Disorders of the Large Intestine
Irritable Bowel Syndrome
Irritable bowel syndrome (IBS) is a chronic non–life-threatening disorder characterized by abdominal pain and alteration in bowel habits. IBS is an extremely common disorder; estimates put the prevalence in the North American population at 10 to 15%, with women affected twice as often as men.1,2 Although only one third of patients who have the clinical syndrome ever seek medical attention, IBS accounts for more than 10% of all visits to primary care physicians and more than 25% of all visits to gastroenterologists. IBS is said to contribute more impairment to quality of life than either diabetes or renal failure.
Principles of Disease
Psychiatric conditions often coexist with IBS, ranging from generalized anxiety disorder to major depression. IBS has been associated with fibromyalgia, chronic fatigue syndrome, and chronic pelvic pain.2 An association with previous sexual abuse also has been reported. In women, symptoms often are related to the menstrual cycle, suggesting a hormonal influence. A familial predisposition for the symptoms of IBS has been described, suggesting a genetic component. Bacterial overgrowth and infection have also been associated with IBS.2
Clinical Features
The diagnosis of IBS is defined by clinical criteria in a patient whose symptoms have no other organic explanation. Several sets of clinical criteria have been published, one set of which is the Rome III criteria (Box 95-1). Patients with IBS experience symptoms intermittently, with the typical patient averaging symptoms on 1 of every 3 days. Complaints include abdominal pain, bloating, and constipation or diarrhea. Pain typically is relieved with defecation; pain that persists suggests another diagnosis. A mucoid discharge from the rectum often accompanies diarrhea. Upper gastrointestinal symptoms such as nausea and dyspepsia can also occur. Patients may come to the ED with an exacerbation of their previous symptoms or with a new abdominal complaint and often report that they are undergoing a period of stress. Physical examination may reveal either mild focal abdominal tenderness that can vary in location, or diffuse tenderness. IBS is subdivided into three categories: constipation-predominant (IBS-C), diarrhea-predominant (IBS-D), and mixed pattern (IBS-M).2
Pain that is progressive or is associated with anorexia or significant abdominal tenderness suggests an alternate diagnosis. Signs and symptoms that point away from the diagnosis of IBS (alarm symptoms) include onset of symptoms after age 50, unintentional weight loss, anorexia, bloody stools, nocturnal diarrhea, or a family history of significant colon disease.2 In the absence of symptoms suggesting another diagnosis, the clinical criteria have a specificity ranging from 87 to 100%, although sensitivity may be only 60%. In patients determined to have IBS through correct use of the clinical criteria, follow-up evaluation over many years rarely leads to a change in the diagnosis.2
A final diagnosis of IBS usually is made in the primary care setting and not in the ED. Studies that are sometimes performed in the evaluation for IBS may include a complete blood count, thyroid studies, stool examination for ova and parasites, evaluation for lactose intolerance, and possibly lower gastrointestinal endoscopy, although current approaches try to limit testing in cases in which the clinical picture is clearly IBS.3 The ED evaluation seeks to exclude other, more urgent causes for the patient’s symptoms. In this setting, testing for pancreatitis, hepatitis, biliary colic, or urologic disorders, including urolithiasis, may be appropriate as indicated by the pattern of the presenting complaints.
Differential Considerations
The differential diagnosis of symptomatic IBS depends on the predominant symptoms and includes a host of disorders (Box 95-2). Patients may report pain, constipation, or diarrhea or any combination of the three.
Management
Not all patients with IBS require treatment. It is recommended that therapy be initiated only if symptoms diminish the quality of life.1 Because no curative therapy is available, treatment is directed toward the relief of symptoms. Diet, behavioral, and pharmacologic therapies all are used in IBS. Specific therapy will be determined by the type of IBS: IBS-C, IBS-D, or IBS-M. Dietary suggestions include a low-fat diet, reduced nondigestible sugars, and avoidance of gas-forming foods, although none of these has any proven benefit. Fiber supplementation may aid IBS-C.
Medications with antispasmodic activity, such as anticholinergics and calcium channel blockers, are used for abdominal cramping, and peripherally acting narcotics, such as loperamide, are used to reduce diarrhea. Osmotic laxatives such as lactulose sometimes are helpful in constipation. Tricyclic antidepressants have been effective in certain classes of patients with IBS. Serotonin receptor antagonists, serotonin receptor agonists, chloride channel activators, and prokinetic agents also are used (Box 95-3), but the serotonin receptor drugs have significant side effects, including adverse cardiovascular events and ischemic colitis, and thus are reserved for patients with severe symptoms unresponsive to other agents. Nonsteroidal anti-inflammatory drugs (NSAIDs) may worsen symptoms.3
Diverticular Disease
Diverticular disease is an affliction of middle age that seems to be a direct consequence of the diet of modern Western civilization. Diverticular disease was virtually unknown in the Western world before the 20th century and is still rare in other cultures. In 1925 only 9% of people older than 50 years of age in the United States had diverticula; by 1968 the percentage had increased to 30%.4 Today it is estimated that 5 to 10% of people older than 45 years and 80% of people older than 85 years have diverticula. Diverticula are less common in people younger than age 40, representing only approximately 2 to 5% of all patients with the disease. The proliferation of this disease was coincident with the invention and widespread use of the flour rolling mill, which removes the fiber-containing outer part of the wheat kernel. This coincidence has prompted the labeling of diverticulosis as a “modern deficiency disease,” which is supported by the fact that adding fiber back into the diet seems to be protective against the development of diverticulosis.4 In rural Africa and Asia, where the diet is high in fiber, diverticular disease is virtually unknown. Over the last decade the incidence of diverticulitis has increased in the United States, especially in people aged 18 to 44.5
Diverticulosis denotes the presence of diverticula in the colon. Most patients with this condition are asymptomatic. Diverticulitis denotes inflammation of diverticular tissue, which is usually painful. It is estimated that 10 to 25% of patients with diverticulosis will develop diverticulitis.5 Complicated diverticulitis is defined by the presence of more extensive disease, including abscess formation, peritonitis, intestinal obstruction, or fistula formation.
Principles of Disease
The wall of the colon is penetrated at regular intervals by blood vessels, collectively known as the vasa recta, that supply the internal intestinal layers. The site of vessel penetration is apparently the weakest part of the colon wall because it is at these sites that diverticula form. Although the exact pathogenic mechanism is unknown, the current theory is that diverticula form in response to increased intracolonic pressures generated when the colon is processing smaller, non–fiber-containing stools. Patients with diverticular disease exhibit normal resting colonic pressure but higher peak pressure than those without diverticular disease5; higher peak pressures lead to a herniation of colonic mucosa through the intestinal wall at the vasa recta, creating small, saclike appendages. These appendages (diverticula) typically measure 5 to 10 mm in diameter but on rare occasions can grow into huge sacs measuring many centimeters across (giant colonic diverticula). Diverticula are generally asymptomatic; symptoms are believed to develop when diverticula become obstructed, presumably with inspissated stool. When obstruction occurs, inflammation sets in and microperforations of the sac develop, resulting in inflammation of pericolonic structures and abdominal pain.
In the Western world, most diverticular disease occurs in the left colon, usually the sigmoid. This is not the case in Japan, where right-sided diverticular disease is more common. Japanese-Hawaiians consuming a low-fiber Western diet have a significantly increased incidence of diverticular disease, but it remains in the right colon.4 This finding suggests that although diet plays a significant role in the formation of diverticula, the location of diverticula is genetically determined.
Diverticula can also bleed, presumably from erosion into the mucosal wall by dried stool trapped in the diverticular sac. Severe hemorrhage occurs in 3 to 5% of all patients with diverticulosis and accounts for approximately 40% of all instances of lower gastrointestinal hemorrhage.4 Bleeding notably occurs in the absence of inflammation and typically is painless. NSAID use is associated with this complication.
Clinical Features
Although approximately 75% remain asymptomatic throughout their lifetime,4,6 patients with diverticulosis sometimes have nonspecific abdominal complaints including bloating, crampy pain, excessive gas, or a change in bowel habits.7 Diverticulitis will develop in approximately 10 to 30% of patients with diverticulosis.
Diverticulitis
Special care must be taken with elders or immunocompromised patients because clinical signs and symptoms are much less dramatic, even with more severe disease. Perforation is more common in these patients (up to 40%), manifests with less significant findings, and carries a high mortality rate.5
Diagnostic Strategies
Complicated Diverticulitis
Abdominal Computed Tomography.: Abdominal CT is the preferred method of evaluation in complicated diverticulitis. CT has the advantage of evaluating the colon and the structures around it, so it can facilitate the diagnosis of diverticulitis and simultaneous evaluation of the extent of disease. CT can also be used to guide percutaneous drainage of diverticular abscesses. Findings on CT consistent with diverticulitis include the presence of diverticula, inflammation of pericolonic fat, thickening of the bowel wall to more than 4 mm, free abdominal air, and abscesses.6,8 CT also can help make an alternative diagnosis when diverticulitis is absent. CT is relatively noninvasive and is well tolerated by ill patients. Sensitivity and specificity for diverticulitis range from 69 to 95% and from 75 to 100%, respectively.8 Negative findings on CT scan cannot absolutely exclude diverticulitis. Small abscesses within the colon or mesocolon can be missed, as can the diverticula themselves. It may also be difficult to differentiate between carcinoma and diverticulitis on CT scan. Marked bowel wall thickening associated with diverticulitis looks like cancer; contrast enema or endoscopy may be required to differentiate the two.
Barium Enema.: Although double-contrast barium examination is the standard for the diagnosis of asymptomatic diverticula, it should be avoided in the setting of diverticulitis. The potential for preexisting occult perforation and subsequent risk of barium peritonitis limits its usefulness. Barium enema may be used after the acute episode to exclude the diagnosis of carcinoma.
Water-Soluble Contrast Enema.: Although now rarely used, a water-soluble contrast enema is the preferred method of imaging if a contrast enema study is needed in the acute setting. Water-soluble contrast material shows less detail than barium, but this modality is still useful. Findings consistent with diverticulitis include the presence of diverticula along with extravasation of contrast material into an abscess cavity or into the peritoneum. This study can also show a fistula or evidence of compression of the colon by an extrinsic mass. Because contrast material usually collects only in the intestinal lumen, contrast enemas give less information than CT about the extent of disease outside of the colon.
Ultrasonography.: Ultrasound examination can detect various pathologic features characteristic of diverticulitis, including fluid collections around the colon, thickened hypoechoic bowel wall, or hyperechoicity adjacent to the bowel wall that suggests pericolonic inflammation. Tenderness to palpation over an abnormal-appearing colon suggests that the colon is the source of the patient’s pain. Diverticula can occasionally be visualized by ultrasound examination. As is often the case, the sensitivity of ultrasound imaging for these findings varies significantly with the experience of the operator. Because gas interferes with ultrasound imaging, adequate visualization of the bowel can be a problem. Currently, the role for ultrasonography in the evaluation of diverticulitis is not well defined.
Endoscopy.: Endoscopy is limited in the acute setting by its more invasive nature, the risk of perforation, and the logistics of arranging this procedure emergently.9,10 Although the endoscope affords visualization of diverticula and other pathologic processes within the lumen of the colon, it does not permit evaluation of the extent of extracolonic disease.
Management
All patients diagnosed with diverticulosis should be placed on a high-fiber diet, which has been shown to reduce abdominal symptoms and recurrent bouts of diverticulitis. The common advice to avoid foods that may obstruct diverticula, such as nuts, small seeds, and popcorn, has recently been discredited.5
Uncomplicated Diverticulitis
Uncomplicated diverticulitis in an immunocompetent, nonelderly patient can be managed on an outpatient basis with oral antibiotics11 (Box 95-4). Coverage for gram-negative aerobic and anaerobic bacteria is required. Patients may be placed on a liquid diet for comfort, although this is not mandatory. NSAIDs or narcotics are appropriate for pain control, but many experts recommend avoiding morphine sulfate because it increases intraintestinal pressure, which can theoretically precipitate perforation. A high-fiber diet prevents recurrent diverticulitis for 5 years in 70% of patients.
Patients with significant comorbid illness or other problems, including inability to tolerate oral liquids, poor social support, and inability to comply with follow-up in a reasonable time frame (2 to 3 days), should be considered for hospital admission. Hospitalized patients generally are treated with intravenous antibiotics (Box 95-5) and placed on bowel rest, although patients hospitalized for psychosocial reasons can be treated with oral medications.
Complicated Diverticulitis
Patients with complicated diverticulitis should be admitted to the hospital and treated with intravenous antibiotics and bowel rest. Emergent surgical intervention is indicated for all patients with peritonitis or perforation. Newer techniques that use a laparoscopic approach with lavage and biologic glue have supplanted open surgical techniques in some patients.12 Continuing clinical decline, sepsis resistant to medical management, or a high level of suspicion for carcinoma warrants urgent surgical consultation.6 Small abscesses (less than 5 cm in diameter) are treated with intravenous antibiotics alone (see Box 95-5), whereas larger abscesses are drained either percutaneously with imaging guidance or surgically.13 Bowel obstruction during an attack of diverticulitis usually is self-limited and resolves with conservative management. Chronic recurrent diverticulitis can result in stricture, necessitating surgical intervention. Fistulae are usually repaired surgically but are typically not emergent.5 A significantly dilated cecum (to greater than 10 cm in diameter) or gas in the bowel wall should prompt early consultation with a surgeon about the possibility of bowel necrosis and impending perforation.
Definitive Management
It is not known whether medical or dietary treatment is of benefit in diverticulitis. The only proven way to eradicate diverticula is to remove the affected segment of colon surgically. Most patients who recover from their first attack of diverticulitis are likely to remain asymptomatic for many years. With subsequent attacks, the likelihood of recurrence increases. Elective resection of diverticula generally is reserved for patients who have had more than one attack of diverticulitis. According to some experts, younger patients (i.e., younger than 40 years) should undergo elective resection after their first bout of diverticulitis because of concerns about a higher risk for a second attack, but this recommendation is controversial, with recent evidence suggesting that outcomes are better without surgery.6 Most resections can be done laparoscopically with a single-stage procedure (no colostomy). Estimates on recurrence of diverticular disease after resection vary, ranging from 3 to 27%.7,14
Disposition
Nonelderly and immunocompetent patients may be sent home on oral antibiotics with referral for follow-up evaluation in 2 to 3 days to determine the success of treatment. Patients are cautioned to return to the ED if their condition worsens. Patients not significantly improved at follow-up should undergo diagnostic imaging to look for an abscess and should be hospitalized for intravenous antibiotic therapy. Of patients treated medically for their first attack of diverticulitis, 95% remain symptom-free for the next 2 years, and 80 to 90% remain symptom-free permanently.14 Patients with recurrent episodes of diverticulitis should be referred to a surgeon for outpatient consultation for elective resection. All patients should undergo an evaluation for colon cancer when the acute episode has resolved because the incidence of coexistent cancer has been reported to be as high as 9%.9
Complicated Diverticulitis
All patients require hospitalization for intravenous antibiotic therapy and bowel rest. Most patients (65-85%) recover with medical management alone; the rest require surgical intervention. Outcomes generally are good, with mortality rates ranging from 1 to 6% for all patients, increasing to 12 to 18% for patients requiring surgery.4
Large Bowel Obstruction
Large bowel obstruction (LBO) is much less common than small bowel obstruction, but LBO is a more ominous condition because it is frequently associated with malignant disease. One half of all operative cases involving LBO in the United States are the result of colorectal cancer, and up to 20% of patients with colon cancer will develop acute obstruction.15 Adhesions, a common cause of small bowel obstruction, cause only a small number of LBOs. Other causes of LBO include volvulus, diverticular disease, fecal impaction, strictures (often related to inflammatory bowel disease [IBD] or chronic colon ischemia), adhesions, hernia, and pseudo-obstruction. Most causes are managed surgically, but pseudo-obstruction responds well to medical management alone.
Principles of Disease
Pseudo-obstruction, also called Ogilvie‘s syndrome, occurs through a completely different mechanism. Pseudo-obstruction is defined as LBO in which no obstructing lesion can be identified. This condition usually is found in patients with significant acute comorbid conditions.16 Patients typically have a history of significant spine or retroperitoneal trauma, severe electrolyte disturbances, or narcotic exposure. Although the exact mechanism is unknown, it is believed to involve malfunction of autonomic control of the bowel. Normal balance between parasympathetic and sympathetic input is disrupted, resulting in changes in motility that lead to obstruction. The pathophysiologic changes observed with pseudo-obstruction are the same as those described for mechanical obstruction.
Diagnostic Strategies
Plain Radiography
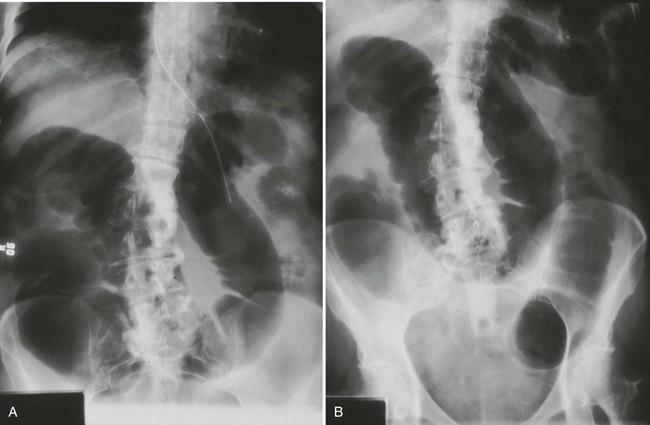
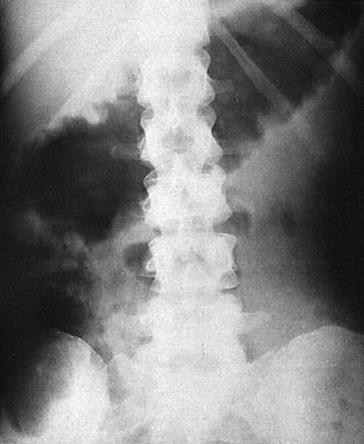
A distended colon is the hallmark of LBO (Fig. 95-1), although small bowel may be distended as well if the ileocecal valve is incompetent. In some cases, gas-filled small bowel may obscure visualization of the colon, leading to the misdiagnosis of small bowel obstruction. An abrupt cutoff at the distal end of the obstructed colonic segment suggests a possible pseudo-obstruction. A cecal diameter exceeding 12 cm is of concern because this finding is associated with a higher risk of perforation.16 The actual location and cause of the LBO are usually not evident on plain films.
Management
Management in the ED is directed at relief of symptoms. Rehydration, electrolyte replacement, and pain management are the first concerns. Gastric decompression with a nasogastric tube may be helpful in cases in which vomiting is prominent or when there is evidence of significant fluid or gas buildup in the small intestine. No additional fluid or solids should be administered by mouth. Antibiotics are indicated if gangrene or perforation is suspected (see Box 95-5). Definitive management depends on the cause of the obstruction. Select diverticular abscesses may be drained percutaneously, whereas a sigmoid volvulus or pseudo-obstruction can be decompressed endoscopically. Diverticular disease and sigmoid volvulus eventually necessitate an elective surgical procedure to prevent recurrence, although this often can be delayed. Carcinoma, cecal volvulus, strictures, intussusception, adhesions, and hernias are dealt with primarily surgically. In malignant obstruction of the left colon, placement of a stent can be done palliatively or as a bridge to surgery.15
As long as the possibility of perforation is not an immediate concern, pseudo-obstruction is managed for the first 24 hours with bowel rest, hydration, and management of any acute comorbid conditions. If the colon fails to decompress, colonoscopic or pharmacologic intervention (neostigmine) may be attempted, with surgery reserved for refractory cases.16
Volvulus
Volvulus of the colon occurs when a loop of bowel twists and obstructs the intestinal lumen. If severe enough, the twist may involve and compromise the vascular supply to the loop of colon. The incidence of colonic volvulus is 2.65 cases per 100,000 population per year, accounting for 1 to 7% of all LBOs.17 Volvulus occurs in all age groups, but older adults are affected most often, with a mean age of 60 to 70 years. One third of cases in the developed world involve institutionalized patients.17 Most cases are divided roughly equally between the sigmoid colon and the cecum, although volvulus can occur in all other areas of the colon. Sigmoid volvulus typically is a disease of older persons. Mortality rates with sigmoid volvulus exceed 50% in patients with gangrenous bowel. In the absence of gangrenous bowel, the risk of death is less than 5%.17
Principles of Disease
The anatomic requirement for a sigmoid volvulus is a long, redundant section of sigmoid that is attached to the abdominal wall by a narrow strip of mesentery. The narrow attachment allows the mesentery to twist on itself, thereby obstructing the intestinal lumen. It is not clear whether this is a congenital condition or occurs as part of the aging process, but the fact that sigmoid volvulus rarely occurs before the third decade suggests it is an acquired condition.17 After the colon twists on itself, the proximal colon continues to force gas and liquid into the obstructed segment, causing a sometimes massive dilation of the distal colon. Significant electrolyte disturbances can occur secondary to third spacing, and respiratory compromise occasionally occurs from massive abdominal distention. If the condition is left untreated, the vascular supply can become compromised, resulting in gangrene and perforation. In one series, approximately 10% of patients developed gangrene of the affected segment.18 The exact precipitator of an acute episode of volvulus is not clear. A high-fiber diet has been implicated, because a significant increase in the disease is noted in patients who are switched to a high-fiber diet. Chronic constipation has been associated with volvulus, but it is unclear how the two conditions are related. Residents of long-term care facilities and patients with neurologic or psychiatric diseases also are predisposed to sigmoid volvulus, possibly as a result of alterations in colonic motility. No association with previous surgery has been observed.
Cecal Volvulus
As in sigmoid volvulus, a mobile segment of cecum is a prerequisite to the disease. This mobility seems to be a result of a congenitally incomplete fusion of the cecal mesentery to the posterior abdominal wall. Cadaver studies show that 11 to 25% of the adult population have ceca that are mobile enough to cause torsion.19 In 10% of the cases, cecal volvulus is a result of a variant called cecal bascule, in which the cecum does not twist but merely folds over on itself; symptoms and management are the same. The tendency for cecal volvulus may be related to “maneuvering room” available for the colon within the abdomen. Women seem to be at a higher risk for cecal volvulus during pregnancy, presumably because of crowding of the abdominal cavity by the enlarged uterus. The condition is still rare, however, occurring in approximately one per million pregnancies.20 Gangrene of the bowel is common with cecal volvulus, occurring in 20% of patients.18
Clinical Features
The hallmark of sigmoid volvulus is the triad of abdominal pain, distention, and constipation. The extent to which the sigmoid colon can twist on itself is recognized to vary, so the presentation of sigmoid volvulus will vary accordingly, from subtle to dramatic. The clinical picture may range from one of minor abdominal discomfort that has been present for many days to an acute onset of severe abdominal pain associated with gross abdominal distention and unstable vital signs. Sometimes the diagnosis of sigmoid volvulus is not made until the patient has been hospitalized for some time. In many instances the history may be suggestive of previous episodes of volvulus that self-reduced. Diarrhea may be present in cases with incomplete obstruction.17
Diagnostic Strategies
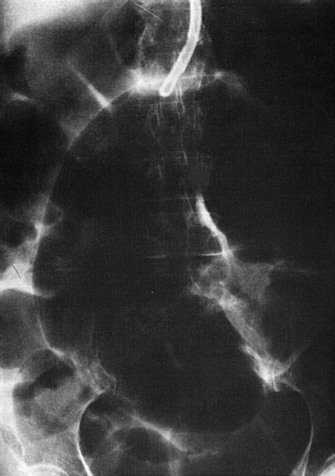
The diagnosis of sigmoid volvulus can be made on the basis of findings on plain radiographs in most cases. A grossly distended loop of colon lacking haustral markings is typical and is seen just as often on the right side of the abdomen as on the left (Fig. 95-2). The bowel may have the appearance of a “bent inner tube.” Free air may be seen on an upright chest radiograph or lateral decubitus radiograph of the abdomen in patients who have a perforation. Gas backing up into the rest of the colon may obscure the typical appearance of sigmoid volvulus on plain radiographs, leading to a significant number of nondiagnostic studies. Cecal volvulus and bowel obstruction from other causes may have a similar radiographic appearance. When the diagnosis is in doubt, contrast enema may be helpful. Contrast material fills up the colon to the tapering point of torsion, giving a “bird’s beak” appearance to the column of contrast material (Fig. 95-3). Sigmoidoscopy is diagnostic in many cases, visualizing a spiral sphincter-like twist in the colonic mucosa.21 CT scan, when used, is also highly accurate, but most diagnoses can be made without it.21,22
Cecal Volvulus
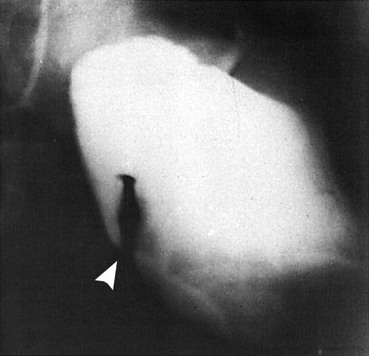
Plain radiographs often are helpful in establishing a diagnosis of cecal volvulus, but the findings are not definitive in 50% of cases. The cecum should be markedly dilated and may contain an air-fluid level. The small bowel often is distended as well. In contrast with the picture in sigmoid volvulus, the distal colon should have a paucity of gas (Fig. 95-4). The classic “coffee bean” sign, a large oval gas shadow with a line down the middle representing bowel bent over on itself, may be seen in the midabdomen. Free air suggests perforation and necessitates emergent surgical consultation. A common mistake is misinterpreting the plain radiograph as showing a sigmoid volvulus. If the diagnosis is unclear, a contrast enema is helpful in showing the site of torsion. Ultrasound imaging generally is unhelpful.20 On CT, a mesocolon “whirl sign” may be seen, indicating a twisted segment of mesentery.23 In many cases, cecal volvulus is definitively diagnosed only at surgery.18
Management
Although spontaneous reduction of a sigmoid volvulus can occur, it is infrequent enough to mandate a proactive approach to treatment. If clinical evidence of gangrenous bowel is lacking, endoscopic detorsion should be attempted by an experienced operator. With the endoscope the bowel is first examined for any signs of gangrene. If the bowel is healthy, the twisted, obstructed proximal end of the bowel lumen is identified, and a lubricated flexible tube is inserted through the obstruction. With decompression of gas and liquid stool, the bowel is able to undergo self-detorsion. Endoscopic decompression is successful in 50 to 90% of cases.21 If the patient has gangrenous bowel or the volvulus does not respond to endoscopic decompression, surgery is indicated. Recurrence rates are estimated at 60%; as a preventative, elective resection of the redundant sigmoid is recommended after resolution of the acute episode. The mortality rate for sigmoid volvulus is 20% overall and exceeds 50% in the subpopulation of patients with gangrene.
Intussusception
Intussusception in adults is rare, accounting for only 1 to 5% of cases of adult bowel obstruction.24 Most adult intussusceptions (80%) are of the small bowel. Although only 10% of children have a pathologic lesion as the cause of the intussusception, 90% of adults do. In the colon, these lesions are malignant 50 to 80% of the time, as opposed to in the small bowel, in which malignant lesions are present approximately one third of the time.25 In adults, the intussusception is often unsuspected before being revealed on a CT scan or during laparotomy. The condition occurs over a wide variety of ages, with a mean age at presentation of 65 years.26
Principles of Disease
The exact mechanism of intussusception is unknown, but it is believed that a lesion (the “lead point”) changes the motility properties of the intestine, allowing a proximal segment to invaginate into a more distal segment. As peristaltic activity pushes the invaginated segment along with its mesentery and mesenteric blood vessels distally down the bowel, the blood supply to the segment can be compromised, and ischemia may occur. Edema associated with the intussusception can lead to a mechanical obstruction of the bowel. In 8 to 20% of cases no lead point can be identified.24
Clinical Features
Intussusception in adults manifests in one of two patterns. The most common is that of acute partial intestinal obstruction. Less than 20% of intussusceptions cause complete obstruction.27 With this pattern, the typical presenting complaint is abdominal pain. Vomiting, bleeding, and constipation may be present but often are not. The abdomen may be distended, and bowel sounds often are decreased. A mass is seldom palpated; the classic triad of abdominal pain, mass, and heme-positive stools noted in children is rarely found in adults. The second presentation is much more subtle, with intermittent abdominal pain for months or years. The diagnosis usually is made only when the pain becomes unrelenting or has been recurrent enough to prompt imaging.
Diagnostic Strategies
Computed Tomography
Typically used in the evaluation of abdominal pain and bowel obstruction, CT is usually the most useful test in suspected intussusception but may not detect the actual intussusception in as many as one half of cases.25,26
Management
Surgery is required in most cases. ED care is supportive and aimed at optimizing fluid status, recognizing gangrene or perforation, administering antibiotics if compromised bowel is suspected, and securing surgical consultation in the appropriate time frame. Because of the high incidence of malignancy, reduction often is not attempted in adults before surgical exploration.26 Occasionally, intussusception may resolve spontaneously, but an evaluation to exclude a pathologic lead point must still be undertaken.
Inflammatory Bowel Disease
IBD includes two clinically similar but distinct diseases: Crohn’s disease (CD) and ulcerative colitis (UC). Both diseases are characterized by chronic and unpredictable relapsing inflammation of the gastrointestinal tract from causes that have not been definitively identified. Significant morbidity occurs from acute exacerbations of inflammation. It is estimated that more than 1 million people in the United States are affected by IBD.25 Cases are divided approximately equally between CD and UC, with a combined annual incidence of approximately 10 cases per 100,000.29 The long-term management of IBD is a complex, stepwise process that involves multiple medications and surgery. The goals of ED evaluation are to (1) recognize potential new cases of IBD, (2) consider and exclude serious complications in patients with IBD, and (3) identify those patients with IBD who need in-hospital care. Treatment plans are best developed in consultation with a physician experienced in the long-term management of IBD. Although life expectancy is slightly decreased for patients with CD, it is normal for patients with UC.30
Principles of Disease
UC causes inflammation and ulceration throughout the colon and rectum but spares the small intestine. Inflammation is more superficial than that found in CD. Typically the inflammation exists as one continuous lesion originating in the rectum and extending a variable distance into the colon, although more recently cases of discontinuous disease (“skip lesions”) similar to that in CD have been reported in UC.31 The concordance rate between identical twins is low (6 to 14%), suggesting that factors other than genetics are involved in the development of UC.32 Stress can trigger exacerbations, and cigarette smoking has a protective effect, suggesting environmental factors at work. Appendectomy at an early age is protective, suggesting that the immune system may play a role. In animal models of IBD, the disease does not occur in animals that are devoid of normal bowel flora, suggesting that bowel flora are a necessary ingredient for disease.33 One unifying theory is that UC represents a genetic predisposition for the development of an inflammatory reaction to normal intestinal flora—in essence, loss of the normal tolerance to these bacteria.
Crohn’s Disease
The cause of CD is unknown, but genetic, environmental, immunologic, and infectious processes have all been implicated as possible causative or contributory factors.29 Concordance between identical twins is 45 to 50%, suggesting a strong genetic predisposition that is modified by other factors.32 Africans have a low incidence of CD, but African Americans have an incidence similar to that in white Americans.29 The first genetic mutation associated with CD was described in 2001 and is associated with 10 to 20% of cases of CD.31 Although the onset of the disease can occur at any time of life, CD affects primarily young patients, with onset of disease typically in the teens and 20s. Inflammation in CD is deep, involving the entire colonic wall. The disease is not limited to the colon and rectum as it is in UC but may affect any part of the gastrointestinal tract. CD most often involves the distal small intestine and colon and less commonly the esophagus, duodenum, or stomach. Because of the transmural nature of the inflammation, the development of intestinal strictures or fistulae to adjacent organs is a potential complication.
Clinical Features
Typical presenting complaints in patients with IBD include abdominal pain, often crampy, and tenesmus with loose or diarrheal stools. Blood may be present in the stool. Patients with CD may have a history of nocturnal diarrhea, a complaint that helps differentiate CD from patients who have IBS. Weight loss is common. The physical examination may reveal significant abdominal tenderness or an abdominal mass representing an abscess. Patients with CD may have fissures, ulcerated hemorrhoids, strictures, or cutaneous abscesses around the anus. Extraintestinal manifestations include inflammatory conditions of the skin, eyes, joints, spine (ankylosing spondylitis), and liver (primary sclerosing cholangitis).34 In children, growth and sexual development may be affected. Onset of symptoms usually occurs before the age of 30 years,28 although the diagnosis can be difficult to make in the early stages.
Patients often come to the ED with a known diagnosis of IBD and worsening abdominal symptoms. A common reason for relapse is interruption of the medications that have kept the disease in remission. Many patients become complacent during quiescent periods and stop taking such medications. IBD requires continuous, lifelong maintenance therapy. Adherence to therapy has been shown to reduce the risk of acute attacks and cancer.28 Common complications of IBD include formation of fistulae, strictures, and abscesses; less common but life-threatening complications include fulminant colitis, toxic megacolon, and intestinal perforation.
Toxic Megacolon
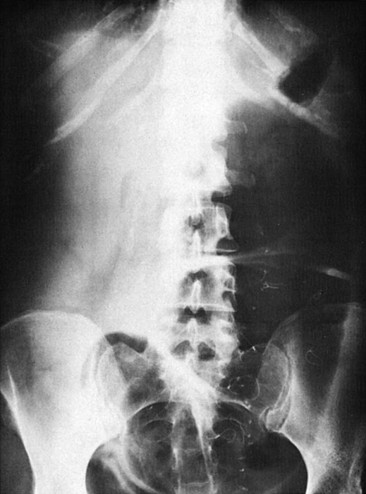
Toxic megacolon is typically associated with IBD or infectious colitis. The triggering event may be recent ingestion of anticholinergics, antimotility agents, narcotics, or antidepressants. Patients usually have experienced symptoms of colitis, often severe, for several days before the onset of toxic megacolon. Abdominal pain, fever, tachycardia, and abdominal distention are present. Plain radiographs are diagnostic and show a colon with a diameter of 6 cm or greater, although this feature may not be present in early stages. Treatment includes aggressive fluid hydration, intravenous corticosteroids, antibiotics covering bowel flora (see Box 95-5), and an evaluation for potential intestinal infections, especially in immunocompromised patients. Hypokalemia or hypomagnesemia should be aggressively corrected if present, as it can exacerbate colonic dilation.34 The mortality rate has decreased over the past four decades to less than 2% as a result of early recognition and aggressive treatment.
Diagnostic Strategies
No specific laboratory tests to diagnose IBD are available, although recent tests targeting antibodies to Saccharomyces cerevisiae or antineutrophil cytoplasm help to differentiate between CD and UC.30 Laboratory abnormalities may occur for a variety of reasons. Electrolyte abnormalities may be secondary to significant diarrhea, or anemia may occur from bloody stools. The erythrocyte sedimentation rate can be elevated and useful for categorizing the severity of the disease. Stools contain fecal leukocytes, but findings on stool cultures and ova and parasite examinations should be normal.
Plain radiography is not helpful in the diagnosis of uncomplicated disease but may show bowel obstruction, toxic megacolon, or free air from a perforation (Fig. 95-5). The use of plain radiographs should be limited to patients suspected of having these complications. Contrast studies can reveal lesions suggestive of IBD, including ulcerations of the mucosal surface, fistulae, and strictures. In Europe, where ultrasound technicians are more experienced in its application, ultrasonography is used to identify active disease and to look for complications.31 Ultrasound imaging is used much less commonly in the United States. Magnetic resonance imaging can locate affected bowel segments and identify fistulae, stenoses, and abscesses and has the advantage of no radiation, which is a consideration in individuals likely to get multiple imaging studies over time. CT is the best study routinely available to evaluate extraluminal complications. CT colonography (“virtual colonoscopy”), although good for identifying cancerous lesions of the colon, does not show the typical lesions of IBD.31 Recently, video capsule endoscopy (VCE) has shown promise in finding small bowel pathology missed by other studies. VCE has the drawback that capsules may get stuck at stricture sites and need to be surgically removed.35 Endoscopic evaluation with biopsy is usually required to confirm the diagnosis.30
Differential Considerations
Symptoms and signs are protean and overlap with those of many common abdominal conditions, including appendicitis, infectious colitis, ischemic colitis, radiation colitis, diverticular disease, cancer, and bowel obstruction. Intestinal infections commonly mimic the symptoms of IBD, and in new patients microbiologic studies for bacterial infection, including Escherichia coli O157 : H7, Clostridium difficile, and amoeba should be considered. Established patients who have recently been hospitalized or on antibiotics should be tested for C. difficile. Several assays may be required owing to the high false-negative rate for testing.34
Management
Medical management is the mainstay of therapy for most patients with IBD. In general, patients are maintained on 5-aminosalicylic acid (5-ASA) agents while asymptomatic and placed on steroids if symptoms recur. Once remission is achieved, steroids are discontinued and the patient is once more maintained on 5-ASA agents. If remission is not achieved with steroids, other agents such as antimetabolites and immunosuppressants are used (Box 95-6). The choice of agents depends on classification of the disease as mild, moderate, or severe (Box 95-7). Surgery is reserved for patients with severe disease who do not respond to medical therapy or for complications such as obstruction or fistula formation. This classic stepwise approach has recently been challenged by evidence that early initiation of immunosuppressants or antibody therapy leads to a more sustained remission and less need for surgical intervention, although this is still controversial.36
Antibiotics may be used for the primary treatment of IBD as well, but their use is controversial. Evidence supporting antibiotic use is stronger in CD than in UC. Metronidazole and ciprofloxacin are the most common antibiotics used, with some evidence suggesting that tobramycin or rifaximin may be beneficial as well.37
Oral corticosteroids are used in patients with moderate to severe disease or patients whose IBD is unresponsive to a 5-ASA agent. Steroids should be tapered when remission is achieved to avoid typical steroid side effects. Intravenous corticosteroids are reserved for hospitalized patients with severe disease. Budesonide, a newer oral corticosteroid, is degraded on its first pass through the bloodstream and has fewer systemic side effects. Prolonged use of steroids can lead to gastrointestinal mucosal injury, problems with wound healing, osteopenia with fractures, and frank osteonecrosis that may be evident only on MRI.34
The immunomodulating drugs azathioprine and 6-mercaptopurine are used in patients resistant to other therapies or to wean steroid-dependent patients. Patients on these medications should be assessed for bone marrow suppression and pancreatitis.38
The immunosuppressant agent cyclosporine is used in severe cases, often when patients are not surgical candidates. Although most patients tolerate it well, cyclosporine has significant potential toxicity, including myelosuppression, electrolyte disturbances, and hepatic and nephrotoxicity.28 Opportunistic infections including Pneumocystis pneumonia have been known to occur.32
Infliximab, an antibody to human tumor necrosis factor alpha, is useful in advanced cases of IBD. It generally has a benign side effect profile but carries an increased risk of opportunistic infections, including tuberculosis and fungal infections.29 Of patients receiving infliximab, 1 to 2% will experience serum sickness–like symptoms including arthralgias, myalgias, fevers, and rash.34 Surgery is reserved for patients with severe disease refractory to medical management and for patients with complications such as intestinal obstruction, significant bleeding, abscess, or fistula. A colectomy is curative for UC and improves quality of life, but there is no curative surgery for CD. Extraintestinal manifestations usually respond to therapy for intestinal disease.30,39
Disposition
Consultation with a gastroenterologist is recommended before patient disposition. Most patients with an uncomplicated mild-to-moderate exacerbation of IBD need only restart their maintenance therapy if it was interrupted, or add oral corticosteroids to their regimen. Patients with severe disease or those in whom oral corticosteroids have failed to effect improvement need hospitalization for administration of parenteral corticosteroids.30 Bowel rest does not seem to be beneficial except as preparation for surgical intervention.40 Emergent surgical consultation should be sought for life-threatening hemorrhage, evidence of perforation, or toxic megacolon. Urgent (not emergent) surgical intervention is indicated if the bowel is obstructed. Abscesses may be treated percutaneously with imaging guidance or surgically. Chronic fistulae initially are treated medically.38 After hospital discharge, close follow-up by the physician monitoring the patient’s disease is indicated to ensure that remission is achieved in a timely fashion and that the patient complies with the suppressive therapy after the acute event. For patients in remission, endoscopic monitoring for cancer is required on an ongoing basis, although the optimal frequency of examination has not yet been defined.38 The estimated prevalence of cancer among patients with CD is significant at 2%, and patients with UC have a 15 times greater risk for the development of colorectal cancer than the general population.32 Up to 25% of patients with UC will eventually require colectomy for uncontrolled disease.36
Colonic Ischemia
Colonic ischemia is the most common of the intestinal ischemic disorders and yet remains poorly understood.41 Estimates place the incidence of colonic ischemia at 1 of every 2000 hospitalizations. Its presentation overlaps with that of many other significant abdominal diseases, and the disorder is difficult to diagnose without endoscopic visualization of the colonic mucosa. Although elders are most at risk, with 90% of cases occurring in those older than 60 years, the condition can occur in all age groups.42 Both sexes are equally affected. In one study more than 50% of persons admitted with colonic ischemia were initially diagnosed with IBD. Because there is no specific treatment, outcomes usually are good and recurrences are rare; difficulty in making the diagnosis does not result in significant morbidity.
Principles of Disease
The exact cause of colonic ischemia is unknown. Isolated ischemia without small bowel involvement usually is a result of nonocclusive microvascular disease of the colon and not large vessel (mesenteric artery) occlusion. The primary insult is a low–blood-flow state associated with a variety of factors, including congestive heart failure, vasoactive drugs, atherosclerosis, renal failure, and recent cardiac or vascular surgery.42–44 Factors that have recently been implicated are infections, especially E. coli O157 : H7, and thrombophilia.41 Younger patients may develop colonic ischemia in the setting of collagen vascular disease, hematologic disorders, long-distance running, or cocaine abuse. Medications associated with colonic ischemia through a variety of mechanisms include digoxin, pseudoephedrine, and sumatriptan, among others. The colonic vascular system generally enjoys significant collateral flow, but in some patients this protective mechanism is tenuous, predisposing the affected person to ischemia from low-flow events.42 In addition, colonic arterioles seem to be particularly sensitive to vasoconstrictive influences, and the rapidly growing intestinal mucosa is especially vulnerable to interruptions in blood flow. High intraluminal pressures that normally develop within the colon can also alter intestinal perfusion significantly. Colonic ischemia can occur in any part of the colon, including the rectum, but for unknown reasons it occurs most often in the left colonic segment.
Colonic ischemia represents a spectrum of disease whose manifestations vary with the extent of the ischemic insult. In most cases the ischemic episode is self-limited, and the condition resolves completely with conservative therapy, but in one third of patients a prolonged or severe insult results in scarring or stricturing of the colon and chronic symptoms.42 If the ischemia is transmural, gangrene and intestinal perforation are possibilities. Chronic mild inflammation results in intermittent symptoms similar to those of IBD.
Clinical Features
The presentation of colonic ischemia typically involves the acute onset of mild crampy abdominal pain in the left lower quadrant with abdominal distention and almost always blood in the stool.45 The typical patient has had recent surgery or has a significant medical illness. Some patients do not have pain. Nausea and vomiting can occur with obstruction secondary to a stricture or an ileus. Tenderness over the affected colon may be present but often is not dramatic. Peritoneal findings, fever, and a significantly elevated WBC count suggest gangrenous bowel and perforation. Toxic megacolon is a recognized complication.
Diagnostic Strategies
No sensitive or specific biochemical markers for colonic ischemia are recognized, although biochemical abnormalities such as elevated serum lactate, phosphate, and alkaline phosphate levels may be present. These abnormalities may be absent in milder disease and are often not observed in more significant disease until after irreversible damage has occurred. A complete blood count to exclude significant anemia and to look for a leukocytosis suggestive of perforation is appropriate. Serum electrolytes should be checked if diarrhea or vomiting has been significant or prolonged. Blood and WBCs in the stool are common findings in several of the entities that present similarly to colonic ischemia, including IBD and infectious colitis. A positive occult stool guaiac test result should ensure that the patient is eventually evaluated for colonic carcinoma. Unfortunately, the definitive diagnosis of colonic ischemia rarely is made in the ED.42
Colonoscopy
Colonoscopy with colonic biopsy is the preferred method to diagnose colonic ischemia because it visualizes the abnormal colonic mucosa better than barium enema and affords the opportunity to take biopsy specimens to differentiate between cancer and other nonischemic causes of colitis. Colonoscopy can also detect necrotic bowel by its distinct cyanotic or black appearance. If colonoscopy is delayed, pathologic changes consistent with colonic ischemia may have already improved or resolved. The diagnosis may be missed on colonoscopy in up to one third of cases.43
Computed Tomography
Although CT does not allow the definitive diagnosis of colonic ischemia, it can exclude other disorders. CT features suggestive of colonic ischemia include thumbprinting, wall thickening, and luminal narrowing and inner wall hypoperfusion (“double halo sign”).42
Angiography
Angiography is usually not helpful in either the diagnosis or the management of colonic ischemia. In most cases the blood flow defect is at the microvascular level and has resolved by the time the patient seeks evaluation.46 The exception is the case in which only the ascending colon is affected, suggesting a superior mesenteric artery thrombosis.
Management
In the absence of surgical complications, the treatment of colonic ischemia is supportive and includes hospitalization for bowel rest, hydration, and pain management, with some authors recommending antibiotic coverage.42 NSAIDs are best avoided, as are oral cathartics or bowel preparation regimens that may lead to perforation. Broad-spectrum antibiotics covering bowel flora are indicated for patients with more significant symptoms (see Box 95-5). If colonic ischemia is precipitated by an episode of hypotension, the underlying cause of the hypotension must be sought and treated aggressively, and cardiac output should be maximized.42 Vasopressors should be avoided to prevent worsening of ischemia; steroids should also be avoided because they may facilitate bowel perforation. Colonic distention, if present, can be relieved acutely through the use of a rectal tube; surgical consultation is recommended in these cases. Decompression of the colon may result in a lowering of transmural pressure and improved colonic perfusion. Sepsis, peritoneal changes, free abdominal air, significant fever, massive bleeding, and a significant leukocytosis suggest bowel necrosis or perforation and should prompt emergent surgical consultation.42,44
Disposition
Patients with mild symptoms and no significant abdominal tenderness or bleeding can be managed on an outpatient basis and referred for colonoscopy. Stool studies including cultures for bacteria, microscopy to look for ova and parasites, and a C. difficile titer are helpful if the diagnosis is uncertain. Patients with more significant findings, especially if the diagnosis of gangrenous bowel cannot be excluded, require hospitalization. A high mortality rate (60%) is expected for patients undergoing emergent surgery, although deaths before the age of 50 years are rare.44 Most patients improve without surgical intervention, and only 5% have a recurrence of colonic ischemia. In those with continuing significant symptoms, colectomy is usually curative.
Radiation Proctocolitis
Radiation proctocolitis is a common side effect of radiation therapy, occurring in 50 to 75% of patients receiving radiation to the pelvis. The disease has two distinct presentations: acute and chronic. Acute radiation proctocolitis begins during or shortly after a course of radiation therapy, is usually easily diagnosed, and is self-limited. Chronic radiation proctocolitis occurs in 5 to 10% of patients who have undergone pelvic radiation therapy and typically begins any time up to 2 years after the end of radiation therapy, although onset of clinical manifestations may be delayed beyond 2 years.48 Some cases have occurred decades later. Patients with more severe acute radiation proctocolitis seem to be prone to chronic proctocolitis.49 Because of its nonspecific presentation and delayed appearance, the diagnosis of chronic radiation proctocolitis can be challenging. Unfortunately there is a paucity of robust research evaluating the best approach to management of this disorder.50
Principles of Disease
Chronic Radiation Proctocolitis
The pathologic mechanism in chronic radiation proctocolitis is entirely different from that in acute radiation proctocolitis. Chronic radiation proctocolitis results from a progressive endarteritis with abnormal tissue collagen deposition. Affected intestine has a decreased microvascular density, with subsequent decreased perfusion.51 Over time, affected bowel gradually becomes more ischemic, leading to ulceration, scarring, and narrowing of the bowel lumen. Frank necrosis and perforation, although uncommon, can occur. Long-term outcomes in chronic radiation proctocolitis have not been well studied, but it seems that patients in whom fistulae and persistent bleeding strictures develop have the poorest prognosis.49
Clinical Features
Acute radiation proctocolitis manifests with abdominal pain, bleeding, and tenesmus. Onset during the course of radiation therapy, typically after several treatments, suggests the diagnosis. Fecal urgency and incontinence can be devastating to quality of life.51
Chronic radiation proctocolitis has a more insidious onset with a variety of presentations, including ulcerative disease, stricture with or without obstruction, fistulae, and bowel perforation. Symptoms may be similar to those in acute disease, with tenesmus, diarrhea, and urgency. Bleeding is common but usually is not hemodynamically significant. Decreased caliber of stool with increased straining or constipation suggests a stricture. Fistulae can develop between affected bowel and any adjacent organ, but the most common fistulae are rectovaginal. Some patients may exhibit anal sphincter dysfunction and loss of bowel control. Symptoms tend to have a significant negative impact on the quality of life.49
Differential Considerations
In chronic radiation proctocolitis, the possibility that symptoms are a result of recurrence of the initial malignancy or a new malignancy induced by radiation exposure must be entertained. Symptoms of chronic radiation proctocolitis generally are clinically indistinguishable from those of other causes of bowel inflammation, including IBD, infectious colitis, and ischemic colitis.52
Management
Chronic radiation proctocolitis treatment also is symptomatic. If rectal involvement is significant, stool softeners, analgesics, anti-inflammatory agents (e.g., sulfasalazine, balsalazide), and sucralfate enemas are helpful. Metronidazole is beneficial when added to anti-inflammatory therapy. Continued rectal bleeding can be controlled with topical formalin or laser photocoagulation.50 Minimally symptomatic strictures can be managed initially with stool softeners and enemas as needed. Some strictures have a reversible edema component, so the extent of narrowing may lessen after treatment. Fistulae and significant strictures generally require surgical repair. Approximately 20% of all patients with chronic radiation injury to the intestinal tract require some type of surgical intervention. Biopsy specimens from ulcerations associated with chronic injury should be obtained to exclude malignancy.
References
1. American College of Gastroenterology Functional Gastrointestinal Disorders Task Force. Evidence-based position statement on the management of irritable bowel syndrome in North America. Am J Gastroenterol. 2002;97:S1.
2. Khan, S, Chang, L. Diagnosis and management of IBS. Nat Rev Gastroenterol Hepatol. 2010;7:565.
3. Agrawal, A, Whorwell, PJ. Irritable bowel syndrome: Diagnosis and management. BMJ. 2006;332:280.
4. Mimura, T, Emanuel, A, Kamm, MA. Pathophysiology of diverticular disease. Best Pract Res Clin Gastroenterol. 2002;16:563.
5. Hall, J, Hammerich, K, Roberts, P. New paradigms in the management of diverticular disease. Curr Probl Surg. 2010;47:680.
6. Janes, SE, Meagher, A, Frizelle, FA. Management of diverticulitis. BMJ. 2006;332:271.
7. Fearnhead, NS, Mortensen, NJ. Clinical features and differential diagnosis of diverticular disease. Best Pract Res Clin Gastroenterol. 2002;16:577.
8. Halligan, S, Saunders, B. Imaging diverticular disease. Best Pract Res Clin Gastroenterol. 2002;16:595.
9. Wong, WD, et al. Practice parameters for the treatment of sigmoid diverticulitis—supporting documentation. The Standards Task Force. The American Society of Colon and Rectal Surgeons. Dis Colon Rectum. 2000;43:290.
10. Buchanan, GN, Kenefick, NJ, Cohen, CR. Diverticulitis. Best Pract Res Clin Gastroenterol. 2002;16:635.
11. Rafferty, J, et al. Practice parameters for sigmoid diverticulitis. Dis Colon Rectum. 2006;49:939.
12. O’Riordan, JM, O’Connell, PR. Practice parameters for sigmoid diverticulitis. Dis Colon Rectum. 2007;50:402.
13. Ambrosetti, P, Chautems, R, Soravia, C, Peiris-Waser, N, Terrier, F. Long-term outcome of mesocolic and pelvic diverticular abscesses of the left colon: A prospective study of 73 cases. Dis Colon Rectum. 2005;48:787.
14. Blair, NP, Germann, E. Surgical management of acute sigmoid diverticulitis. Am J Surg. 2002;183:525.
15. Trompetas, V. Emergency management of malignant acute left-sided colonic obstruction. Ann R Coll Surg Engl. 2008;90:181.
16. Saunders, MD, Kimmey, MB. Colonic pseudo-obstruction: The dilated colon in the ICU. Semin Gastrointest Dis. 2003;14:20.
17. Raveenthiran, V, Madiba, TE, Atamanalp, SS, De, U. Volvulus of the sigmoid colon. Colorectal Dis. 2010;12:e1.
18. Lau, KC, Miller, BJ, Schache, DJ, Cohen, JR. A study of large-bowel volvulus in urban Australia. Can J Surg. 2006;49:203.
19. Habre, J, Sautot-Vial, N, Marcotte, C, Benchimol, D. Caecal volvulus. Am J Surg. 2008;196:e48.
20. Hogan, BA, Brown, CJ, Brown, JA. Cecal volvulus in pregnancy: Report of a case and review of the safety and utility of medical diagnostic imaging in the assessment of the acute abdomen during pregnancy. Emerg Radiol. 2008;15:127–131.
21. Clinical presentation and diagnosis of sigmoid volvulus. Outcomes of 40-year and 859-patient experience. J Gastroenterol Hepatol. 2009;24:1154.
22. Selvaraj, DR, Palaniswamy, C. Sigmoid volvulus. J Hosp Med. 2010;5:E36.
23. Madiba, TE, Thomson, SR. The management of cecal volvulus. Dis Colon Rectum. 2002;45:264.
24. Wang, N, et al. Adult intussusception: A retrospective review of 41 cases. World J Gastroenterol. 2009;15:3303.
25. Zubaidi, A, Al-Saif, F, Silverman, R. Adult intussusception: A retrospective review. Dis Colon Rectum. 2006;49:1546.
26. Takeuchi, K, et al. The diagnosis and treatment of adult intussusception. J Clin Gastroenterol. 2003;36:18.
27. Haas, EM, Etter, EL, Ellis, S, Taylor, TV. Adult intussusception. Am J Surg. 2003;186:75.
28. Hanauer, SB, Present, DH. The state of the art in the management of inflammatory bowel disease. Rev Gastroenterol Disord. 2003;3:81.
29. Knutson, D, Greenberg, G, Cronau, H. Management of Crohn’s disease—a practical approach. Am Fam Physician. 2003;68:707.
30. Baumgart, DC, Sandborn, WJ. Inflammatory bowel disease: Clinical aspects and established and evolving therapies. Lancet. 2007;369:1641.
31. Scholmerich, J. Inflammatory bowel disease. Endoscopy. 2003;35:164.
32. Farrell, RJ, Peppercorn, MA. Ulcerative colitis. Lancet. 2002;359:331.
33. Xavier, RJ, Podolsky, DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427.
34. Kornbluth, A, Sachar, DB. Ulcerative colitis practice guidelines in adults: American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010;105:501.
35. Lichtenstein, GR, Hanauer, SB, Sandborn, WJ. Management of Crohn’s disease in adults. Am J Gastroenterol. 2009;104:465.
36. Panaccione, R, et al. Review article: Treatment algorithms to maximize remission and minimize corticosteroid dependence in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2008;28:674.
37. Gionchetti, P, et al. Antibiotics and probiotics in treatment of inflammatory bowel disease. World J Gastroenterol. 2006;12:3306.
38. Hanauer, SB, Sandborn, W. Management of Crohn’s disease in adults. Am J Gastroenterol. 2001;96:635.
39. Cohen, JL, et al. Practice parameters for the surgical treatment of ulcerative colitis. Dis Colon Rectum. 2005;48:1997.
40. Rizzello, F, Gionchetti, P, Venturi, A, Campieri, M. Review article: Medical treatment of severe ulcerative colitis. Aliment Pharmacol Ther. 2003;17(Suppl 2):7.
41. Brandt, LJ. Colon ischemia: Respice, adspice, prospice. Surgery. 2010;148:3.
42. Baixauli, J, Kiran, RP, Delaney, CP. Investigation and management of ischemic colitis. Cleve Clin J Med. 2003;70:920.
43. Scharff, JR, et al. Ischemic colitis: Spectrum of disease and outcome. Surgery. 2003;134:624.
44. Acosta, S, Ogren, M, Sternby, NH, Bergqvist, D, Björck, M. Fatal colonic ischemia: A population-based study. Scand J Gastroenterol. 2006;41:1312.
45. Ullery, BS, Boyko, AT, Banet, GA, Lewis, LM. Colonic ischemia: An under-recognized cause of lower gastrointestinal bleeding. J Emerg Med. 2004;27:1.
46. Brandt, LJ, Boley, SJ. AGA technical review on intestinal ischemia. American Gastrointestinal Association. Gastroenterology. 2000;118:954.
47. American Gastroenterological Association Medical Position Statement. Guidelines on intestinal ischemia. Gastroenterology. 2000;118:951.
48. Otchy, DP, Nelson, H. Radiation injuries of the colon and rectum. Surg Clin North Am. 1993;73:1017.
49. Denton, A, Forbes, A, Andreyev, J, Maher, EJ. Non-surgical interventions for late radiation proctitis in patients who have received radical radiotherapy to the pelvis. Cochrane Database Syst Rev. (1):2002.
50. Wong, MT, et al. Radiation proctitis: A decade’s experience. Singapore Med J. 2010;51:315.
51. O’Brien, PC. Radiation injury of the rectum. Radiother Oncol. 2001;60:1.
52. Leupin, N, et al. Acute radiation colitis in patients treated with short-term preoperative radiotherapy for rectal cancer. Am J Surg Pathol. 2002;26:498.