54 Disorders of Thrombosis and Hemostasis
Etiology And Pathogenesis
Congenital Bleeding Disorders
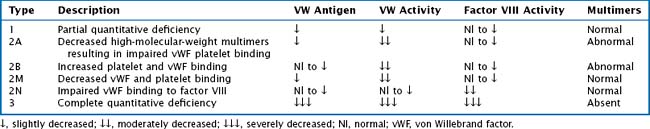
vWF is also a carrier protein for factor VIII; factor VIII unbound to vWF is rapidly degraded. vWD is classified into six types: 1, 2A, 2B, 2M, 2N, and 3. Table 54-1 provides a description and laboratory findings for all types of vWD.
Disseminated Intravascular Coagulation
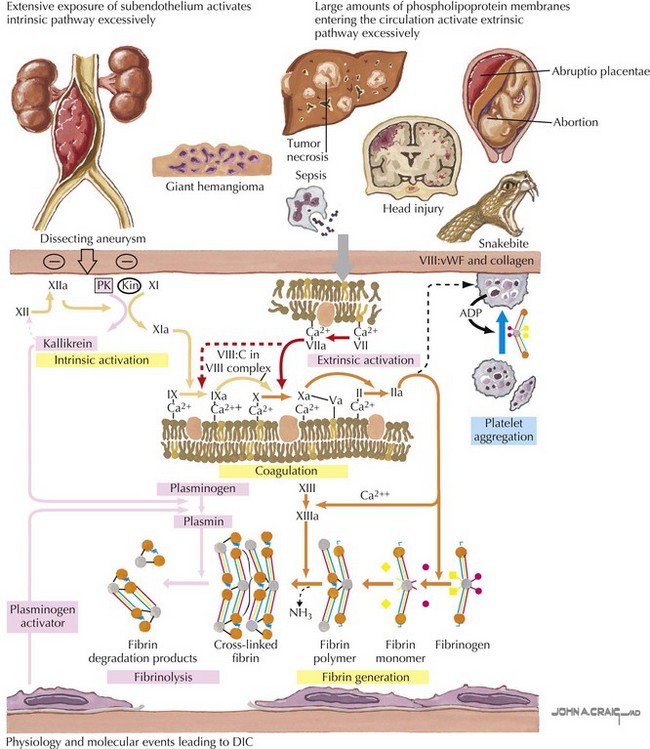
In disseminated intravascular coagulation (DIC), the normal physiology of coagulation is disturbed with resultant bleeding and thrombosis within the microvasculature. DIC is always secondary to a precipitating condition such as infection, tissue injury, malignancy, venom or toxin, or microangiopathic disorders. There is widespread deposition of intravascular fibrin leading to compromised organ function and capillary leak. Continued activation of this system leads to depletion of platelets and clotting factors, resulting in clinical bleeding (Figure 54-1).
Thrombosis
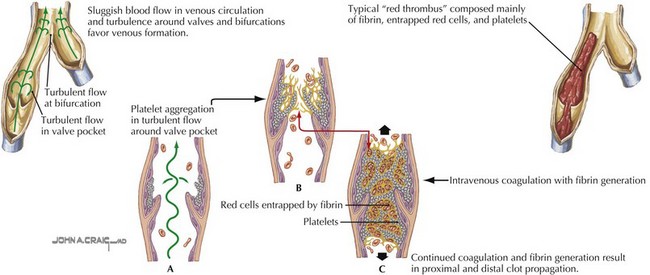
Deep venous thrombosis (DVT) is a consequence of Virchow’s triad of venous stasis, endothelial injury, and a hypercoagulable state. More than 90% of children with DVT have an identifiable prothrombotic risk factor. The most common risk factor is the presence of a central venous catheter (CVC). More than 50% of DVTs in children and more than 80% in neonates are associated with a CVC. Venous stasis in children may be caused by a postoperative state, casting or splinting, or other causes of prolonged immobility. Endothelial injury may be caused by trauma, infection, inflammation, or CVCs. Children may also have an inherited thrombophilia such as factor V Leiden or prothrombin gene mutations, deficiencies in the anticoagulant proteins including C, S, or antithrombin, hyperhomocysteinemia, or an elevated lipoprotein A. Acquired causes of thrombophilia include dehydration, antiphospholipid antibodies, malignancy, inflammatory states, anticoagulant deficiencies from consumption, or loss and exposure to certain medications such as estrogen-containing oral contraceptive pills or L-asparaginase (Figure 54-2).
Clinical Presentation
Congenital Bleeding Disorders
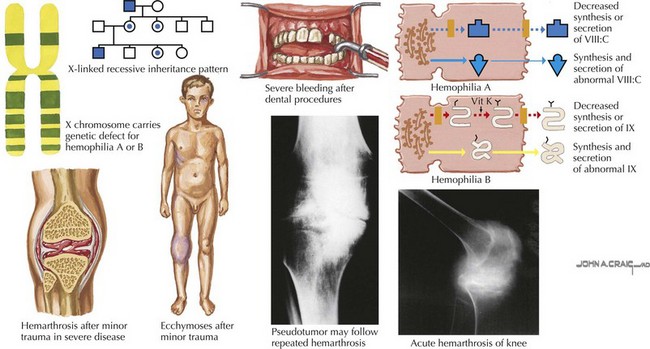
In the newborn period, about 50% of boys with hemophilia have excessive bleeding with circumcision, and 3% to 4% have an intracranial hemorrhage. Muscle bleeds can also occur at the site of intramuscular immunization administration. Hemarthrosis is unusual in an infant and typically occurs after a child is ambulating. Patients with hemarthroses have pain, tenderness, warmth, swelling, and decreased range of motion of the affected joint. Muscle bleeds present with pain and swelling, and any muscle can be affected. Intracranial hemorrhage is the most serious type of bleeding for patients with hemophilia with the highest mortality rate and can be the result of head trauma or spontaneous in patients with severe hemophilia (Figure 54-3).
Evaluation And Management
Thrombosis
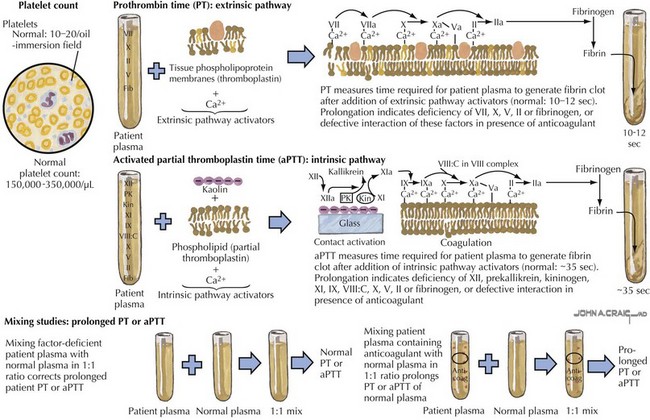
Laboratory studies for evaluation of DVT and PE include a PT and PTT as well as a CBC (Figure 54-4). Renal function should be assessed. A D-dimer can be elevated in the presence of a DVT or PE but is not specific and can be elevated in other clinical settings and therefore is not diagnostic of the presence of thrombosis. A pregnancy test should be obtained in menstruating women. In many cases, it may be appropriate to evaluate a child for a predisposing thrombophilia, including factor V Leiden and prothrombin G20210 mutation analyses, protein C activity, protein S activity, antithrombin, homocysteine, lipoprotein(a) levels, dilute Russell Venom time, anticardiolipin, and anti-β2-glycoprotein antibodies.
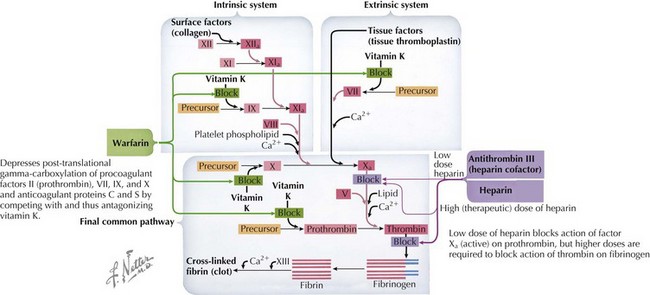
When a DVT or PE is identified, anticoagulation is the definitive treatment but a decision must be made as to whether anticoagulation is appropriate, weighing the risk of bleeding versus the risk of clot propagation, embolization, or both. Acutely, therapy should be initiated with a continuous infusion of unfractionated heparin (UFH) or a subcutaneous administration of a low-molecular-weight heparin (LMWH) (Figure 54-5). Warfarin is an alternative anticoagulant that may be considered for maintenance therapy after adequate anticoagulation has been established with either heparin or a LMWH. The duration of anticoagulation depends on the clinical circumstances, size, site, and resolution of clot, as well as the presence of any thrombophilia.
Dunn AL, Abshire TC. Recent advances in the management of the child who has hemophilia. Hematol Oncol Clin North Am. 2004;18(6):1249-1276. viii
Goldberg NA, Bernard TJ. Venous thromboembolism in children. Pediatr Clin North Am. 2008;55(2):305-322.
Levi M. Disseminated intravascular coagulation. Crit Care Med. 2007;35(9):2191-2195.
Robertson J, Lillicrap D, James PD. von Willebrand Disease. Pediatr Clin North Am. 2008;55:377-392.