
52 Lieb WE, Merton DA, Shields JA, et al. Colour Doppler imaging in the demonstration of an orbital varix. Br J Ophthalmol. 1990;74(5):305-308.
53 Aung T, Oen FT, Fu ER. Orbital colour Doppler imaging in carotid–cavernous sinus fistula. Aust N Z J Ophthalmol. 1996;24(2):121-126.
54 Stefanczyk L, Kaurzel Z, Kazanek M, et al. [Carotid–cavernous fistula – diagnostic possibilities of color doppler ultrasonography]. Klin Oczna. 1996;98(1):51-53.
55 Costa VP, Molnar LJ, Cerri GG. Diagnosing and monitoring carotid cavernous fistulas with color Doppler imaging. J Clin Ultrasound. 1997;25(8):448-452.
56 Chiou HJ, Chou YH, Guo WY, et al. Verifying complete obliteration of carotid artery–cavernous sinus fistula: role of color Doppler ultrasonography. J Ultrasound Med. 1998;17(5):289-295.
57 Zhang W, Zhao H, Song G. [The value of color Doppler imaging ultrasound in diagnosis of orbital diseases]. Zhonghua Yan Ke Za Zhi. 2001;37(6):447-450.
58 Gobel W, Lieb WE. [Changes in orbital hemodynamics caused by nitroglycerin and nifedipine. A study using color duplex ultrasound]. Ophthalmologe. 1995;92(2):206-211.
59 Erdogmus B, Yazici S, Yazici B, et al. Orbital blood flow velocities in patients with rheumatoid arthritis. J Clin Ultrasound. 2007;35(7):367-371.
60 Fujioka S, Karashima K, Inoue A, et al. Case of infectious endocarditis predicted by orbital color Doppler imaging. Jpn J Ophthalmol. 2005;49(1):46-48.
61 Guven D, Ozdemir H, Hasanreisoglu B. Hemodynamic alterations in diabetic retinopathy. Ophthalmology. 1996;103(8):1245-1249.
62 Wright SA, O’Prey FM, Hamilton PK, et al. Colour Doppler ultrasound of the ocular circulation in patients with systemic lupus erythematosus identifies altered microcirculatory haemodynamics. Lupus. 2009;18(11):950-957.
63 Krasnicki P, Proniewska-Skretek E, Mariak Z, et al. [Embolic central retinal artery occlusion as a complication of percutaneous coronary angioplasty – case report]. Klin Oczna. 2008;110(1–3):64-66.
64 Erkin EF, Tarhan S, Kayikcioglu OR, et al. Effects of betaxolol and latanoprost on ocular blood flow and visual fields in patients with primary open-angle glaucoma. Eur J Ophthalmol. 2004;14(3):211-219.
65 Gherghel D, Orgul S, Gugleta K, et al. Retrobulbar blood flow in glaucoma patients with nocturnal over-dipping in systemic blood pressure. Am J Ophthalmol. 2001;132(5):641-647.
66 Harris A, Migliardi R, Rechtman E, et al. Comparative analysis of the effects of dorzolamide and latanoprost on ocular hemodynamics in normal tension glaucoma patients. Eur J Ophthalmol. 2003;13(1):24-31.
67 Harris A, Spaeth GL, Sergott RC, et al. Retrobulbar arterial hemodynamic effects of betaxolol and timolol in normal-tension glaucoma. Am J Ophthalmol. 1995;120(2):168-175.
68 Zeitz O, Matthiessen ET, Reuss J, et al. Effects of glaucoma drugs on ocular hemodynamics in normal tension glaucoma: a randomized trial comparing bimatoprost and latanoprost with dorzolamide [ISRCTN18873428]. BMC Ophthalmol. 2005;5:6.
69 Hedges TR. Ophthalmic artery blood flow in humans. Br J Ophthalmol. 2002;86(11):1197.
70 Baxter GM, Williamson TH. The value of serial Doppler imaging in central retinal vein occlusion: correlation with visual recovery. Clin Radiol. 1996;51(6):411-414.
71 Williamson TH, Baxter GM. Central retinal vein occlusion, an investigation by color Doppler imaging. Blood velocity characteristics and prediction of iris neovascularization. Ophthalmology. 1994;101(8):1362-1372.
72 Flaharty PM, Sergott RC, Lieb W, et al. Optic nerve sheath decompression may improve blood flow in anterior ischemic optic neuropathy. Ophthalmology. 1993;100(3):297-302. discussion 303–5
73 de Keizer R. Carotid–cavernous and orbital arteriovenous fistulas: ocular features, diagnostic and hemodynamic considerations in relation to visual impairment and morbidity. Orbit. 2003;22(2):121-142.
74 Wu Z, Yang H. Color Doppler imaging in the diagnosis and follow–up of carotid cavernous sinus fistulas. Yan Ke Xue Bao. 1993;9(3):153-157.
75 Soulier–Sotto V, Beaufrere L, Laroche JP, et al. [Diagnosis by Doppler color echography of dural carotid–cavernous fistula of ophthalmological manifestation]. J Fr Ophtalmol. 1992;15(1):38-42.
76 Zuravleff JJ, Johnson MH. An ophthalmic surgeon’s view of orbital imaging techniques. Semin Ultrasound CT MR. 1997;18(6):395-402.
77 Belden CJ, Abbitt PL, Beadles KA. Color Doppler US of the orbit. Radiographics. 1995;15(3):589-608.
78 Correas JM, Bridal L, Lesavre A, et al. Ultrasound contrast agents: properties, principles of action, tolerance, and artifacts. Eur Radiol. 2001;11(8):1316-1328.
79 Sirsi S, Borden M. Microbubble compositions, properties and biomedical applications. Bubble Sci Eng Technol. 2009;1(1–2):3-17.
80 Gramiak R, Shah PM. Echocardiography of the aortic root. Invest Radiol. 1968;3(5):356-366.
81 Dayton PA, Morgan KE, Klibanov AL, et al. Optical and acoustical observations of the effects of ultrasound on contrast agents. IEEE Trans Ultrason Ferroelectr Freq Control. 1999;46(1):220-232.
82 Qin S, Caskey CF, Ferrara KW. Ultrasound contrast microbubbles in imaging and therapy: physical principles and engineering. Phys Med Biol. 2009;54(6):R27-R57.
83 Uhlendorf V, Scholle FD, Reinhardt M. Acoustic behaviour of current ultrasound contrast agents. Ultrasonics. 2000;38(1–8):81-86.
84 Leclercq F, Messner-Pellenc P, Descours Q, et al. Combined assessment of reflow and collateral blood flow by myocardial contrast echocardiography after acute reperfused myocardial infarction. Heart. 1999;82(1):62-67.
85 Dijkmans PA, Senior R, Becher H, et al. Myocardial contrast echocardiography evolving as a clinically feasible technique for accurate, rapid, and safe assessment of myocardial perfusion: the evidence so far. J Am Coll Cardiol. 2006;48(11):2168-2177.
86 Correas JM, Tranquart F, Claudon M. [Guidelines for contrast enhanced ultrasound (CEUS) – update 2008]. J Radiol. 2009;90(1 Pt 2):123-138. quiz 139–40
87 Ries F, Honisch C, Lambertz M, et al. A transpulmonary contrast medium enhances the transcranial Doppler signal in humans. Stroke. 1993;24(12):1903-1909.
88 Testa AC, Timmerman D, Van Belle V, et al. Intravenous contrast ultrasound examination using contrast-tuned imaging (CnTI) and the contrast medium SonoVue for discrimination between benign and malignant adnexal masses with solid components. Ultrasound Obstet Gynecol. 2009;34(6):699-710.
89 Rickes S, Monkemuller K, Malfertheiner P. Contrast–enhanced ultrasound in the diagnosis of pancreatic tumors. JOP. 2006;7(6):584-592.
90 Forte R, Cennamo G, Staibano S, et al. Echographic examination with new generation contrast agent of choroidal malignant melanomas. Acta Ophthalmol Scand. 2005;83(3):347-354.
91 Exacoustos C, Di Giovanni A, Szabolcs B, et al. Automated sonographic tubal patency evaluation with three-dimensional coded contrast imaging (CCI) during hysterosalpingo-contrast sonography (HyCoSy). Ultrasound Obstet Gynecol. 2009;34(5):609-612.
92 Price RJ, Skyba DM, Kaul S, et al. Delivery of colloidal particles and red blood cells to tissue through microvessel ruptures created by targeted microbubble destruction with ultrasound. Circulation. 1998;98(13):1264-1267.
93 Miller MW. Gene transfection and drug delivery. Ultrasound Med Biol. 2000;26(Suppl. 1):S59-S62.
94 Wu Y, Unger EC, McCreery TP, et al. Binding and lysing of blood clots using MRX–408. Invest Radiol. 1998;33(12):880-885.
95 Lemke AJ, Hosten N, Richter M, et al. Contrast-enhanced color Doppler sonography of uveal melanomas. J Clin Ultrasound. 2001;29(4):205-211.
96 Coppola V, Vallone G, Verrengia D, et al. [Doppler color ultrasonography with contrast media in the study of eye and orbit neoplasms]. Radiol Med. 1997;93(4):367-373.
97 Montanari P, Bianchi R, Oldani A, et al. Study of optic nerve head perfusion in glaucomatous patients by color Doppler imaging with a contrast agent. Acta Ophthalmol Scand Suppl. 2000;232:35-36.