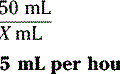CHAPTER 5 Dispensing
I. Definitions and Purpose
A. Dispensing is the physical act of giving, providing, or delivering a drug, chemical, device, or medication for later oral ingestion, insertion, application, injection, or other use.
B. The goal of dispensing is to select and dispense medications in a manner that promotes safe and effective use.
1. Use the National Drug Code (NDC) number and other attributes to identify the correct drug product.
a. The key identifier when selecting a product in the U.S. is the NDC number, which is unique to every drug product.
b. NDC is an 11-digit, three-segment number. The format for all medications follows 55555-4444-22. The first segment of five numbers is a labeler code assigned by the Food and Drug Administration (FDA). A labeler is any firm that manufactures, repackages, or distributes a drug product. The second segment of four numbers is the product code, which indicates a specific strength, dosage form, and formulation for a particular product. Lastly, the third segment of two numbers identifies the package size.
2. Use barcode technology: Newer systems can include barcode technology that can scan for the appropriate product and even prevent dispensing unless the correct product is selected. This practice is used in hospitals and retail settings.
4. Check for drug interactions. When checking for interactions, a pharmacist must review the patient’s medication, evaluate and consider the indication, consider the patient’s age and hepatic/renal function, and whether or not the patient is pregnant. These factors may alter drug therapy.
b. Many pharmacy computer systems will have a drug-interaction screening software program in place. The integrated applications are usually provided through vendors such as First DataBank. As each prescription is filled, the system automatically checks the medication against other medications the patient is taking. However, this system is not always accurate because some people get prescriptions filled at multiple pharmacies and the computers are not on the same network. Ask patients about their use of all prescriptions, over-the-counter (OTC) medications, herbal remedies, vitamins, minerals, and other supplements. Determine if the patient has any known allergies.
c. There are other resources available to check for drug interactions including Clinical Pharmacology, Facts and Comparisons, Micromedex, and many others.
d. Herb-drug interactions are a concern due to uncertainty as to how herbs and supplements will interfere with other medication. Resources and data are limited in this area.
1) An example of an herb-drug interaction is St. John’s wort and cyclosporine. The mechanism for this interaction is proposed to be induction of cytochrome P-450 enzymes by St. John’s wort.
2) It is important to counsel patients on the possibility of food-drug interactions. Grapefruit or grapefruit juice has the potential to alter the effects of various medications, including antiarrhythmic agents, immunosuppressive agents, statins, and calcium channel blockers. The interaction is likely the result of inhibition of intestinal or liver metabolism by cytochrome P-450.
5. Identify and verify drugs by their generic, brand, and/or common names. Most drugs have several names: a chemical name, a generic name, and a brand name. For example: [R-(R*, R*)]-2-(4-fluorophenyl)-ß, δ-dihydroxy-5-(1-methylethyl)-3-phenyl-4-[(phenylamino) carbonyl]-1H-pyrrole-1-heptanoic acid, calcium salt (2:1) trihydrate is the chemical name for atorvastatin calcium, the generic name for Lipitor®.
a. Generic products contain the same active ingredient but are not likely to contain the same excipients (inactive ingredients). Generic drugs may differ in shape, scoring, configuration, release mechanisms, packaging, colors, flavors, and preservatives from the brand name product. The generic version delivers the same amount of its active ingredient and must have the same dosage form, safety, strength, route of administration, and conditions of use as the innovator/brand name product. Generic drugs are subject to the same FDA standards as all drugs and must be manufactured under the same strict standards of the FDA’s good manufacturing practice (GMP) regulations. Generic drugs must pass stringent bioequivalency tests in humans to ensure the generic version delivers the same amount of active ingredient as the innovator/brand equivalent.
b. Excipients, or inactive ingredients in drug products, include fillers, binders, colors, and coatings. An individual may be allergic or sensitive to a specific excipient. Patients should be asked about all of their allergies, not just allergies to medications.
c. Therapeutic interchange is the process of dispensing prescribed medications that are chemically different but are therapeutically similar to the medication prescribed. Normally there are approved written guidelines or protocols in a formulary system.
1) Therapeutic interchange is common in the hospital setting. For example, a doctor may prescribe esomeprazole (Nexium®) 40 mg, but the hospital pharmacy substitutes the preferred drug, pantoprazole (Protonix®). However, this practice varies from institution to institution and state to state. Some states do not allow any therapeutic interchange unless the prescriber is contacted. Other states do not address the issue at all.
d. Generic interchange is the process of dispensing a medication produced by another manufacturer that is the exact same chemical entity as the brand name prescribed.
1) This practice also varies from state to state. Some use positive formularies, meaning that generics may be dispensed if the drug appears on the formulary. Other states use negative formularies that prohibit generic interchange of selected drugs. Using generic medications saves money because the price can be 30% to 80% less than the brand name.
6. Determine whether a particular drug dosage strength or dosage form is commercially available and whether it is available on a nonprescription basis.
a. Some medications are available as a prescription and an OTC product. Ibuprofen 200 mg (Motrin®, Advil®) is available OTC; ibuprofen 400 mg, 600 mg, and 800 mg are available by prescription. Other medications have become OTC products after previously being a prescription-only product. Cetirizine (Zyrtec®), loratadine (Claritin®), and omeprazole (Prilosec®) are a few of the medications that have made the switch from prescription to OTC.
b. Often there are different routes of administration are available for a therapeutic agent. The route of administration is determined by the therapeutic objective and the properties of the drug used. Each route has advantages and disadvantages, and the administration should be suited to the patient’s needs. The two most common routes of administration are enteral and parenteral.
1) Enteral: Oral is the most common route of administration. It is the easiest, most convenient, and least expensive. There are some disadvantages to the oral route: slower onset of absorption and action; variation in rate and degree of absorption with gastrointestinal contents and motility; cannot be used with nausea and vomiting; cannot be used with patients who are unconscious, have difficulty swallowing, or can take nothing by mouth (NPO). The patient’s ability and willingness to swallow a solid dosage form is also a factor.
2) Rectal: Rectal administration may be used in patients who have difficulty swallowing or have nausea. In this form, the drug is not inactivated by intestinal enzymes if the drug is placed properly in the rectum.
3) Parenteral: Some examples of the parenteral route are intravenous, subcutaneous, and intramuscular. Some drugs must be given by this route to stay in their active form. Insulin glargine (Lantus®) can only be given as an injection because oral administration would break down the medication before absorption could occur. Bioavailability of drugs administered parenterally is usually more rapid, extensive, and predictable. Another advantage is that a parenteral route can be used during emergency therapy when a patient is unable to take medications by mouth.
a) Intravenous is a common parenteral route. Drug absorption is not dependent on the GI tract and the effects are rapid. However, rapid administration may cause hemolysis and other adverse effects.
b) Intramuscular injections permit the administration of more irritating drugs and larger volumes of solutions that cannot be tolerated by other routes.
E. Identify commercially available drug products by their characteristic physical attributes.
1. Imprint codes
a. Imprint codes are used for quick identification of solid dosage forms in drug overdose cases, to identify unknown drug products, and to allow patients to check that they have been dispensed the correct medication. Until 1995, there were no regulations regarding imprint data on solid-dosage forms of medications. Drugs exempt from federal regulations are in Table 5-1.2
b. The FDA only requires drug firms to provide their imprint information, along with their listing forms, to the agency’s Drug Listing Team, where it is entered into a database. The data captured include identifiers such as shape, size, color, imprint code, scoring, and coating. The database also incorporates imprint graphics, which describe a logo that does not consist of conventional characters.
c. For example, tadalafil 10 mg (Cialis®) is a teardrop shaped, yellow tablet imprinted with C 10; tadalafil 20 mg (Cialis®) is a teardrop shaped, yellow tablet imprinted with C 20.
d. The Division of Drug Information can identify oral-dosage drugs based on physical appearance and markings. This service offered by the FDA is free to the American public. Drug-identification inquiries can be sent to the Division of Drug Information via telephone at 888-INFOFDA (888-463-6332), via fax (301-827-4577), or via e-mail (druginfo@cder.fda.gov).
2. Packaging and labeling
a. For some medications, the original package is important to the proper storage of the medication, or to reference manufacturer labeling. For example, nitroglycerin sublingual tablets must be stored in their original, tightly closed, glass bottle because potency can be lost by adsorption if repackaged. Packaging can vary between different strengths or types of medications.
3. OTC medications: The drug facts label format was based on the nutrition facts food label. It uses an easy to read format and includes:
4. Dietary supplements:
a. The FDA regulates dietary supplements (defined by the FDA as being composed only of essential nutrients, such as vitamins, minerals, proteins, herbs, or similar nutritional sources) differently than food or OTC/prescription drug products. Dietary supplement manufacturers do not have to get FDA approval or register their products before producing or selling them. The Dietary Supplement Health and Education Act of 1994 (DSHEA) states that the dietary supplement manufacturer is responsible for ensuring that the supplement is safe before it is marketed. The FDA is responsible for monitoring safety via adverse event reporting and product information. The manufacturers must ensure that the label is truthful and not misleading. Good Manufacturing Processes (GMP), determined by the FDA, must be in place. These govern the preparation, packing, and holding of dietary supplements under conditions that ensure their safety. The manufacturer, however, does not have to prove supplement quality.4
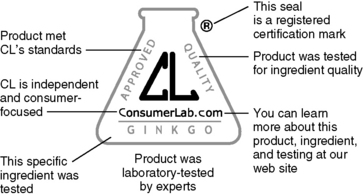
b. There are third-party testing organizations that certify dietary supplements, which include USP, NSF International, and Consumer Lab. Certifications are important because many herbs and supplements have no identifying features on the pill or capsule. Keeping the consumer well informed about herbs and supplements is an important role for a pharmacist.
1) USP is an independent, nongovernmental, nonprofit public health organization that verifies the identity, strength, purity, and quality of dietary supplements. Products that pass USP scrutiny receive a USP Verified mark on the label (Figure 5-1).
F. Interpret and apply pharmacokinetic parameters and quality assurance data to determine bioequivalence among manufactured drug products and identify products for which documented evidence of inequivalence exists.
1. Drug products are considered pharmaceutical equivalents if they contain the same active ingredient, are of the same dosage form and route of administration, and are identical in strength or concentration.
2. Generic drugs must pass stringent pharmacokinetic and bioequivalency tests in humans to be noted as bioequivalent to the innovator/brand product. The tests ensure the generic version delivers the same amount of active ingredient as the innovator/brand equivalent.
3. The Orange Book is published by the FDA and aides in determining bioequivalence between drug products made by different manufacturers.3 The Orange Book uses a two-letter coding system to help determine which drug products are therapeutically equivalent.
a. Codes that begin with A are considered to be therapeutically equivalent to other pharmaceutically equivalent products.
1) Drugs that have no known equivalence or suspected bioequivalence problems are designated AA, AN, AO, AP, or AT, depending on the dosage form.
2) The Orange Book has a list with therapeutic equivalence (TE) evaluations for FDA-approved drug products. TE codes are composed of 2 letters (e.g., AB, AB2, BX). The first letter indicates whether the approved product is therapeutically equivalent to the reference-listed drug (RLD). If it is, then the drug will be designated with the letter “A.” Drug products with a TE code starting with “B” are not considered to be therapeutically equivalent, or there is a problem in bioequivalence. The second letter provides additional information on the basis of the FDA’s evaluations, such as route of administration or formulation.
b. Drug products with codes that begin with B are not considered therapeutically equivalent to other pharmaceutically equivalent products. Drugs fall under this category for one of three reasons: 1) the drug has documented bioequivalence problems or significant potential for problems, 2) quality standards are inadequate or the FDA has insufficient basis to determine therapeutic equivalence, or 3) the drug products are under regulatory review. B* indicates that the drug previously received an A or B code, but new information has been received by the FDA that raises questions regarding therapeutic equivalence, and the FDA will not take a position on the drug until it completes an investigation and review.
G. Identify and communicate appropriate information regarding packaging, storage, handling, administration, and disposal of medications.
a. Packaging
b. Storage
1. Expiration date: The expiration date of a medication as determined by USP states that the expiration date must be no later than the expiration date on the manufacturer’s container or one year from the date the drug is dispensed, whichever date is earlier. The expiration date for certain products such as insulin is different. For example, the expiration date on insulin products is 24 months from the date of manufacture. However, the stability of the insulin is altered once the product is opened and it therefore bears a new expiration date. The expiration information can be found in the product’s package insert. For example, insulin glargine (Lantus®) and other insulin vials should discarded 28 days after the product is opened. Other drug products may also have new expiration dates when opened. For example, Latanoprost (Xalatan®) needs to be stored in the refrigerator until first use, then may be stored at room temperature for 6 weeks.
c. Handling
d. Administration
1. When a medication is dispensed to the patient, the prescription label must have specific information*:
2. Most medications are to be discarded in the trash, not flushed down the toilet. This should be done by taking the medications out of their original container and mixing with an undesirable substance such as coffee grounds or cat litter. However, the Office of National Drug Control Policy states that certain medications (e.g., fentanyl) can be disposed of in the toilet.
H. Identify and describe the use of equipment and apparatus required to administer medications.
a. The pharmacist should be able to describe in patient-appropriate language, how each medication should be used. This is particularly important for describing proper use of inhalers, nebulizers, insulin administration, auto injectors (e.g., EpiPen®), and ophthalmic and otic preparations.
Table 5-1 Exemptions to Imprint Code Regulations
| Drug products used in clinical investigations | When physical characteristics of the drug make it impossible to imprint |
| Drug products intended for use in bioequivalence studies | When the medication is dispensed in a controlled health care setting (i.e., doctor’s office) |
| Prescribed drug products compounded extemporaneously by pharmacists | When the drug is not dispensed to patients for self administration. |
| Drugs classified as radiopharmaceutical drug products |
Kiliany B.J., Kremzner M., Nelson T. The evolution of imprint identification, Pharm Times. Available at http://www.pharmacytimes.com/issue/pharmacy/2006/2006-03/2006-03-5374 Accessed June 2009
FDA FDA. Electronic orange book. Available at http://www.fda.gov/cder/ob/default.htm Accessed September 2008
FDA FDA. Dietary Supplement Health and Education Act of 1994. Available at http://www.cfsan.fda.gov/~dms/dietsupp.html Accessed September 2008
REVIEW QUESTIONS
(Answers and Rationales on page 324.)
4. A consulting pharmacist in a nursing home is asked by a nurse for advice regarding selegiline and sertraline administration for an 85-year-old patient. The patient has received the following new orders:
5. A 62-year-old patient is transferred from a skilled nursing facility to the emergency department after a fall. The emergency department doctor writes an order for IV meperidine 15 mg/h. During medication reconciliation, the pharmacist notices that the patient has been taking 10 mg selegiline q am.
6. A patient brings in a vial of cloudy NPH insulin. Examination of the medication profile reveals simultaneous use of NPH and regular insulin. Which of the following is the MOST PROBABLE explanation for the cloudy appearance of the NPH insulin?
8. A patient has a prescription for Lansoprazole, which is not on the formulary of his insurance. The pharmacist calls the prescriber to recommend a change to a similar medication that is on the formulary. Which of the following would be the most appropriate recommendation?
9. An uninsured patient has a prescription for Lipitor® 10 mg daily. After discussing the cost of the prescription with the patient, the pharmacist calls the prescriber to recommend a change to a similar medication that is less expensive. Which of the following would be the most appropriate recommendation?
13. A patient who has been seizure free on phenytoin suspension 3.5 mL PO twice a day is now receiving feedings and medication through a nasogastric tube. What would be the most appropriate recommendation to ensure that the patient’s phenytoin level stays at a therapeutic level?
14. Which of the following brand(s) can be used for the treatment of pruritus that is associated with partial biliary obstruction?
22. It is recommended that patients take prazosin just before bedtime to minimize which side effect?
36. Which of the following products is/are not appropriate for a patient taking warfarin (Coumadin®)?
52. A patient has been taking prednisone for 5 days following an exacerbation of asthma symptoms. He begins treatment with cromolyn sodium. True or False: He should immediately stop prednisone with the first dose of cromolyn.
64. A 70-year-old man with renal insufficiency is to be treated with a tetracycline. Which of the following will not accumulate to a great degree in this patient’s blood?
73. Which of the following is the most appropriate treatment for conjunctival herpes simplex virus (HSV) infection?
77. Compared with other NSAIDs, which of the following is a benefit associated with the use of piroxicam?
85. A 65-year-old woman with congestive heart failure and atrial fibrillation is diagnosed with glaucoma. What is the most appropriate topical treatment?
86. What is the rationale for the preferred use of inhaled corticosteroids over oral corticosteroids in the treatment of asthma?
88. True or False: It takes 2 to 6 weeks after initiation of cromolyn sodium to see therapeutic effects for the maintenance treatment of asthma.
89. True or False: Angiotensin-converting enzyme (ACE) inhibitors combined with angiotensin-receptor blocker (ARB) provide greater efficacy then either alone.
90. True or False: Antihypertensive agents of different classes may be combined in patients refractory to single-drug treatment.
93. Which of the following is the most appropriate initial treatment for a patient with newly diagnosed type 2 diabetes?
125. What is the correct dose of cyclobenzaprine for the treatment of pain associated with muscle spasms?
145. Of the following, which amount best represents an initial total daily target for quetiapine in the treatment of schizophrenia?
177. True or False: Clotrimazole is available for administration as an oral troche, topical cream, topical solution, and vaginal cream.
187. Which of the following are the correct contents and quantity of medication contained in each teaspoonful of Tussionex®?
194. True or False: Oxymetazoline nasal spray should be used for at least 2 weeks for the patient to experience relief.
198. Which of the following medications should be avoided in pregnant women due to its properties as an abortifacient?