Chapter 9 Do Bone Morphogenetic Proteins Improve Spinal Fusion?
BACKGROUND
Lumbar spinal fusion is a common surgical treatment for many degenerative, traumatic, deformity, and destructive conditions of the lumbar spine. It has been shown to improve outcomes in disabling conditions such as spinal stenosis, degenerative and isthmic spondylolisthesis, and degenerative disc disease (DDD).1 With advances in diagnostic tools, fusion techniques, subspecialty training, raised patient and physician awareness, and better understanding of the causes and consequences of these disorders, as well as their management, there has been a dramatic increase in overall rates of spinal fusion.2 Spinal fusion surgery can be technically demanding, surgical costs are high, and complications can be significant.3 Population-based reoperation rates after spinal fusion in the early 1990s were 10% to 20%.4,5 Nonunion of the spine is a common reason for pain and reoperation after spinal fusion.6 Harvesting iliac crest autograft for spinal fusion is another common causeof persistent pain and reduced functional outcome after lumbar spinal fusion surgery.7–9
Bone morphogenetic proteins (BMPs), first identified by Urist in 1965,10 consist of protein components of bone matrix that can be extracted to induce the differentiation of osteoprogenitor cells into bone-producing osteogenic cells, thus stimulating the production of new bone.11 Further research has been able to identify many different BMPs, of which BMP-2, BMP-6, and BMP-7 appear to have the most important roles.12
Earlier preclinical and clinical studies demonstrated that BMPs increase fusion rates. In a comprehensive review, Sandhu suggests that recombinant BMPs can be used as substitutes for autograft, and that in some circumstances, their efficacy for inducing fusion is superior.13
SYSTEMATIC REVIEW
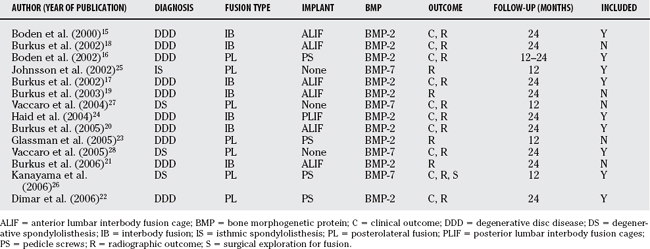
From our systematic review (Table 9-1), we identified 75 articles, of which 14 were considered potentially relevant from abstract review. One additional published randomized, controlled trial (RCT) was identified from a manual bibliography search; however, abstract review excluded it based on inadequate length of follow-up.14 Hand searches of major meeting proceedings identified an additional RCT; however, this study has not yet been submitted for publication. The 14 potentially relevant trials were retrieved in full for data abstraction.15–28 The baseline characteristics of these trials are presented in Table 9-2. Once all data were abstracted, the trials were then reviewed in an unblinded fashion. It became apparent that many of them included the same randomized patients presented in different publications.
| KEYWORD | MEDLINE (1950–2007 WEEK 11) | EMBASE (1980–2007 WEEK 11) |
|---|---|---|
| Clinical trials | Clinical trials/or clinical trials, phase i/or clinical trials, phase ii/or clinical trials, phase iii/or clinical trials, phase iv/or controlled clinical trials/or randomized controlled trials/or multicenter studies/or cross-over studies/or double-blind method/or meta-analysis/or random allocation/or single-blind method/or systematic review$. ti, ab. or ((singl$ or doubl$ or tripl$) adj (mask$ or blind$)).ti, ab. or (blind$ or random$).ti, ab. | Randomized controlled trial/or ((controlled study/or comparative study/) and (clinical trial/or phase 1 clinical trial/or phase 2 clinical trial/or phase 3 clinical trial/or phase 4 clinical trial/or major clinical study/or prospective study/)) or “systematic review”/or randomization/or double blind procedure/or single blind procedure/or triple blind procedure/or ((singl$ or doubl$ or tripl$) adj (mask$ or blind$)).ti, ab. or (blind$ or random$). ti, ab. |
| Spinal fusion | Spinal Fusion/or spondylosyndesis.ti, ab. or spondylodesis.ti, ab. or (spin$ adj2 fusion$). ti, ab. | Exp spine fusion/ |
| Bone morphoge-netic protein | Exp Bone Morphogenetic Proteins/or bone morphogenetic protein.mp or bone morphogenic protein or bone derived growth factor.mp or bmp.mp or osteogenic protein. mp | Bone Morphogenetic Protein/or bone morphogenetic protein.mp or Bone Morphogenetic Protein 2/or bone morphogenetic protein 2.mp or Bone Morphogenetic Protein 6/or bone morphogenetic protein 6.mp or Bone Morphogenetic Protein 9/or bone morphogenetic protein 9.mp or bmp.mp or bone derived growth factor.mp or osteogenic protein.mp or bone morphogenetic protein$.ti, ab. |
We attempted to consolidate the trials that included the same patients to avoid over-reporting and to obtain mutually exclusive trials. Two trials included patients with DDD who underwent anterior lumbar interbody fusion (ALIF) with tapered cylindrical cages randomized to BMP-2 or autograft.17,19 One trial included 45 patients at 1 center, whereas the larger of the two reported on 279 patients at 16 investigational sites. Because follow-up was similar in both trials and the smaller trial reported only radiographic outcomes, we decided to include only the larger of the two trials.17
Three trials included patients with DDD who underwent ALIF using threaded cortical allografts randomized to BMP-2 or autograft.18,20, 21 The smallest trial reported a pilot series that was reanalyzed in the two other larger studies. Of the larger studies, only one of them reported radiographic outcomes and focused on the healing patterns, whereas the other reported both clinical and radiographic outcomes for the entire cohort. Thus, we included only the larger trial with both clinical and radiographic outcomes.20
Two trials of patients with degenerative spondylolisthesis who underwent posterolateral noninstr-umented fusion randomized to BMP-7 or autograft were identified.27,28 One was a longer follow-up (to 24 months) of the same cohort and, therefore, the one only included for analysis.28 Finally, two trialsof patients with DDD who underwent posterolateral fusion with pedicle screw fixation randomized to BMP-2 or autograft were identified.22,23 The smaller of the two reported only radiographic results for a single institution of a multicenter trial with 12-month follow-up. The larger trial that included clinical and radiographic outcomes at 24 months for two of the investigating centers was retained for analysis.22 In all cases of trials that were eliminated, information abstracted was used to complete missing information for those retained. Therefore, our results are reported in detail for the nine “mutually exclusive” trials.15–17,20,22,24–26,28
Overall, descriptions of randomization methods were poor. Only one of the nine trials (Burkus and colleagues, 200520) described an adequate method of randomization of subjects. One other trial (Johnsson and coworkers, 200225) indicated that randomization was blind to patient and surgeon; however, only until the time of surgery. Also, the method of randomization was not discussed to ensure allocation concealment. The other eight trials had unclear or no discussion of randomization methods.
Six of the nine trials had over 24-month follow-up data, and three trials had at least 12-month follow-up data. Four trials had near-complete follow-up with minimal dropouts. One trial (Vaccaro and coauthors, 200528) had incomplete 24-month follow-up data but imputed the missing values with 36-month data. One trial (Haid and colleagues, 200424) had unclear accounting of the final numbers of patients analyzed, although the discrepancy was relatively small. Two trials (Burkus and colleagues, 200217; Burkus and colleagues, 200520) failed to perform intention-to-treat analyses and had removed patients who underwent reoperations from subsequent analyses. One other trial (Dimar and investigators, 200622) had a follow-up rate of less than 67% without a proper account of the dropouts.
Patients and investigators were not blinded to treatment in any of the trials at the time of outcome assessment. Radiologists and orthopedic surgeons who assessed radiographic fusion were blinded in seven of the trials. Two trials (Johnsson and coworkers, 200225; Kanayama and colleagues, 200626) did not mention whether the radiologists were blinded to treatment assignment at the time of their assessment.
Eight of the trials provided validated patient-oriented clinical outcome measures (ODI, 36-Item Short Form Health Survey [SF-36]). Six trials reported both outcome measures, and two of them (Burkus and colleagues, 200217; Kanayama and colleagues, 200626) reported only one (Oswestry Disability Index [ODI]). One trial (Johnsson and coworkers, 200225) had no validated patient-oriented clinical outcome measure. Seven of the nine trials reported on adverse surgical events, and eight of the nine trials reported on the need for reoperation.
Of the five posterolateral fusions, two trials used BMP-2 for patients with DDD in instrumented fusions. The other three trials all used BMP-7. One was in noninstrumented fusions in patients with isthmic spondylolisthesis. Two trials evaluated BMP-7 in posterolateral fusions in patients with degenerative spondylolisthesis, one noninstrumented and one instrumented. Commercial funding sources and/or conflicts of interest were reported for all of the trials. Commercial funding solely designated to research and education was reported in only one of the trials (Kanayama and colleagues, 200626).
EVIDENCE
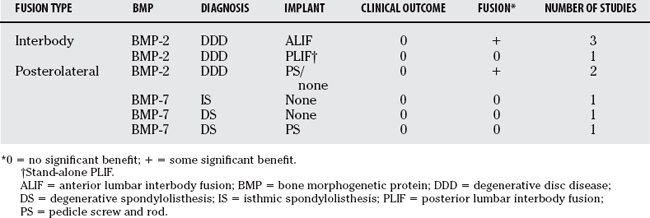
A summary of our findings is shown in Table 9-3. The evidence is presented for three main clinical applications. The first is BMP-2 in interbody fusion for patients with DDD (four trials). The second application is the use of BMP-2 in posterolateral fusion for patients with DDD (two trials), and the final is the use of BMP-7 in posterolateral fusion for patients with spondylolisthesis (three trials).
Interbody Fusion Using Bone Morphogenetic Protein-2 in Degenerative Disc Disease
Boden and coauthors (2000)15 investigated the efficacy of recombinant human BMP-2 (rhBMP-2) versus iliac crest autograft in patients with DDD who underwent ALIF using tapered cylindrical threaded fusion cages. Fourteen patients were randomized in a 3:1 ratio (intervention/control ratio). Eleven patients who were randomized to the intervention received 1.5 mg/mL rhBMP-2 on an absorbable collagen sponge giving a total dose of 1.3 or 2.6 mL depending on the cage size. The three control patients received iliac crest autograft bone.
In a large multicenter trial, Burkus and colleagues (2002)17 compared rhBMP-2 with iliac crest autograft in patients with single-level DDD who underwent ALIF using tapered interbody cages. Two hundred and seventy-nine patients were enrolled in the study. The 143 patients who were randomized to receive rhBMP-2 were given 1.5 mg/mL on an absorbable collagen sponge for a total dose ranging from 4.2 to 8.4 mg depending on the size of the cage used. The 136 control patients received autograft. Intention-to-treat analyses were not performed.
Burkus and colleagues (2005)20 report on the results of a two-part multicenter trial evaluating rhBMP-2 versus iliac crest autograft in another series of patients with single-level DDD who underwent ALIF using threaded cortical allograft bone dowels. A total of 131 patients were enrolled at 13 sites (46 patients in the pilot phase and 85 in the pivotal phase). Seventy-nine patients randomized to rhBMP-2 received 1.5 mg/mL rhBMP-2 on an absorbable collagen sponge giving a total dose between 8.4 and 12 mg depending on the size of dowels. The 52 control patients received autograft. Intention-to-treat analysis was not performed because patients who underwent secondary surgery were removed from subsequent analyses.
Haid and colleagues (2004)24 report the results of multicenter trial evaluating rhBMP-2 versus autograft in patients with DDD undergoing PLIF via a posterior approach. A total of 67 patients were enrolled in this 14-center trial. The trial suspended enrollment prematurely because of concerns about ectopic bone formation posterior to the cages in investigational patients. Thirty-four patients were randomized to receive 1.5 mg/mL rhBMP-2 on a collagen sponge for total doses between 4 and 8 mg, and 33 patients to iliac crest autograft as control. Follow-up at the 24-month period was 85% of rhBMP-2 patients from enrollment and 91% of control patients.
Posterolateral Fusion Using Bone Morphogenetic Protein-2 in Degenerative Disc Disease
Boden and coauthors (2002)16 assessed the outcome of rhBMP-2 in patients with DDD undergoing instrumented and noninstrumented posterior fusion. Twenty-seven patients were enrolled at six investigational centers. Eleven patients were randomized to receive a total dose of 40 mg rhBMP-2 (concentration, 2.0 mg/mL) on a 60% hydroxyapatite/40% tricalcium phosphate (HA/TCP) granule carrier with pedicle screw and rod instrumentation. Nine patients were randomized to receive the same rhBMP-2 with carrier and no internal fixation, and five additional patients were randomized to iliac crest autograft and pedicle screw fixation. Follow-up was to a minimum of 12 months but up to 24 months for some patients.
Dimar and investigators (2006)22 also compared rhBMP-2 with autograft in patients with DDD undergoing posterior pedicle screw and rod instrumented fusions. From this multicenter randomized trial, 150 patients were enrolled but only 98 of them (65%) were available for review at 2 years after surgery. Of the 98 patients, 53 received a total dose of 40 mg rhBMP-2 (concentration, 2.0 mg/mL) combined with a bovine collagen and an HA/TCP compression-resistant matrix, and 45 patients received autograft bone from the iliac crest. At 2 years follow-up, the mean improvement in ODI for the rhBMP-2 group was 24.5 points compared with 21.4 points in the control group. The mean improvement in SF-36 PCS score was 8.6 and 10.7 points for the rhBMP-2 and control groups, respectively. No clinical improvements were significantly different for the two groups. Radiographic fusion, based on plain radiographs and CT, was observed in 48 of 53 rhBMP-2 patients (90.6%) and 33 of the 45 control patients (73.3%). This difference was found to be significant at the P < 0.05 level. Three control patients underwent reoperation for revision of malpositioned screws. No reoperations were reported for the rhBMP-2 group.
Posterolateral Fusion Using Bone Morphogenetic Protein-7 in Spondylolisthesis
Johnsson and coworkers (2002)25 evaluated the use of recombinant human BMP-7, also termed osteogenic protein-1 (OP-1) in patients with isthmic low-grade spondylolisthesis (no more than 50% vertebral slip) undergoing noninstrumented posterolateral fusion. This trial of 20 patients primarily evaluated outcomes by radiostereometric analysis. The only clinical assessment was subjective back pain; however, the investigators assessed plain radiographic evidence of fusion. Ten patients were randomized to receive 3.5 mg rhOP-1 reconstituted with a bone collagen carrier into a paste for each side of the fusion (total dose, 7 mg). Ten other patients received iliac crest autograft through the initial incision. At 12-month follow-up, fusion (bilateral bridging bone) was observed in 6 of 10 rhOP-1 patients and 8 of 10 control patients. Radiostereometric analysis did not show any significant differences between the groups.
Vaccaro and coauthors (2005)28 compared OP-1 with autograft in a series of patients with degenerative spondylolisthesis (no more than 50% slip) undergoing decompression and noninstrumented posterolateral fusion. This multicenter trial enrolled 36 patients at five centers and randomized them in a 2:1 ratio (OP-1/control ratio) such that 24 received OP-1 and 12 received iliac crest autograft. OP-1 patients were given 3.5 mg rhOP-1 with 1 g bovine bone collagen and 200 mg carboxymethylcellulose on each side of the fusion (total dose, 7 mg).
Kanayama and colleagues (2006)26 also evaluated the use of OP-1 in patients with degenerative spondylolisthesis (no more than 25% slip). These patients underwent pedicle screw instrumentation and posterolateral fusion. Twenty patients were enrolled in this study, and 10 were randomized to receive 3.5 mg OP-1, type I bovine collage, and carboxymethylcellulose per side (total dose, 7 mg). The other 10 patients randomized to control treatment received 5 g HA/TCP mixed with locally harvested autograft bone for each side of the fusion. Clinical and radiographic assessments were made at 12 months, and surgical exploration for fusion and hardware removal was planned when radiographic fusion criteria were met.
SUMMARY
The radiographic fusion benefit of BMP-2 without a concomitant clinical benefit illustrates the well-known concept of discrepancy between physician-based outcome measures (fusion) and patient-based measures. It would be reassuring to see corresponding results from both of these outcomes. However, this is not the case. The question then arises, how do we resolve differences between patient-based and physician-based outcomes?
The clinical benefit of a definite fusion mass is controversial. Randomized trials of spinal fusions with and without instrumentation found increased rates of fusion but no differences in clinical improvement.29,30 However, in a study of patients who had noninstrumented fusions, clinical outcomes were superior at longer term follow-up for those with solid fusions compared with those who experienced development of pseudarthroses.31 Potentially, clinical benefit may not have been realized by the current BMP studies because of short follow-up periods. Negative clinical outcomes associated with pseudarthroses may not become evident for many years.
From an economic consideration, with a safety profile free from adverse events, and a price lower than the cost-equivalent of donor-site morbidity, the use of BMPs may be justifiable. Economic evaluation performed from the payer’s perspective (i.e., not accounting for health-related quality of life or lost productivity) has suggested that with obviating donor-site complications and considering other cost offsets, a 3.6% increase in fusion success rate with a $3000 price for BMP would be all that is required to achieve cost neutrality over iliac crest autograft.32 The authors also suggest that economic analyses accounting from a societal perspective (i.e., accounting for health-related quality of life and lost productivity) would require smaller increases in fusion success to achieve similar cost neutrality.
Our recommendations are summarized in Table 9-4. All of the RCTs were found to be of lesser quality (Level II) with regard to adequacy of random allocation, how patients were accounted for, and blinding to intervention.
Furthermore, in patients considered high risk for nonunion (heavy smokers, revision surgery, rheumatoid arthritis, other medical comorbidities, etc.), marginal increases in fusion rates of BMP over autograft, even in the absence of clinical improvements, may justify its use.33
1 Bambakidis NC, Feiz-Erfan I, Klopfenstein JD, et al. Indications for surgical fusion of the cervical and lumbar motion segment. Spine. 2005;30:S2-S6.
2 Weinstein JN, Lurie JD, Olson PR, et al. United States’ trends and regional variations in lumbar spine surgery: 1992-2003. Spine. 2006;31:2707-2714.
3 Katz JN. Lumbar spinal fusion. Surgical rates, costs, and complications. Spine. 1995;20:78S-83S.
4 Martin BI, Mirza SK, Comstock BA, et al. Reoperation rates following lumbar spine surgery and the influence of spinal fusion procedures. Spine. 2007;32:382-387.
5 Hu RW, Jaglal S, Axcell T, et al. A population-based study of reoperations after back surgery. Spine. 1997;22:2265-2271.
6 Lee C, Dorcil J, Radomisli TE. Nonunion of the spine: A review. Clin Orthop. 2004;419:71-75.
7 Robertson PA, Wray AC. Natural history of posterior iliac crest bone graft donation for spinal surgery: A prospective analysis of morbidity. Spine. 2001;26:1473-1476.
8 Goulet JA, Senunas LE, DeSilva GL, et al. Autogenous iliac crest bone grafts. Complications and functional assessment. Clin Orthop Relat Res. 1997;339:76-81.
9 Sasso RC, LeHuec JC, Shaffrey C, et al. Iliac crest bone graft donor site pain after anterior lumbar interbody fusion: A prospective patient satisfaction outcome assessment. J Spinal Disord Tech. 2005;18(suppl):S77-S81.
10 Urist MR. Bone: Formation by autoinduction. Science. 1965;150:893-899.
11 Urist MR, Silverman BF, Buring K, et al. The bone induction principle. Clin Orthop. 1967;53:243-283.
12 Cheng H, Jiang W, Phillips FM, et al. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs). J Bone Joint Surg Am. 2003;85:1544-1552.
13 Sandhu HS. Bone morphogenetic proteins and spinal surgery. Spine. 2003;28:S64-S73.
14 Mummaneni PV, Pan J, Haid RW, et al. Contribution of recombinant human bone morphogenetic protein-2 to the rapid creation of interbody fusion when used in transforaminal lumbar interbody fusion: A preliminary report. J Neurosurg Spine. 2004;1:19-23.
15 Boden SD, Zdeblick TA, Sandhu HS, et al. The use of rhBMP-2 in interbody fusion cages. Definitive evidence of osteoinduction in humans: A preliminary report. Spine. 2000;25:376-381.
16 Boden SD, Kang J, Sandhu HS, et al. Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans. A prospective, randomized clinical pilot trial: 2002 Volvo Award in Clinical Studies. Spine. 2002;27:2662-2673.
17 Burkus JK, Gornet MF, Dickman CA, et al. Anterior lumbar interbody fusion using rhBMP-2 with tapered interbody cages. J Spinal Disord Tech. 2002;15:337-349.
18 Burkus JK, Transfeldt EE, Kitchel SH, et al. Clinical and radiographic outcomes of anterior lumbar interbody fusion using recombinant human bone morphogenetic protein-2. Spine. 2002;27:2396-2408.
19 Burkus JK, Dorchak JD, Sanders DL. Radiographic assessment of interbody fusion using recombinant human bone morphogenetic protein type 2. Spine. 2003;28:372-377.
20 Burkus JK, Sandhu HS, Gornet MF, et al. Use of rhBMP-2 in combination with structural cortical allografts: Clinical and radiographic outcomes in anterior lumbar spinal surgery. J Bone Joint Surg Am. 2005;87:1205-1212.
21 Burkus JK, Sandhu HS, Gornet MF. Influence of rhBMP-2 on the healing patterns associated with allograft interbody constructs in comparison with autograft. Spine. 2006;31:775-781.
22 Dimar JR, Glassman SD, Burkus JK, et al. Clinical outcomes and radiographic fusion success at 2 years of single-level instrumented posterolateral fusions with recombinant human bone morphogenetic protein-2/compression resistant matrix versus iliac crest bone graft. Spine. 2006;31:2534-2539.
23 Glassman SD, Dimar JR, Carreon LY, et al. Initial fusion rates with recombinant human bone morphogenetic protein-2/compression resistant matrix and a hydroxyapatite and tricalcium phosphate/collagen carrier in posterolateral spinal fusion. Spine. 2005;30:1694-1698.
24 Haid RW, Branch CL, Alexander JT, et al. Posterior lumbar interbody fusion using recombinant human bone morphogenetic protein type 2 with cylindrical interbody cages. Spine J. 2004;4:527-539.
25 Johnsson R, Stromqvist B, Aspenberg P. Randomized radiostereometric study comparing Osteogenic Protein-1 (BMP-7) and autograft bone in human noninstrumented posterolateral lumbar fusion: 2002 Volvo Award in Clinical Studies. Spine. 2002;27:2654-2661.
26 Kanayama M, Hashimoto T, Shigenobu K, et al. A prospective randomized study of posterolateral lumbar fusion using Osteogenic Protein-1 (OP-1) versus local autograft with ceramic bone substitute: Emphasis of surgical exploration and histologic assessment. Spine. 2006;31:1067-1074.
27 Vaccaro AR, Patel T, Fischgrund J, et al. A pilot study evaluating the safety and efficacy of OP-1 Putty (rhBMP-7) as a replacement for iliac crest autograft in posterolateral lumbar arthrodesis for degenerative spondylolisthesis. Spine. 2004;29:1885-1892.
28 Vaccaro AR, Anderson DG, Patel T, et al. Comparison of OP-1 Putty (rhBMP-7) to iliac crest autograft for posterolateral lumbar arthrodesis: A minimum 2-year follow-up pilot study. Spine. 2005;30:2709-2716.
29 Fischgrund JS, Mackay M, Herkowitz HN, et al. Degenerative lumbar spondylolisthesis with spinal stenosis: A prospective, randomized study comparing decompressive laminectomy and arthrodesis with and without spinal instrumentation. Spine. 1997;22:2807-2812.
30 Thomsen K, Christensen FB, Eiskjaer SP, et al. The effect of pedicle screw instrumentation on functional outcome and fusion rates in posterolateral lumbar spinal fusion: A prospective, randomized clinical study. Spine. 1997;22:2813-2822.
31 Kornblum MB, Fischgrund JS, Herkowitz HN, et al. Degenerative lumbar spondylolisthesis with spinal stenosis: A prospective long-term study comparing fusion and pseudarthrosis. Spine. 2004;29:726-733.
32 Ackerman SJ, Mafilios MS, Polly DWJr. Economic evaluation of bone morphogenetic protein versus autogenous iliac crest bone graft in single-level anterior lumbar fusion: An evidence-based modeling approach. Spine. 2002;27:S94-S99.
33 Govender PV, Rampersaud YR, Rickards L, et al. Use of osteogenic protein-1 in spinal fusion: Literature review and preliminary results in a prospective series of high-risk cases. Neurosurg Focus. 2002;13:e4.