FIGURE 404-4 Simplified approach to the differential diagnosis of diabetes insipidus. When symptoms suggest diabetes insipidus (DI), the syndrome should be differentiated from a genitourinary (GU) abnormality by measuring the 24-h urine volume and osmolarity on unrestricted fluid intake. If DI is confirmed, basal plasma arginine vasopressin (AVP) should be measured on unrestricted fluid intake. If AVP is normal or elevated (>1 pg/mL), the patient probably has nephrogenic DI. However, if plasma AVP is low or undetectable, the patient has either pituitary DI or primary polydipsia. In that case, magnetic resonance imaging (MRI) of the brain can be performed to differentiate between these two conditions by determining whether or not the normal posterior pituitary bright spot is visible on T1-weighted midsagittal images. In addition, the MRI anatomy of the pituitary hypothalamic area can be examined to look for evidence of pathology that sometimes causes pituitary DI or the dipsogenic form of primary polydipsia. MRI is not reliable for differential diagnosis unless nephrogenic DI has been excluded because the bright spot is also absent, small, or faint in this condition.
If MRI and/or AVP assays with the requisite sensitivity and specificity are unavailable and a fluid deprivation test is impractical or undesirable, a third way to differentiate between pituitary DI, nephrogenic DI, and primary polydipsia is a trial of desmopressin therapy. Such a trial should be conducted with very close monitoring of serum sodium as well as urine output, preferably in hospital, because desmopressin will produce hyponatremia in 8–24 h if the patient has primary polydipsia.
|
TREATMENT |
DIABETES INSIPIDUS |
The signs and symptoms of uncomplicated pituitary DI can be eliminated by treatment with desmopressin (DDAVP), a synthetic analogue of AVP (Fig. 404-1). DDAVP acts selectively at V2 receptors to increase urine concentration and decrease urine flow in a dose-dependent manner. It is also more resistant to degradation than is AVP and has a three- to fourfold longer duration of action. DDAVP can be given by IV or SC injection, nasal inhalation, or orally by means of a tablet of melt. The doses required to control pituitary DI completely vary widely, depending on the patient and the route of administration. However, among adults, they usually range from 1–2 μg qd or bid by injection, 10–20 μg bid or tid by nasal spray, or 100–400 μg bid or tid orally. The onset of antidiuresis is rapid, ranging from as little as 15 min after injection to 60 min after oral administration. When given in a dose that normalizes 24-h urinary osmolarity (400–800 mosmol/L) and volume (15–30 mL/kg body weight), DDAVP produces a slight (1–3%) increase in total body water and a decrease in plasma osmolarity/sodium that rapidly eliminates thirst and polydipsia (Fig. 404-5). Consequently, water balance is maintained within the normal range. Hyponatremia does not develop unless urine volume is reduced too far (to less than 10 mL/kg per day) or fluid intake is excessive due to an associated abnormality in thirst or cognition. Fortunately, thirst abnormalities are rare, and if the patient is taught to drink only when truly thirsty, DDAVP can be given safely in doses sufficient to normalize urine output (~15–30 mL/kg per day) without the need for allowing intermittent escape to prevent water intoxication.
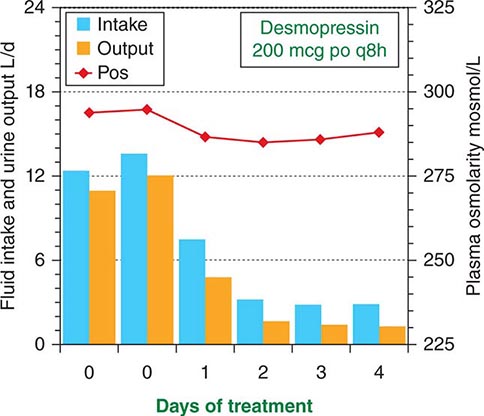
FIGURE 404-5 Effect of desmopressin therapy on fluid intake (blue bars), urine output (orange bars), and plasma osmolarity (red line) in a patient with uncomplicated pituitary diabetes insipidus. Note that treatment rapidly reduces fluid intake and urine output tonormal, with only a slight increase in body water as evidenced by the slight decrease in plasma osmolarity.
Primary polydipsia cannot be treated safely with DDAVP or any other antidiuretic drug because eliminating the polyuria does not eliminate the urge to drink. Therefore, it invariably produces hyponatremia and/or other signs of water intoxication, usually within 8–24 h if urine output is normalized completely. There is no consistently effective way to correct dipsogenic or psychogenic polydipsia, but the iatrogenic form may respond to patient education. To minimize the risk of water intoxication, all patients should be warned about the use of other drugs such as thiazide diuretics or carbamazepine (Tegretol) that can impair urinary free-water excretion directly or indirectly.
The polyuria and polydipsia of nephrogenic DI are not affected by treatment with standard doses of DDAVP. If resistance is partial, it may be overcome by tenfold higher doses, but this treatment is too expensive and inconvenient for long-term use. However, treatment with conventional doses of a thiazide diuretic and/or amiloride in conjunction with a low-sodium diet and a prostaglandin synthesis inhibitor (e.g., indomethacin) usually reduces the polyuria and polydipsia by 30–70% and may eliminate them completely in some patients. Side effects such as hypokalemia and gastric irritation can be minimized by the use of amiloride or potassium supplements and by taking medications with meals.
HYPODIPSIC HYPERNATREMIA
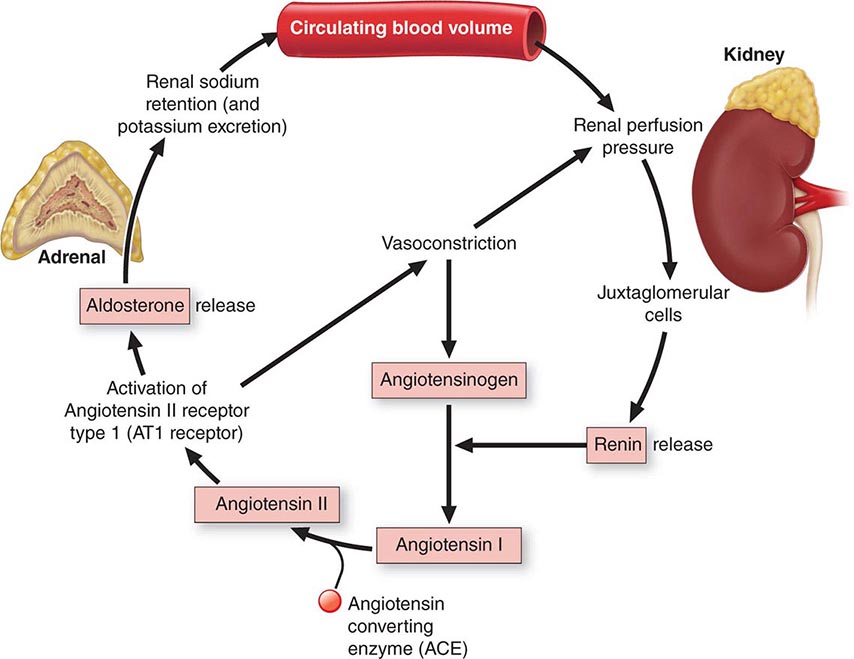
An increase in plasma osmolarity/sodium above the normal range (hypertonic hypernatremia) can be caused by either a decrease in total body water or an increase in total body sodium. The former results from a failure to drink enough to replace normal or increased urinary and insensible water loss. The deficient intake can be due either to water deprivation or a lack of thirst (hypodipsia). The most common cause of an increase in total body sodium is primary hyperaldosteronism (Chap. 406). Rarely, it can also result from ingestion of hypertonic saline in the form of sea water or incorrectly prepared infant formula. However, even in these forms of hypernatremia, inadequate intake of water also contributes. This chapter focuses on hypodipsic hypernatremia, the form of hypernatremia due to a primary defect in the thirst mechanism.
Clinical Characteristics Hypodipsic hypernatremia is a syndrome characterized by chronic or recurrent hypertonic dehydration. The hypernatremia varies widely in severity and usually is associated with signs of hypovolemia such as tachycardia, postural hypotension, azotemia, hyperuricemia, and hypokalemia due to secondary hyperaldosteronism. Muscle weakness, pain, rhabdomyolysis, hyperglycemia, hyperlipidemia, and acute renal failure may also occur. Obtundation or coma may be present but are often absent. Despite inappropriately low levels of plasma AVP, DI usually is not evident at presentation but may develop during rehydration as blood volume, blood pressure, and plasma osmolarity/sodium return toward normal, further reducing plasma AVP.
Etiology Hypodipsia is usually due to hypogenesis or destruction of the osmoreceptors in the anterior hypothalamus that regulate thirst. These defects can result from various congenital malformations of midline brain structures or may be acquired due to diseases such as occlusions of the anterior communicating artery, primary or metastatic tumors in the hypothalamus, head trauma, surgery, granulomatous diseases such as sarcoidosis and histiocytosis, AIDS, and cytomegalovirus encephalitis. Because of their proximity, the osmoreceptors that regulate AVP secretion also are usually impaired. Thus, AVP secretion responds poorly or not at all to hyperosmotic stimulation (Fig. 404-6) but, in most cases, increases normally to nonosmotic stimuli such as nausea or large reductions in blood volume or blood pressure, indicating that the neurohypophysis is intact.
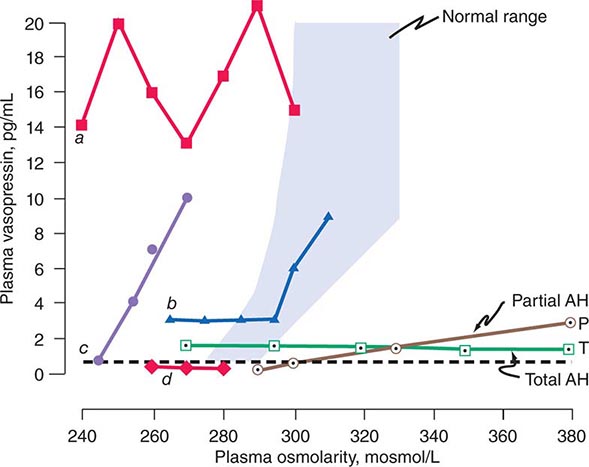
FIGURE 404-6 Heterogeneity of osmoregulatory dysfunction in adipsic hypernatremia (AH) and the syndrome of inappropriate antidiuresis (SIAD). Each line depicts schematically the relationship of plasma arginine vasopressin (AVP) to plasma osmolarity during water loading and/or infusion of 3% saline in a patient with either AH (open symbols) or SIAD (closed symbols). The shaded area indicates the normal range of the relationship. The horizontal broken line indicates the plasma AVP level below which the hormone is undetectable and urinary concentration usually does not occur. Lines P and T represent patients with a selective deficiency in the osmoregulation of thirst and AVP that is either partial (![]() ) or total (
) or total (![]() ). In the latter, plasma AVP does not change in response to increases or decreases in plasma osmolarity but remains within a range sufficient to concentrate the urine even if overhydration produces hypotonic hyponatremia. In contrast, if the osmoregulatory deficiency is partial (
). In the latter, plasma AVP does not change in response to increases or decreases in plasma osmolarity but remains within a range sufficient to concentrate the urine even if overhydration produces hypotonic hyponatremia. In contrast, if the osmoregulatory deficiency is partial (![]() ), rehydration of the patient suppresses plasma AVP to levels that result in urinary dilution and polyuria before plasma osmolarity and sodium are reduced to normal. Lines a –d represent different defects in the osmoregulation of plasma AVP observed in patients with SIADH or SIAD. In a (
), rehydration of the patient suppresses plasma AVP to levels that result in urinary dilution and polyuria before plasma osmolarity and sodium are reduced to normal. Lines a –d represent different defects in the osmoregulation of plasma AVP observed in patients with SIADH or SIAD. In a (![]() ), plasma AVP is markedly elevated and fluctuates widely without relation to changes in plasma osmolarity, indicating complete loss of osmoregulation. In b (
), plasma AVP is markedly elevated and fluctuates widely without relation to changes in plasma osmolarity, indicating complete loss of osmoregulation. In b (![]() ), plasma AVP remains fixed at a slightly elevated level until plasma osmolarity reaches the normal range, at which point it begins to rise appropriately, indicating a selective defect in the inhibitory component of the osmoregulatory mechanism. In c (
), plasma AVP remains fixed at a slightly elevated level until plasma osmolarity reaches the normal range, at which point it begins to rise appropriately, indicating a selective defect in the inhibitory component of the osmoregulatory mechanism. In c (![]() ), plasma AVP rises in close correlation with plasma osmolarity before the latter reaches the normal range, indicating downward resetting of the osmostat. In d (
), plasma AVP rises in close correlation with plasma osmolarity before the latter reaches the normal range, indicating downward resetting of the osmostat. In d (![]() ), plasma AVP appears to be osmoregulated normally, suggesting that the inappropriate antidiuresis is caused by some other abnormality.
), plasma AVP appears to be osmoregulated normally, suggesting that the inappropriate antidiuresis is caused by some other abnormality.
Pathophysiology Hypodipsia results in a failure to drink enough water to replenish obligatory renal and extrarenal losses. Consequently, plasma osmolarity and sodium rise often to extremely high levels before the disorder is recognized. In most cases, urinary loss of water contributes little, if any, to the dehydration because AVP continues to be secreted in the small amounts necessary to concentrate the urine. In some patients this appears to be due to hypovolemic stimulation and/or incomplete destruction of AVP osmoreceptors because plasma AVP declines and DI develops during rehydration (Fig. 404-6). In others, however, plasma AVP does not decline during rehydration even if they are overhydrated. Consequently, they develop a hyponatremic syndrome indistinguishable from inappropriate antidiuresis. This suggests that the AVP osmoreceptors normally provide inhibitory and stimulatory input to the neurohypophysis and the patients can no longer osmotically stimulate or suppress tonic secretion of the hormone because both inputs have been totally eliminated by the same pathology that destroyed the osmoregulation of thirst. In a few patients, the neurohypophysis is also destroyed, resulting in a combination of chronic pituitary DI and hypodipsia that is particularly difficult to manage.
Differential Diagnosis Hypodipsic hypernatremia usually can be distinguished from other causes of inadequate fluid intake (e.g., coma, paralysis, restraints, absence of fresh water) by the clinical history and setting. Previous episodes and/or denial of thirst and failure to drink spontaneously when the patient is conscious, unrestrained, and hypernatremic are virtually diagnostic. The hypernatremia caused by excessive retention or intake of sodium can be distinguished by the presence of thirst as well as the physical and laboratory signs of hypervolemia rather than hypovolemia.
|
TREATMENT |
HYPODIPSIC HYPERNATREMIA |
Hypodipsic hypernatremia should be treated by administering water orally if the patient is alert and cooperative or by infusing hypotonic fluids (0.45% saline or 5% dextrose and water) if the patient is not. The amount of free water in liters required to correct the deficit (ΔFW) can be estimated from body weight in kg (BW) and the serum sodium concentration in mmol/L (SNa) by the formula ΔFW = 0.5BW × ([SNa – 140]/140). If serum glucose (SGlu) is elevated, the measured S Na should be corrected (SNa*) by the formula SNa* = SNa + ([SGlu – 90]/36). This amount plus an allowance for continuing insensible and urinary losses should be given over a 24- to 48-h period. Close monitoring of serum sodium as well as fluid intake and urinary output is essential because, depending on the extent of osmoreceptor deficiency, some patients will develop AVP-deficient DI, requiring DDAVP therapy to complete rehydration; others will develop hyponatremia and a syndrome of inappropriate antidiuresis (SIAD)-like picture if overhydrated. If hyperglycemia and/or hypokalemia are present, insulin and/or potassium supplements should be given with the expectation that both can be discontinued soon after rehydration is complete. Plasma urea/creatinine should be monitored closely for signs of acute renal failure caused by rhabdomyolysis, hypovolemia, and hypotension.
Once the patient has been rehydrated, an MRI of the brain and tests of anterior pituitary function should be performed to look for the cause and collateral defects in other hypothalamic functions. A long-term management plan to prevent or minimize recurrence of the fluid and electrolyte imbalance also should be developed. This should include a practical method to regulate fluid intake in accordance with variations in water balance as indicated by changes in body weight or serum sodium determined by home monitoring analyzers. Prescribing a constant fluid intake is ineffective and potentially dangerous because it does not take into account the large, uncontrolled variations in insensible loss that inevitably result from changes in ambient temperature and physical activity.
HYPONATREMIA DUE TO INAPPROPRIATE ANTIDIURESIS
A decrease in plasma osmolarity/sodium below the normal range (hypotonic hyponatremia) can be due to any of three different types of salt and water imbalance: (1) an increase in total body water that exceeds the increase in total body sodium (hypervolemic hyponatremia); (2) a decrease in body sodium greater than the decrease in body water (hypovolemic hyponatremia); or (3) an increase in body water with little or no change in body sodium (euvolemic hyponatremia) (Chap. 63). All three forms are associated with a failure to fully dilute the urine and mount a water diuresis in the face of hypotonic hyponatremia. The hypervolemic form typically occurs in disorders like severe congestive heart failure or cirrhosis. The hypovolemic form typically occurs in disorders such as severe diarrhea, diuretic abuse, or mineralocorticoid deficiency. Euvolemic hyponatremia, however, is due mainly to expansion of total body water caused by excessive intake in the face of a defect in urinary dilution. The impaired dilution is usually caused by a defect in the osmotic suppression of AVP that can have either of two causes. One is a nonhemodynamic stimulus such as nausea or a cortisol deficiency, which can be corrected quickly by treatment with antiemetics or cortisol. The other is a primary defect in osmoregulation caused by another disorder such as malignancy, stroke, or pneumonia that cannot be easily or quickly corrected. The latter is commonly known as the syndrome of inappropriate antidiuretic hormone (SIADH). Much less often, euvolemic hyponatremia can also result from AVP-independent activation of renal V2 receptors, a variant known as nephrogenic inappropriate antidiuresis or NSIAD. Both of the latter will be discussed in this chapter.
Clinical Characteristics Antidiuresis of any cause decreases the volume and increases the concentration of urine. If not accompanied by a commensurate reduction in fluid intake or an increase in insensible loss, the reduction in urine output results in excess water retention which expands and dilutes body fluids. If the hyponatremia develops gradually or has been present for more than a few days, it may be largely asymptomatic. However, if it develops acutely, it is usually accompanied by symptoms and signs of water intoxication that may include mild headache, confusion, anorexia, nausea, vomiting, coma, and convulsions. Severe acute hyponatremia may be lethal. Other clinical signs and symptoms vary greatly, depending on the type of hyponatremia. The hypervolemic form is characterized by generalized edema and other signs of marked volume expansion. The opposite is evident in the hypovolemic form. However, overt signs of volume expansion or contraction are absent in SIADH, SIAD, and other forms of euvolemic hyponatremia.
Etiology In SIADH, the inappropriate secretion of AVP can have many different causes. They include ectopic production of AVP by lung cancer or other neoplasms; eutopic release induced by various diseases or drugs; and exogenous administration of AVP, DDAVP, or large doses of oxytocin (Table 404-2). The ectopic forms result from abnormal expression of the AVP-NPII gene by primary or metastatic malignancies. The eutopic forms occur most often in patients with acute infections or strokes but have also been associated with many other neurologic diseases and injuries. The mechanisms by which these diseases interfere with osmotic suppression of AVP are not known. The defect in osmoregulation can take any of four distinct forms (Fig. 404-6). In one of the most common (reset osmostat), AVP secretion remains fully responsive to changes in plasma osmolarity/sodium, but the threshold, or set point, of the osmoregulatory system is abnormally low. These patients differ from those with the other types of SIADH in that they are able to maximally suppress plasma AVP and dilute their urine if their fluid intake is high enough to reduce their plasma osmolarity and/or sodium to the new set point. In most patients, SIADH is self-limited and remits spontaneously within 2–3 weeks, but about 10% of cases are chronic. Another, smaller subgroup (~10% of the total) has inappropriate antidiuresis without a demonstrable defect in the osmoregulation of plasma AVP (Fig. 404-6). In some of them, all young boys, the inappropriate antidiuresis has been traced to a constitutively activating mutation of the V2 receptor gene. This unusual variant may be referred to as familial nephrogenic SIAD (NSIAD) to distinguish it from other possible causes of the syndrome. The inappropriate antidiuresis in these patients appears to be permanent, although the hyponatremia is variable owing presumably to individual differences in fluid intake.
|
CAUSES OF SYNDROME OF INAPPROPRIATE ANTIDIURETIC HORMONE (SIADH) |
Pathophysiology Impaired osmotic suppression of antidiuresis results in excessive retention of water and dilution of body fluids only if water intake exceeds insensible and urinary losses. The excess intake is sometimes due to an associated defect in the osmoregulation of thirst (dipsogenic) but can also be psychogenic or iatrogenic, including excessive IV administration of hypotonic fluids. In SIADH and other forms of euvolemic hyponatremia, the decrease in plasma osmolarity/sodium and the increase in extracellular and intracellular volume are proportional to the amount of water retained. Thus, an increase in body water of 10% (~4 L in a 70-kg adult) reduces plasma osmolarity and sodium by approximately 10% (~28 mosmol/L or 14 meq/L). An increase in body water of this magnitude is rarely detectable on physical examination but will be reflected in a weight gain of about 4 kg. It also increases glomerular filtration and atrial natriuretic hormone and suppresses plasma renin activity, thereby increasing urinary sodium excretion. The resultant reduction in total body sodium decreases the expansion of extracellular volume but aggravates the hyponatremia and further expands intracellular volume. The latter further increases brain swelling and intracranial pressure, which probably produces most of the symptoms of acute water intoxication. Within a few days, this swelling may be counteracted by inactivation or elimination of intracellular solutes, resulting in the remission of symptoms even though the hyponatremia persists.
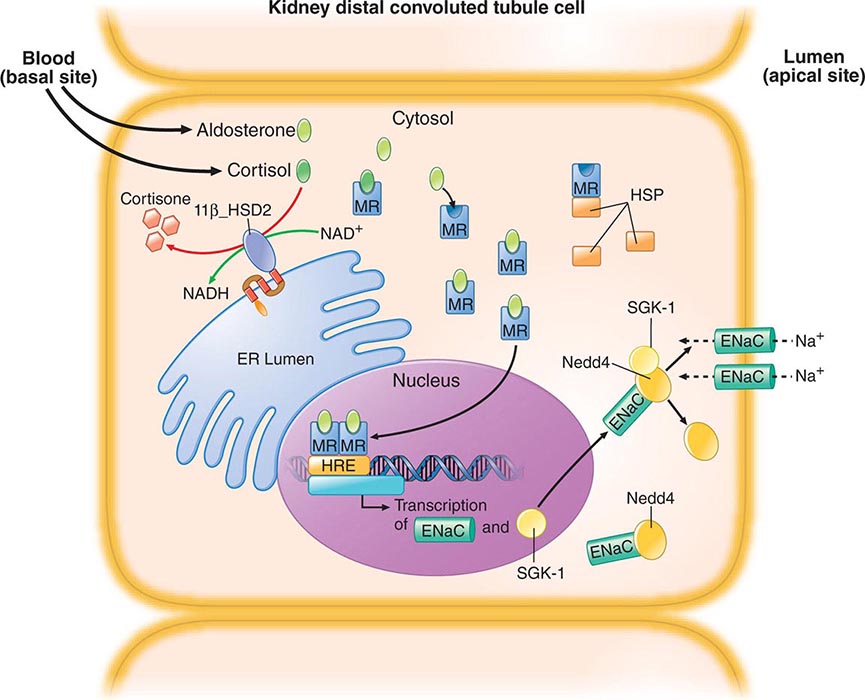
In type I (hypervolemic) or type II (hypovolemic) hyponatremia, osmotic suppression of AVP secretion appears to be counteracted by a hemodynamic stimulus resulting from a large reduction in cardiac output and/or effective blood volume. The resultant antidiuresis is enhanced by decreased distal delivery of glomerular filtrate that results from increased reabsorption of sodium in proximal nephron. If the reduction in urine output is not associated with a commensurate reduction in water intake or an increase in insensible loss, body fluids are expanded and diluted, resulting in hyponatremia despite an increase in body sodium. Unlike SIADH and other forms of euvolemic hyponatremia, however, glomerular filtration is reduced and plasma renin activity and aldosterone are elevated. Thus, the rate of urinary sodium excretion is low (unless sodium reabsorption is impaired by a diuretic), and the hyponatremia is usually accompanied by edema, hypokalemia, azotemia, and hyperuricemia. In type II (hypovolemic) hyponatremia, sodium and water are also retained as an appropriate compensatory response to the severe depletion.
Differential Diagnosis SIADH is a diagnosis of exclusion that usually can be made from the history, physical examination, and basic laboratory data. If hyperglycemia is present, its contribution to the reduction in plasma sodium can be estimated either by measuring plasma osmolarity for a more accurate estimate of the true “effective” tonicity of body fluids or by correcting the measured plasma sodium for the reduction caused by the hyperglycemia using the simplified formula
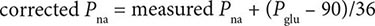
where Pna = plasma sodium in meq/L and Pglu = plasma glucose in mg/dL.
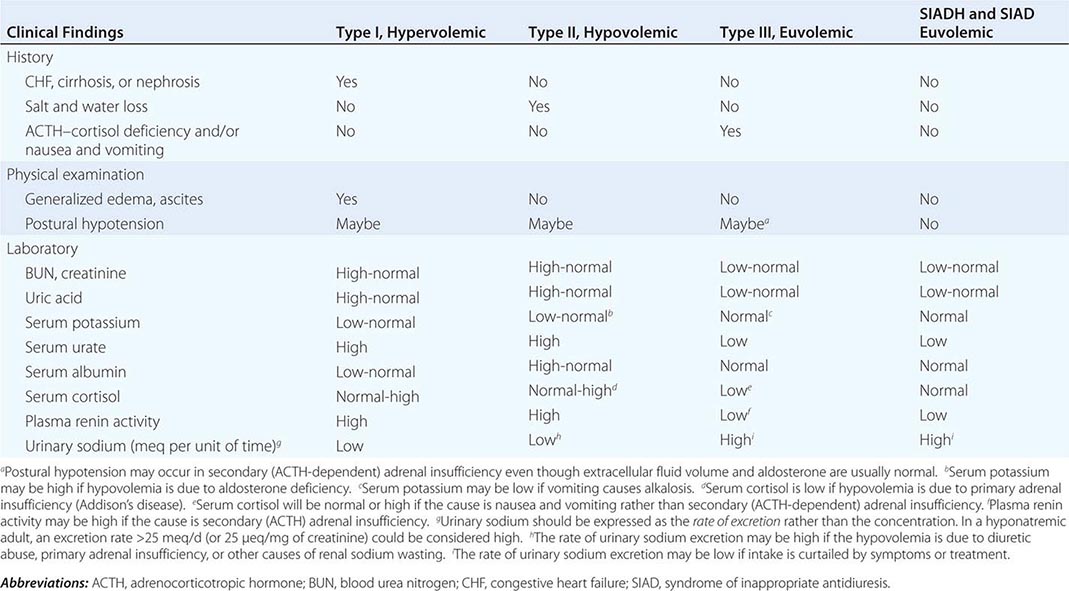
If the plasma osmolarity and/or corrected plasma sodium are below normal limits, hypotonic hyponatremia is present and further evaluation to determine the type should be undertaken in order to administer safe and effective treatment. This differentiation is usually possible by evaluating standard clinical indicators of the extracellular fluid volume (Table 404-3). If these findings are ambiguous or contradictory, measuring plasma renin activity or the rate of urinary sodium excretion may be helpful provided that the hyponatremia is not in the recovery phase or is due to a primary defect in renal conservation of sodium, diuretic abuse, or hyporeninemic hypoaldosteronism. The latter may be suspected if serum potassium is elevated instead of low, as it usually is in types I and II hyponatremia. Measurements of plasma AVP are currently of no value in differentiating SIADH from the other types of hyponatremia since the plasma levels are elevated similarly in all. In patients who fulfill the clinical criteria for type III (euvolemic) hyponatremia, morning plasma cortisol should also be measured to exclude secondary adrenal insufficiency. If it is normal and there is no history of nausea/vomiting, the diagnosis of SIADH is confirmed, and a careful search for occult lung cancer or other common causes of the syndrome (Table 404-2) should be undertaken.
|
DIFFERENTIAL DIAGNOSIS OF HYPONATREMIA BASED ON CLINICAL ASSESSMENT OF EXTRACELLULAR FLUID VOLUME (ECFV) |
SIAD due to an activating mutation of the V2 receptor gene should be suspected if the hyponatremia occurs in a child or several members of the family or is refractory to treatment with a vaptan (see below). In that case, plasma AVP should be measured to confirm that it is appropriately suppressed while the hyponatremia and antidiuresis are present, and the V2 receptor gene should be sequenced, if possible.
|
TREATMENT |
HYPONATREMIA |
The management of hyponatremia differs depending on the type and the severity and duration of symptoms. In acute symptomatic SIADH, the aim should be to raise plasma osmolarity and/or plasma sodium at a rate approximating 1% an hour until they reach levels of about 270 mosmol/L or 130 meq/L, respectively. This can be accomplished in either of two ways. One is to infuse hypertonic (3%) saline at a rate of about 0.05 mL/kg body weight per minute. This treatment also has the advantage of correcting the sodium deficiency that is partly responsible for the hyponatremia and often produces a solute diuresis that serves to remove some of the excess water. The other treatment is to reduce body water by giving an AVP receptor-2 antagonist (vaptan) to block the antidiuretic effect of AVP and increase urine output (Fig. 404-7). One of the vaptans, a combined V2/V1a antagonist (Conivaptan), has been approved for short-term, in-hospital IV treatment of SIADH, and others are in various stages of development. With either approach, fluid intake should be restricted to less than urine output, and serum sodium should be checked at least once every 2h to ensure it is not raised too fast or too far. Doing so may result in central pontine myelinolysis, an acute, potentially fatal neurologic syndrome characterized by quadriparesis, ataxia, and abnormal extraocular movements.
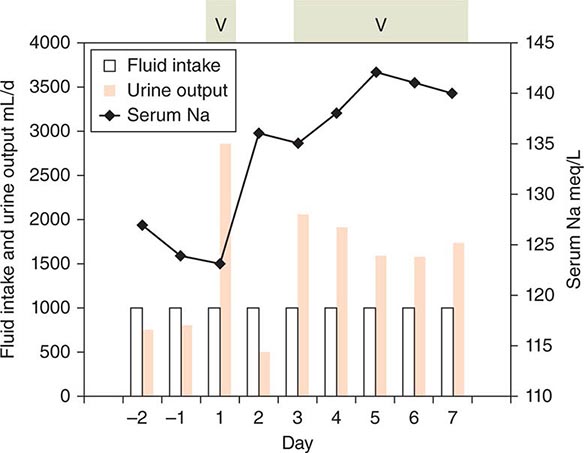
FIGURE 404-7 The effect of vaptan therapy on water balance in a patient with chronic syndrome of inappropriate antidiuretic hormone (SIADH). The periods of vaptan (V) therapy are indicated by the green shaded boxes at the top. Urine output is indicated by orange bars. Fluid intake is shown by the open bars. Intake was restricted to 1 L/d throughout. Serum sodium is indicated by the black line. Note that sodium increased progressively when vaptan increased urine output to levels that clearly exceeded fluid intake.
In chronic and/or minimally symptomatic SIADH, the hyponatremia can and should be corrected more gradually. This can be achieved by restricting total fluid intake to less than the sum of urinary and insensible losses. Because the water derived from food (300–700 mL/d) usually approximates basal insensible losses in adults, the aim should be to reduce total discretionary intake (all liquids) to approximately 500 mL less than urinary output. Adherence to this regimen is often problematic and, even if achieved, usually reduces body water and increases serum sodium by only about 1–2% per day. Hence, additional approaches are usually desirable if not necessary. The best approach for treatment of chronic SIADH is the administration of an oral vaptan, tolvaptan, a selective V2 antagonist that also increases urinary water excretion by blocking the antidiuretic effect of AVP. Some restriction of fluid intake may also be necessary to achieve satisfactory control of the hyponatremia. It is approved for treatment of nonemergent SIADH with initial in-hospital dosing. Other approaches include demeclocycline, 150–300 mg PO tid or qid, or fludrocortisone, 0.05–0.2 mg PO bid. The effect of the demeclocycline manifests in 7–14 days and is due to induction of a reversible form of nephrogenic DI. Potential side effects include phototoxicity and azotemia. The effect of fludrocortisone also requires 1–2 weeks and is partly due to increased retention of sodium and possibly inhibition of thirst. It also increases urinary potassium excretion, which may require replacement through dietary adjustments or supplements and may induce hypertension, occasionally necessitating discontinuation of the treatment.
In euvolemic hyponatremia caused by protracted nausea and vomiting or isolated glucocorticoid deficiency (type III), all abnormalities can be corrected quickly and completely by giving an antiemetic or stress doses of hydrocortisone (for glucocorticoid deficiency). As with other treatments, care must be taken to ensure that serum sodium does not rise too quickly or too far.
In SIAD due to an activating mutation of the V2 receptor, the V2 antagonists usually do not block the antidiuresis or raise plasma osmolarity/sodium. In that condition, use of an osmotic diuretic such as urea is reported to be effective in preventing or correcting hyponatremia. However, some vaptans may be effective in patients with a different type of activating mutation so the response to this therapy may be neither predictable nor diagnostic.
In hypervolemic hyponatremia, fluid restriction is also appropriate and somewhat effective if it can be maintained. However, infusion of hypertonic saline is contraindicated because it further increases total body sodium and edema and may precipitate cardiovascular decompensation. However, as in SIADH, the V2 receptor antagonists are also safe and effective in the treatment of hypervolemic hyponatremia caused by congestive heart failure. Tolvaptan is approved by the Food and Drug Administration for this indication with the caveat that treatment should be initiated or reinitiated in hospital. Its use should also be limited to 30 days at a time because of reports that longer periods may be associated with abnormal liver chemistries.
In hypovolemic hyponatremia, the defect in AVP secretion and water balance usually can be corrected easily and quickly by stopping the loss of sodium and water and/or replacing the deficits by mouth or IV infusion of normal or hypertonic saline. As with the treatment of other forms of hyponatremia, care must be taken to ensure that plasma sodium does not increase too rapidly or too far. Fluid restriction and administration of AVP antagonists are contraindicated in type II hyponatremia because they would only aggravate the underlying volume depletion and could result in hemodynamic collapse.
GLOBAL PERSPECTIVES
 The incidence, clinical characteristics, etiology, pathophysiology, differential diagnosis, and treatments of fluid and electrolyte disorders in tropical and nonindustrialized countries differ in some respects from those in the United States and other industrialized parts of the world. Hyponatremia, for example, appears to be more common and is more likely to be due to infectious diseases such as cholera, shigellosis, and other diarrheal disorders. In these circumstances, hyponatremia is probably due to gastrointestinal losses of salt and water (hypovolemia type II), but other abnormalities, including undefined infectious toxins, also may contribute. The causes of DI are similar worldwide except that malaria and venoms from snake or insect bites are much more common.
The incidence, clinical characteristics, etiology, pathophysiology, differential diagnosis, and treatments of fluid and electrolyte disorders in tropical and nonindustrialized countries differ in some respects from those in the United States and other industrialized parts of the world. Hyponatremia, for example, appears to be more common and is more likely to be due to infectious diseases such as cholera, shigellosis, and other diarrheal disorders. In these circumstances, hyponatremia is probably due to gastrointestinal losses of salt and water (hypovolemia type II), but other abnormalities, including undefined infectious toxins, also may contribute. The causes of DI are similar worldwide except that malaria and venoms from snake or insect bites are much more common.
405 |
Disorders of the Thyroid Gland |
The thyroid gland produces two related hormones, thyroxine (T4) and triiodothyronine (T3) (Fig. 405-1). Acting through thyroid hormone receptors α and β, these hormones play a critical role in cell differentiation during development and help maintain thermogenic and metabolic homeostasis in the adult. Autoimmune disorders of the thyroid gland can stimulate overproduction of thyroid hormones (thyrotoxicosis) or cause glandular destruction and hormone deficiency (hypothyroidism). In addition, benign nodules and various forms of thyroid cancer are relatively common and amenable to detection by physical examination.
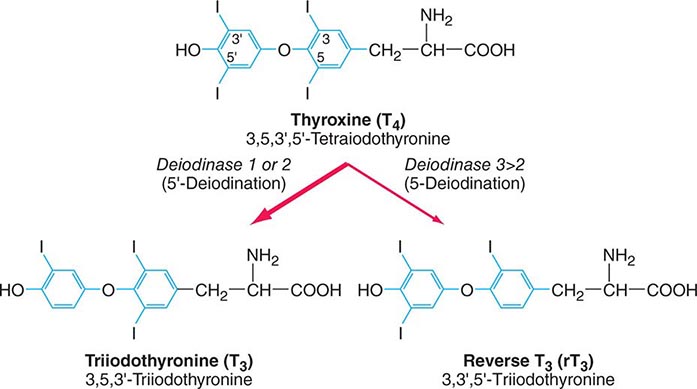
FIGURE 405-1 Structures of thyroid hormones. Thyroxine (T4) contains four iodine atoms. Deiodination leads to production of the potent hormone triiodothyronine (T3) or the inactive hormone reverse T3.
ANATOMY AND DEVELOPMENT
The thyroid (Greek thyreos, shield, plus eidos, form) consists of two lobes connected by an isthmus. It is located anterior to the trachea between the cricoid cartilage and the suprasternal notch. The normal thyroid is 12–20 g in size, highly vascular, and soft in consistency. Four parathyroid glands, which produce parathyroid hormone (Chap. 424), are located posterior to each pole of the thyroid. The recurrent laryngeal nerves traverse the lateral borders of the thyroid gland and must be identified during thyroid surgery to avoid injury and vocal cord paralysis.
The thyroid gland develops from the floor of the primitive pharynx during the third week of gestation. The developing gland migrates along the thyroglossal duct to reach its final location in the neck. This feature accounts for the rare ectopic location of thyroid tissue at the base of the tongue (lingual thyroid) as well as the occurrence of thyroglossal duct cysts along this developmental tract. Thyroid hormone synthesis normally begins at about 11 weeks’ gestation.
Neural crest derivatives from the ultimobranchial body give rise to thyroid medullary C cells that produce calcitonin, a calcium-lowering hormone. The C cells are interspersed throughout the thyroid gland, although their density is greatest in the juncture of the upper one-third and lower two-thirds of the gland. Calcitonin plays a minimal role in calcium homeostasis in humans but the C-cells are important because of their involvement in medullary thyroid cancer.
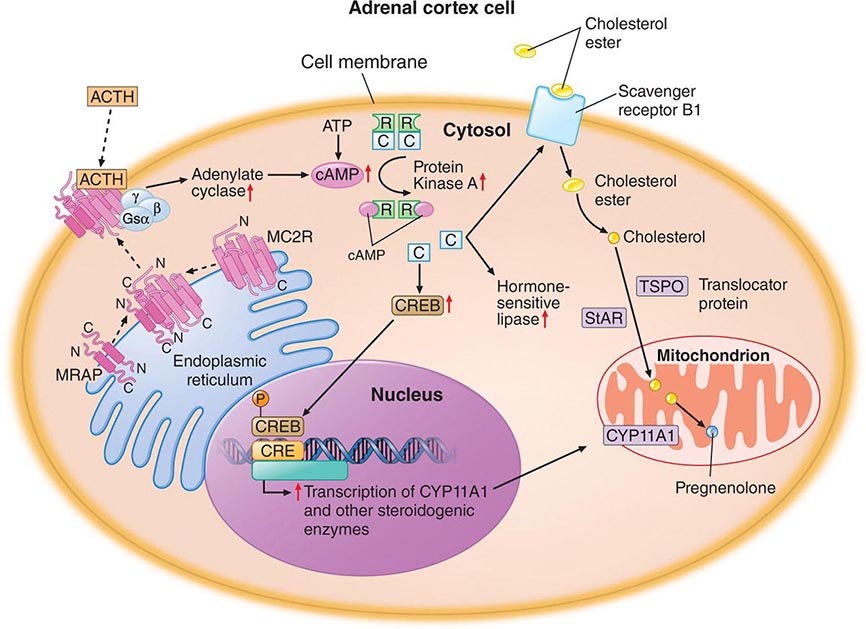
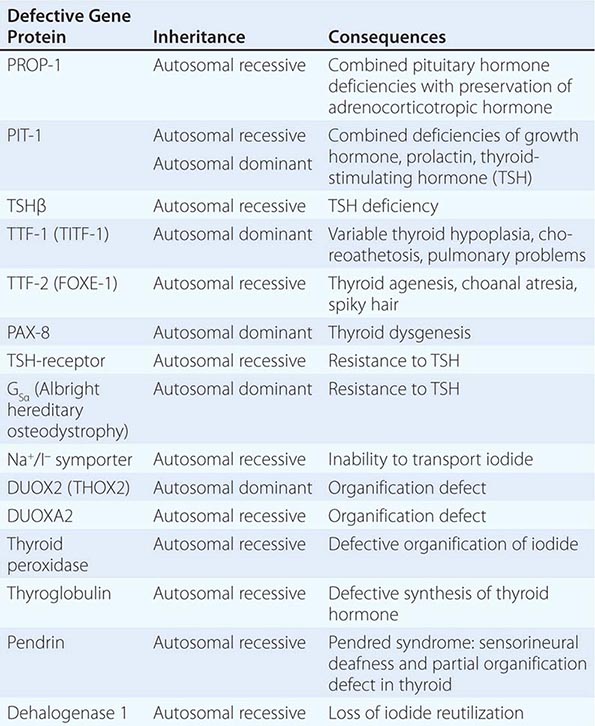
Thyroid gland development is orchestrated by the coordinated expression of several developmental transcription factors. Thyroid transcription factor (TTF)-1, TTF-2, and paired homeobox-8 (PAX-8) are expressed selectively, but not exclusively, in the thyroid gland. In combination, they dictate thyroid cell development and the induction of thyroid-specific genes such as thyroglobulin (Tg), thyroid peroxidase (TPO), the sodium iodide symporter (Na+/I–, NIS), and the thyroid-stimulating hormone receptor (TSH-R). Mutations in these developmental transcription factors or their downstream target genes are rare causes of thyroid agenesis or dyshormonogenesis, although the causes of most forms of congenital hypothyroidism remain unknown (Table 405-1). Because congenital hypothyroidism occurs in approximately 1 in 4000 newborns, neonatal screening is now performed in most industrialized countries (see below). Transplacental passage of maternal thyroid hormone occurs before the fetal thyroid gland begins to function and provides partial hormone support to a fetus with congenital hypothyroidism. Early thyroid hormone replacement in newborns with congenital hypothyroidism prevents potentially severe developmental abnormalities.
|
GENETIC CAUSES OF CONGENITAL HYPOTHYROIDISM |
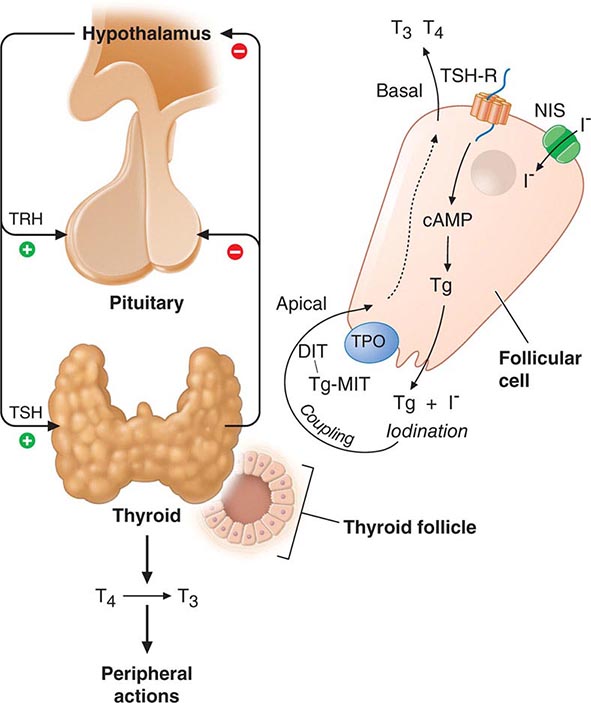
The thyroid gland consists of numerous spherical follicles composed of thyroid follicular cells that surround secreted colloid, a proteinaceous fluid containing large amounts of thyroglobulin, the protein precursor of thyroid hormones (Fig. 405-2). The thyroid follicular cells are polarized—the basolateral surface is apposed to the bloodstream and an apical surface faces the follicular lumen. Increased demand for thyroid hormone is regulated by thyroid-stimulating hormone (TSH), which binds to its receptor on the basolateral surface of the follicular cells. This binding leads to Tg reabsorption from the follicular lumen and proteolysis within the cytoplasm, yielding thyroid hormones for secretion into the bloodstream.
FIGURE 405-2 Regulation of thyroid hormone synthesis. Left. Thyroid hormones T4 and T3 feed back to inhibit hypothalamic production of thyrotropin-releasing hormone (TRH) and pituitary production of thyroid-stimulating hormone (TSH). TSH stimulates thyroid gland production of T4 and T3. Right. Thyroid follicles are formed by thyroid epithelial cells surrounding proteinaceous colloid, which contains thyroglobulin. Follicular cells, which are polarized, synthesize thyroglobulin and carry out thyroid hormone biosynthesis (see text for details). DIT, diiodotyrosine; MIT, monoiodotyrosine; NIS, sodium iodide symporter; Tg, thyroglobulin; TPO, thyroid peroxidase; TSH-R, thyroid-stimulating hormone receptor.
REGULATION OF THE THYROID AXIS
TSH, secreted by the thyrotrope cells of the anterior pituitary, plays a pivotal role in control of the thyroid axis and serves as the most useful physiologic marker of thyroid hormone action. TSH is a 31-kDa hormone composed of α and β subunits; the α subunit is common to the other glycoprotein hormones (luteinizing hormone, follicle-stimulating hormone, human chorionic gonadotropin [hCG]), whereas the TSH β subunit is unique to TSH. The extent and nature of carbohydrate modification are modulated by thyrotropin-releasing hormone (TRH) stimulation and influence the biologic activity of the hormone.
The thyroid axis is a classic example of an endocrine feedback loop. Hypothalamic TRH stimulates pituitary production of TSH, which, in turn, stimulates thyroid hormone synthesis and secretion. Thyroid hormones act via negative feedback predominantly through thyroid hormone receptor β2 (TRβ2) to inhibit TRH and TSH production (Fig. 405-2). The “set-point” in this axis is established by TSH. TRH is the major positive regulator of TSH synthesis and secretion. Peak TSH secretion occurs ~15 min after administration of exogenous TRH. Dopamine, glucocorticoids, and somatostatin suppress TSH but are not of major physiologic importance except when these agents are administered in pharmacologic doses. Reduced levels of thyroid hormone increase basal TSH production and enhance TRH-mediated stimulation of TSH. High thyroid hormone levels rapidly and directly suppress TSH gene expression secretion and inhibit TRH stimulation of TSH, indicating that thyroid hormones are the dominant regulator of TSH production. Like other pituitary hormones, TSH is released in a pulsatile manner and exhibits a diurnal rhythm; its highest levels occur at night. However, these TSH excursions are modest in comparison to those of other pituitary hormones, in part, because TSH has a relatively long plasma half-life (50 min). Consequently, single measurements of TSH are adequate for assessing its circulating level. TSH is measured using immunoradiometric assays that are highly sensitive and specific. These assays readily distinguish between normal and suppressed TSH values; thus, TSH can be used for the diagnosis of hyperthyroidism (low TSH) as well as hypothyroidism (high TSH).
THYROID HORMONE SYNTHESIS, METABOLISM, AND ACTION
THYROID HORMONE SYNTHESIS
Thyroid hormones are derived from Tg, a large iodinated glycoprotein. After secretion into the thyroid follicle, Tg is iodinated on tyrosine residues that are subsequently coupled via an ether linkage. Reuptake of Tg into the thyroid follicular cell allows proteolysis and the release of newly synthesized T4 and T3.
Iodine Metabolism and Transport Iodide uptake is a critical first step in thyroid hormone synthesis. Ingested iodine is bound to serum proteins, particularly albumin. Unbound iodine is excreted in the urine. The thyroid gland extracts iodine from the circulation in a highly efficient manner. For example, 10–25% of radioactive tracer (e.g., 123I) is taken up by the normal thyroid gland over 24 h; this value can rise to 70–90% in Graves’ disease. Iodide uptake is mediated by NIS, which is expressed at the basolateral membrane of thyroid follicular cells. NIS is most highly expressed in the thyroid gland, but low levels are present in the salivary glands, lactating breast, and placenta. The iodide transport mechanism is highly regulated, allowing adaptation to variations in dietary supply. Low iodine levels increase the amount of NIS and stimulate uptake, whereas high iodine levels suppress NIS expression and uptake. The selective expression of NIS in the thyroid allows isotopic scanning, treatment of hyperthyroidism, and ablation of thyroid cancer with radioisotopes of iodine, without significant effects on other organs. Mutation of the NIS gene is a rare cause of congenital hypothyroidism, underscoring its importance in thyroid hormone synthesis. Another iodine transporter, pendrin, is located on the apical surface of thyroid cells and mediates iodine efflux into the lumen. Mutation of the pendrin gene causes Pendred syndrome, a disorder characterized by defective organification of iodine, goiter, and sensorineural deafness.
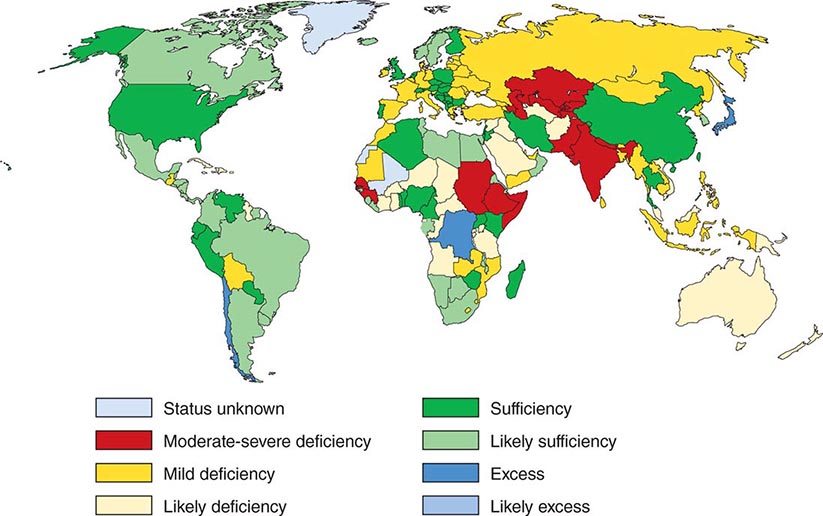
 Iodine deficiency is prevalent in many mountainous regions and in central Africa, central South America, and northern Asia (Fig. 405-3). Europe remains mildly iodine-deficient, and health surveys indicate that iodine intake has been falling in the United States and Australia. The World Health Organization (WHO) estimates that about 2 billion people are iodine-deficient, based on urinary excretion data. In areas of relative iodine deficiency, there is an increased prevalence of goiter and, when deficiency is severe, hypothyroidism and cretinism. Cretinism is characterized by mental and growth retardation and occurs when children who live in iodine-deficient regions are not treated with iodine or thyroid hormone to restore normal thyroid hormone levels during early life. These children are often born to mothers with iodine deficiency, and it is likely that maternal thyroid hormone deficiency worsens the condition. Concomitant selenium deficiency may also contribute to the neurologic manifestations of cretinism. Iodine supplementation of salt, bread, and other food substances has markedly reduced the prevalence of cretinism. Unfortunately, however, iodine deficiency remains the most common cause of preventable mental deficiency, often because of societal resistance to food additives or the cost of supplementation. In addition to overt cretinism, mild iodine deficiency can lead to subtle reduction of IQ. Oversupply of iodine, through supplements or foods enriched in iodine (e.g., shellfish, kelp), is associated with an increased incidence of autoimmune thyroid disease. The recommended average daily intake of iodine is 150–250 μg/d for adults, 90–120 μg/d for children, and 250 μg/d for pregnant and lactating women. Urinary iodine is >10 μg/dL in iodine-sufficient populations.
Iodine deficiency is prevalent in many mountainous regions and in central Africa, central South America, and northern Asia (Fig. 405-3). Europe remains mildly iodine-deficient, and health surveys indicate that iodine intake has been falling in the United States and Australia. The World Health Organization (WHO) estimates that about 2 billion people are iodine-deficient, based on urinary excretion data. In areas of relative iodine deficiency, there is an increased prevalence of goiter and, when deficiency is severe, hypothyroidism and cretinism. Cretinism is characterized by mental and growth retardation and occurs when children who live in iodine-deficient regions are not treated with iodine or thyroid hormone to restore normal thyroid hormone levels during early life. These children are often born to mothers with iodine deficiency, and it is likely that maternal thyroid hormone deficiency worsens the condition. Concomitant selenium deficiency may also contribute to the neurologic manifestations of cretinism. Iodine supplementation of salt, bread, and other food substances has markedly reduced the prevalence of cretinism. Unfortunately, however, iodine deficiency remains the most common cause of preventable mental deficiency, often because of societal resistance to food additives or the cost of supplementation. In addition to overt cretinism, mild iodine deficiency can lead to subtle reduction of IQ. Oversupply of iodine, through supplements or foods enriched in iodine (e.g., shellfish, kelp), is associated with an increased incidence of autoimmune thyroid disease. The recommended average daily intake of iodine is 150–250 μg/d for adults, 90–120 μg/d for children, and 250 μg/d for pregnant and lactating women. Urinary iodine is >10 μg/dL in iodine-sufficient populations.
FIGURE 405-3 Worldwide iodine nutrition. Data are from the World Health Organization and the International Council for the Control of Iodine Deficiency Disorders (http://indorgs.virginia.edu/iccidd/mi/cidds.html).
Organification, Coupling, Storage, and Release After iodide enters the thyroid, it is trapped and transported to the apical membrane of thyroid follicular cells, where it is oxidized in an organification reaction that involves TPO and hydrogen peroxide produced by dual oxidase (DUOX) and DUOX maturation factor (DUOXA). The reactive iodine atom is added to selected tyrosyl residues within Tg, a large (660 kDa) dimeric protein that consists of 2769 amino acids. The iodotyrosines in Tg are then coupled via an ether linkage in a reaction that is also catalyzed by TPO. Either T4 or T3 can be produced by this reaction, depending on the number of iodine atoms present in the iodotyrosines. After coupling, Tg is taken back into the thyroid cell, where it is processed in lysosomes to release T4 and T3. Uncoupled mono- and diiodotyrosines (MIT, DIT) are deiodinated by the enzyme dehalogenase, thereby recycling any iodide that is not converted into thyroid hormones.
Disorders of thyroid hormone synthesis are rare causes of congenital hypothyroidism. The vast majority of these disorders are due to recessive mutations in TPO or Tg, but defects have also been identified in the TSH-R, NIS, pendrin, hydrogen peroxide generation, and dehalogenase. Because of the biosynthetic defect, the gland is incapable of synthesizing adequate amounts of hormone, leading to increased TSH and a large goiter.
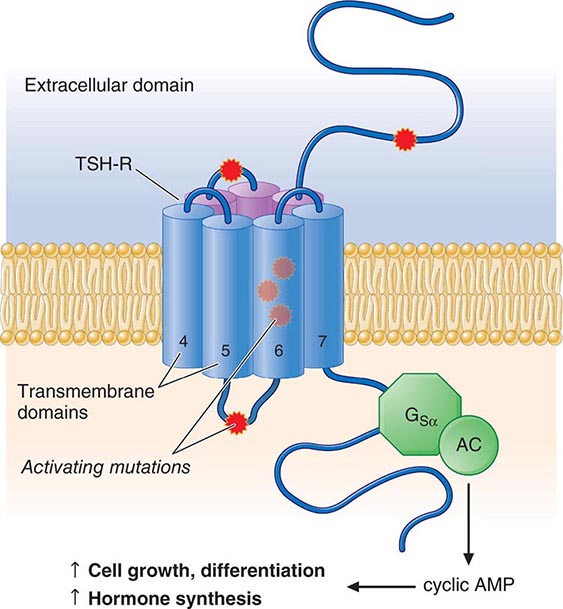
TSH Action TSH regulates thyroid gland function through the TSH-R, a seven-transmembrane G protein–coupled receptor (GPCR). The TSH-R is coupled to the α subunit of stimulatory G protein (GSα), which activates adenylyl cyclase, leading to increased production of cyclic adenosine monophosphate (AMP). TSH also stimulates phosphatidylinositol turnover by activating phospholipase C. The functional role of the TSH-R is exemplified by the consequences of naturally occurring mutations. Recessive loss-of-function mutations cause thyroid hypoplasia and congenital hypothyroidism. Dominant gain-of-function mutations cause sporadic or familial hyperthyroidism that is characterized by goiter, thyroid cell hyperplasia, and autonomous function. Most of these activating mutations occur in the transmembrane domain of the receptor. They mimic the conformational changes induced by TSH binding or the interactions of thyroid-stimulating immunoglobulins (TSI) in Graves’ disease. Activating TSH-R mutations also occur as somatic events, leading to clonal selection and expansion of the affected thyroid follicular cell and autonomously functioning thyroid nodules (see below).
Other Factors That Influence Hormone Synthesis and Release Although TSH is the dominant hormonal regulator of thyroid gland growth and function, a variety of growth factors, most produced locally in the thyroid gland, also influence thyroid hormone synthesis. These include insulin-like growth factor I (IGF-I), epidermal growth factor, transforming growth factor β (TGF-β), endothelins, and various cytokines. The quantitative roles of these factors are not well understood, but they are important in selected disease states. In acromegaly, for example, increased levels of growth hormone and IGF-I are associated with goiter and predisposition to multinodular goiter (MNG). Certain cytokines and interleukins (ILs) produced in association with autoimmune thyroid disease induce thyroid growth, whereas others lead to apoptosis. Iodine deficiency increases thyroid blood flow and upregulates the NIS, stimulating more efficient iodine uptake. Excess iodide transiently inhibits thyroid iodide organification, a phenomenon known as the Wolff-Chaikoff effect. In individuals with a normal thyroid, the gland escapes from this inhibitory effect and iodide organification resumes; the suppressive action of high iodide may persist, however, in patients with underlying autoimmune thyroid disease.
THYROID HORMONE TRANSPORT AND METABOLISM
Serum Binding Proteins T4 is secreted from the thyroid gland in about twentyfold excess over T3 (Table 405-2). Both hormones are bound to plasma proteins, including thyroxine-binding globulin (TBG), transthyretin (TTR, formerly known as thyroxine-binding prealbumin, or TBPA), and albumin. The plasma-binding proteins increase the pool of circulating hormone, delay hormone clearance, and may modulate hormone delivery to selected tissue sites. The concentration of TBG is relatively low (1–2 mg/dL), but because of its high affinity for thyroid hormones (T4 > T3), it carries about 80% of the bound hormones. Albumin has relatively low affinity for thyroid hormones but has a high plasma concentration (~3.5 g/dL), and it binds up to 10% of T4 and 30% of T3. TTR carries about 10% of T4 but little T3.
|
CHARACTERISTICS OF CIRCULATING T4 AND T3 |
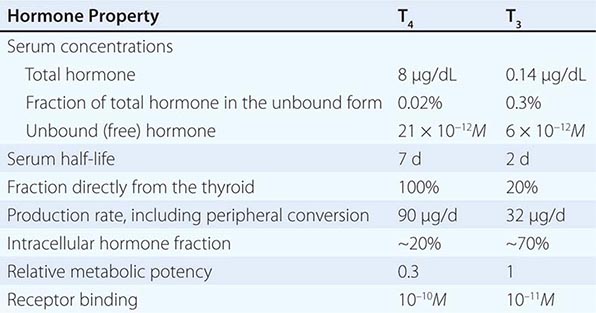
When the effects of the various binding proteins are combined, approximately 99.98% of T4 and 99.7% of T3 are protein-bound. Because T3 is less tightly bound than T4, the fraction of unbound T3 is greater than unbound T4, but there is less unbound T3 in the circulation because it is produced in smaller amounts and cleared more rapidly than T4. The unbound or “free” concentrations of the hormones are ~2 × 10–11 M for T4 and ~6 × 10–12 M for T3, which roughly correspond to the thyroid hormone receptor binding constants for these hormones (see below). The unbound hormone is thought to be biologically available to tissues. Nonetheless, the homeostatic mechanisms that regulate the thyroid axis are directed toward maintenance of normal concentrations of unbound hormones.
Abnormalities of Thyroid Hormone Binding Proteins A number of inherited and acquired abnormalities affect thyroid hormone binding proteins. X-linked TBG deficiency is associated with very low levels of total T4 and T3. However, because unbound hormone levels are normal, patients are euthyroid and TSH levels are normal. It is important to recognize this disorder to avoid efforts to normalize total T4 levels, because this leads to thyrotoxicosis and is futile because of rapid hormone clearance in the absence of TBG. TBG levels are elevated by estrogen, which increases sialylation and delays TBG clearance. Consequently, in women who are pregnant or taking estrogen-containing contraceptives, elevated TBG increases total T4 and T3 levels; however, unbound T4 and T3 levels are normal. These features are part of the explanation for why women with hypothyroidism require increased amounts of L-thyroxine replacement as TBG levels are increased by pregnancy or estrogen treatment. Mutations in TBG, TTR, and albumin may increase the binding affinity for T4 and/or T3 and cause disorders known as euthyroid hyperthyroxinemia or familial dysalbuminemic hyperthyroxinemia (FDH) (Table 405-3). These disorders result in increased total T4 and/or T3, but unbound hormone levels are normal. The familial nature of the disorders, and the fact that TSH levels are normal rather than suppressed, should suggest this diagnosis. Unbound hormone levels (ideally measured by dialysis) are normal in FDH. The diagnosis can be confirmed by using tests that measure the affinities of radiolabeled hormone binding to specific transport proteins or by performing DNA sequence analyses of the abnormal transport protein genes.
|
CONDITIONS ASSOCIATED WITH EUTHYROID HYPERTHYROXINEMIA |
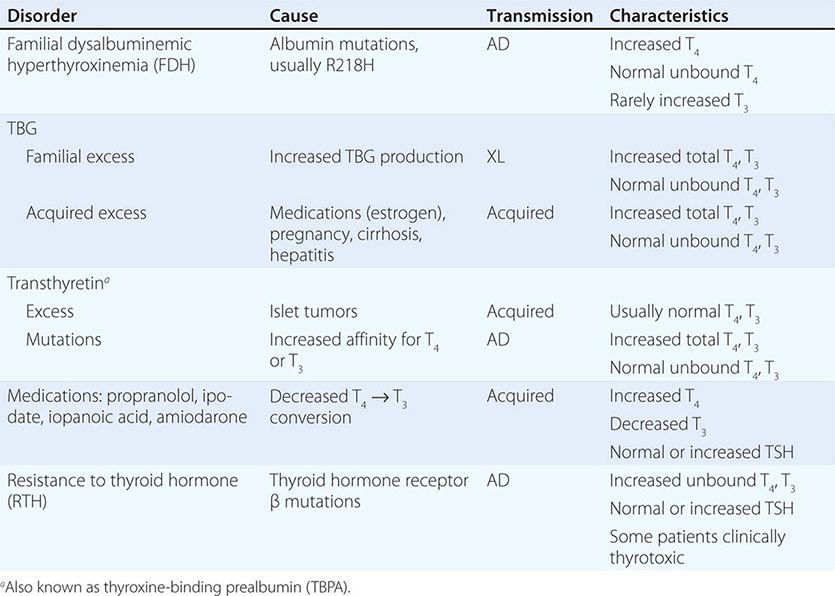
Abbreviations: AD, autosomal dominant; TBG, thyroxine-binding globulin; TSH, thyroid-stimulating hormone; XL, X-linked.
Certain medications, such as salicylates and salsalate, can displace thyroid hormones from circulating binding proteins. Although these drugs transiently perturb the thyroid axis by increasing free thyroid hormone levels, TSH is suppressed until a new steady state is reached, thereby restoring euthyroidism. Circulating factors associated with acute illness may also displace thyroid hormone from binding proteins (see “Sick Euthyroid Syndrome,” below).
Deiodinases T4 may be thought of as a precursor for the more potent T3. T4 is converted to T3 by the deiodinase enzymes (Fig. 405-1). Type I deiodinase, which is located primarily in thyroid, liver, and kidneys, has a relatively low affinity for T4. Type II deiodinase has a higher affinity for T4 and is found primarily in the pituitary gland, brain, brown fat, and thyroid gland. Expression of type II deiodinase allows it to regulate T3 concentrations locally, a property that may be important in the context of levothyroxine (T4) replacement. Type II deiodinase is also regulated by thyroid hormone; hypothyroidism induces the enzyme, resulting in enhanced T4 → T3 conversion in tissues such as brain and pituitary. T4 → T3 conversion is impaired by fasting, systemic illness or acute trauma, oral contrast agents, and a variety of medications (e.g., propylthiouracil, propranolol, amiodarone, glucocorticoids). Type III deiodinase inactivates T4 and T3 and is the most important source of reverse T3 (rT3), including in the sick euthyroid syndrome. This enzyme is expressed in the human placenta but is not active in healthy individuals. In the sick euthyroid syndrome, especially with hypoperfusion, the type III deiodinase is activated in muscle and liver. Massive hemangiomas that express type III deiodinase are a rare cause of hypothyroidism in infants.
THYROID HORMONE ACTION
Thyroid Hormone Transport Circulating thyroid hormones enter cells by passive diffusion and via specific transporters such as the monocarboxylate 8 transporter (MCT8), MCT10, and organic anion-transporting polypeptide 1C1. Mutations in the MCT8 gene have been identified in patients with X-linked psychomotor retardation and thyroid function abnormalities (low T4, high T3, and high TSH). After entering cells, thyroid hormones act primarily through nuclear receptors, although they also have nongenomic actions through stimulating mitochondrial enzymatic responses and may act directly on blood vessels and the heart through integrin receptors.
Nuclear Thyroid Hormone Receptors Thyroid hormones bind with high affinity to nuclear thyroid hormone receptors (TRs) α and β. Both TRα and TRβ are expressed in most tissues, but their relative expression levels vary among organs; TRα is particularly abundant in brain, kidneys, gonads, muscle, and heart, whereas TRβ expression is relatively high in the pituitary and liver. Both receptors are variably spliced to form unique isoforms. The TRβ2 isoform, which has a unique amino terminus, is selectively expressed in the hypothalamus and pituitary, where it plays a role in feedback control of the thyroid axis (see above). The TRα2 isoform contains a unique carboxy terminus that precludes thyroid hormone binding; it may function to block the action of other TR isoforms.
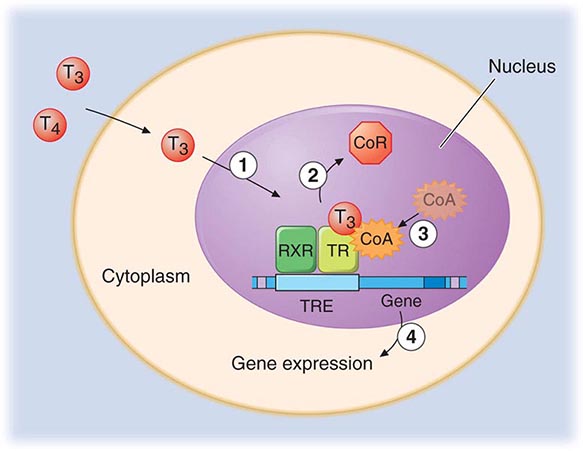
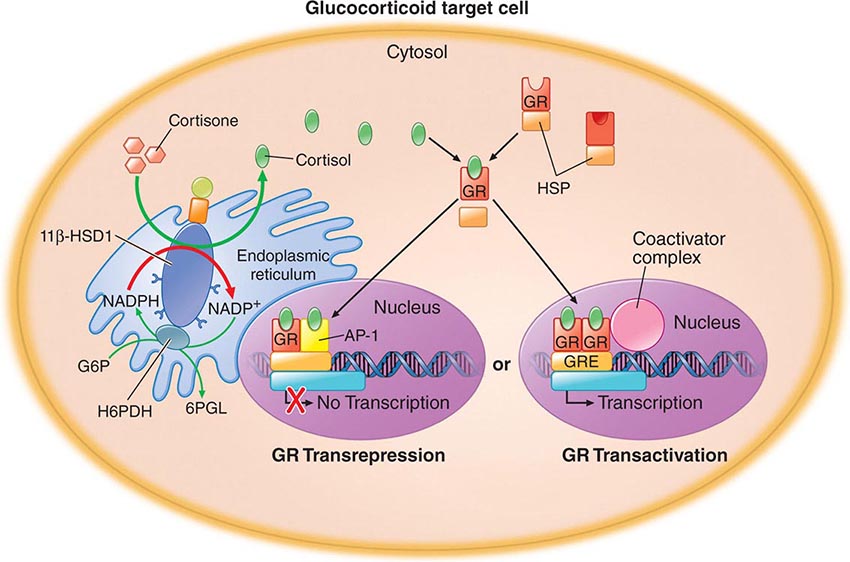
The TRs contain a central DNA-binding domain and a C-terminal ligand-binding domain. They bind to specific DNA sequences, termed thyroid response elements (TREs), in the promoter regions of target genes (Fig. 405-4). The receptors bind as homodimers or, more commonly, as heterodimers with retinoic acid × receptors (RXRs) (Chap. 400e). The activated receptor can either stimulate gene transcription (e.g., myosin heavy chain α) or inhibit transcription (e.g., TSH β-subunit gene), depending on the nature of the regulatory elements in the target gene.
FIGURE 405-4 Mechanism of thyroid hormone receptor action. The thyroid hormone receptor (TR) and retinoid × receptor (RXR) form heterodimers that bind specifically to thyroid hormone response elements (TRE) in the promoter regions of target genes. In the absence of hormone, TR binds co-repressor (CoR) proteins that silence gene expression. The numbers refer to a series of ordered reactions that occur in response to thyroid hormone: (1) T4 or T3 enters the nucleus; (2) T3 binding dissociates CoR from TR; (3) co-activators (CoA) are recruited to the T3-bound receptor; and (4) gene expression is altered.
Thyroid hormones (T3 and T4) bind with similar affinities to TRα and TRβ. However, structural differences in the ligand binding domains provide the potential for developing receptor-selective agonists or antagonists, and these are under investigation. T3 is bound with 10–15 times greater affinity than T4, which explains its increased hormonal potency. Although T4 is produced in excess of T3, receptors are occupied mainly by T3, reflecting T4 → T3 conversion by peripheral tissues, greater T3 bioavailability in the plasma, and the greater affinity of receptors for T3. After binding to TRs, thyroid hormone induces conformational changes in the receptors that modify its interactions with accessory transcription factors. Importantly, in the absence of thyroid hormone binding, the aporeceptors bind to co-repressor proteins that inhibit gene transcription. Hormone binding dissociates the co-repressors and allows the recruitment of co-activators that enhance transcription. The discovery of TR interactions with co-repressors explains the fact that TR silences gene expression in the absence of hormone binding. Consequently, hormone deficiency has a profound effect on gene expression because it causes gene repression as well as loss of hormone-induced stimulation. This concept has been corroborated by the finding that targeted deletion of the TR genes in mice has a less pronounced phenotypic effect than hormone deficiency.
Thyroid Hormone Resistance Resistance to thyroid hormone (RTH) is an autosomal dominant disorder characterized by elevated thyroid hormone levels and inappropriately normal or elevated TSH. Individuals with RTH do not, in general, exhibit signs and symptoms that are typical of hypothyroidism because hormone resistance is partial and is compensated by increased levels of thyroid hormone. The clinical features of RTH can include goiter, attention deficit disorder, mild reduction in IQ, delayed skeletal maturation, tachycardia, and impaired metabolic responses to thyroid hormone.
Classical forms of RTH are caused by mutations in the TRβ gene. These mutations, located in restricted regions of the ligand-binding domain, cause loss of receptor function. However, because the mutant receptors retain the capacity to dimerize with RXRs, bind to DNA, and recruit co-repressor proteins, they function as antagonists of the remaining normal TRβ and TRα receptors. This property, referred to as “dominant negative” activity, explains the autosomal dominant mode of transmission. The diagnosis is suspected when unbound thyroid hormone levels are increased without suppression of TSH. Similar hormonal abnormalities are found in other affected family members, although the TRβ mutation arises de novo in about 20% of patients. DNA sequence analysis of the TRβ gene provides a definitive diagnosis. RTH must be distinguished from other causes of euthyroid hyperthyroxinemia (e.g., FDH) and inappropriate secretion of TSH by TSH-secreting pituitary adenomas (Chap. 403). In most patients, no treatment is indicated; the importance of making the diagnosis is to avoid inappropriate treatment of mistaken hyperthyroidism and to provide genetic counseling.
A distinct form of RTH is caused by mutations in the TRα gene. Affected patients have many clinical features of congenital hypothyroidism including growth retardation, skeletal dysplasia, and severe constipation. In contrast to RTH caused by mutations in TRβ, thyroid function tests include normal TSH, low or normal T4, and normal or elevated T3 levels. These distinct clinical and laboratory features underscore the different tissue distribution and functional roles of TRβ and TRα. Optimal treatment of patients with RTH caused by TRα mutations has not been established.
PHYSICAL EXAMINATION
In addition to the examination of the thyroid itself, the physical examination should include a search for signs of abnormal thyroid function and the extrathyroidal features of ophthalmopathy and dermopathy (see below). Examination of the neck begins by inspecting the seated patient from the front and side and noting any surgical scars, obvious masses, or distended veins. The thyroid can be palpated with both hands from behind or while facing the patient, using the thumbs to palpate each lobe. It is best to use a combination of these methods, especially when nodules are small. The patient’s neck should be slightly flexed to relax the neck muscles. After locating the cricoid cartilage, the isthmus, which is attached to the lower one-third of the thyroid lobes, can be identified and then followed laterally to locate either lobe (normally, the right lobe is slightly larger than the left). By asking the patient to swallow sips of water, thyroid consistency can be better appreciated as the gland moves beneath the examiner’s fingers.
Features to be noted include thyroid size, consistency, nodularity, and any tenderness or fixation. An estimate of thyroid size (normally 12–20 g) should be made, and a drawing is often the best way to record findings. However, ultrasound is the method of choice when it is important to determine thyroid size accurately. The size, location, and consistency of any nodules should also be defined. A bruit or thrill over the gland, located over the insertion of the superior and inferior thyroid arteries (supero- or inferolaterally), indicates increased vascularity, as occurs in hyperthyroidism. If the lower borders of the thyroid lobes are not clearly felt, a goiter may be retrosternal. Large retrosternal goiters can cause venous distention over the neck and difficulty breathing, especially when the arms are raised (Pemberton’s sign). With any central mass above the thyroid, the tongue should be extended, as thyroglossal cysts then move upward. The thyroid examination is not complete without assessment for lymphadenopathy in the supraclavicular and cervical regions of the neck.
LABORATORY EVALUATION
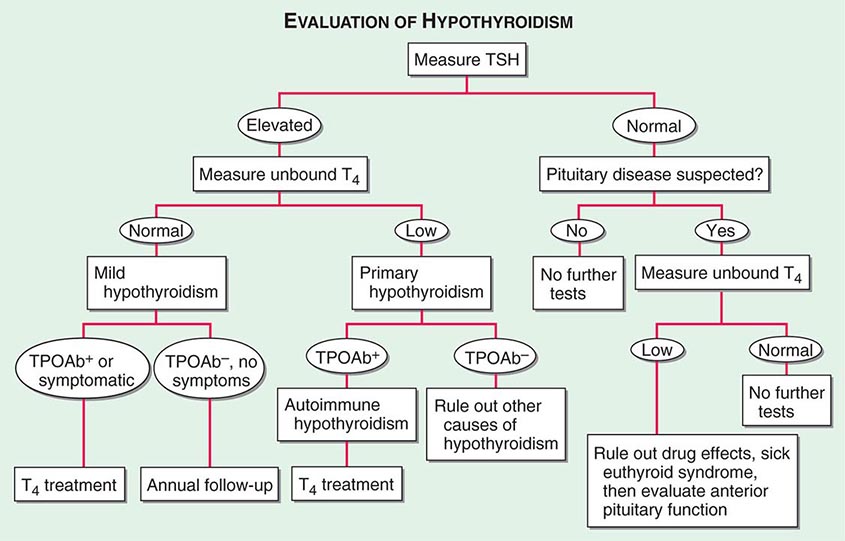
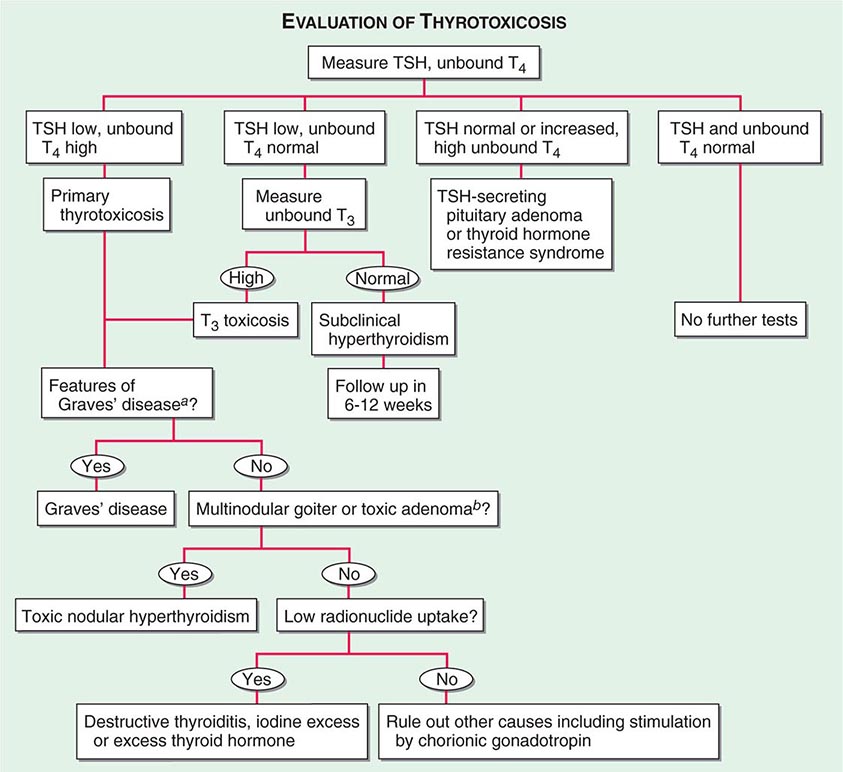
Measurement of Thyroid Hormones The enhanced sensitivity and specificity of TSH assays have greatly improved laboratory assessment of thyroid function. Because TSH levels change dynamically in response to alterations of T4 and T3, a logical approach to thyroid testing is to first determine whether TSH is suppressed, normal, or elevated. With rare exceptions (see below), a normal TSH level excludes a primary abnormality of thyroid function. This strategy depends on the use of immunochemiluminometric assays (ICMAs) for TSH that are sensitive enough to discriminate between the lower limit of the reference range and the suppressed values that occur with thyrotoxicosis. Extremely sensitive (fourth-generation) assays can detect TSH levels ≤0.004 mIU/L, but, for practical purposes, assays sensitive to ≤0.1 mIU/L are sufficient. The widespread availability of the TSH ICMA has rendered the TRH stimulation test obsolete, because the failure of TSH to rise after an intravenous bolus of 200–400 μg TRH has the same implications as a suppressed basal TSH measured by ICMA.
The finding of an abnormal TSH level must be followed by measurements of circulating thyroid hormone levels to confirm the diagnosis of hyperthyroidism (suppressed TSH) or hypothyroidism (elevated TSH). Radioimmunoassays are widely available for serum total T4 and total T3. T4 and T3 are highly protein-bound, and numerous factors (illness, medications, genetic factors) can influence protein binding. It is useful, therefore, to measure the free, or unbound, hormone levels, which correspond to the biologically available hormone pool. Two direct methods are used to measure unbound thyroid hormones: (1) unbound thyroid hormone competition with radiolabeled T4 (or an analogue) for binding to a solid-phase antibody, and (2) physical separation of the unbound hormone fraction by ultracentrifugation or equilibrium dialysis. Although early unbound hormone immunoassays suffered from artifacts, newer assays correlate well with the results of the more technically demanding and expensive physical separation methods. An indirect method that is now less commonly used to estimate unbound thyroid hormone levels is to calculate the free T3 or free T4 index from the total T4 or T3 concentration and the thyroid hormone binding ratio (THBR). The latter is derived from the T3-resin uptake test, which determines the distribution of radiolabeled T3 between an absorbent resin and the unoccupied thyroid hormone binding proteins in the sample. The binding of the labeled T3 to the resin is increased when there is reduced unoccupied protein binding sites (e.g., TBG deficiency) or increased total thyroid hormone in the sample; it is decreased under the opposite circumstances. The product of THBR and total T3 or T4 provides the free T3 or T4 index. In effect, the index corrects for anomalous total hormone values caused by abnormalities in hormone-protein binding.
Total thyroid hormone levels are elevated when TBG is increased due to estrogens (pregnancy, oral contraceptives, hormone therapy, tamoxifen, selective estrogen receptor modulators, inflammatory liver disease) and decreased when TBG binding is reduced (androgens, nephrotic syndrome). Genetic disorders and acute illness can also cause abnormalities in thyroid hormone binding proteins, and various drugs (phenytoin, carbamazepine, salicylates, and nonsteroidal anti-inflammatory drugs [NSAIDs]) can interfere with thyroid hormone binding. Because unbound thyroid hormone levels are normal and the patient is euthyroid in all of these circumstances, assays that measure unbound hormone are preferable to those for total thyroid hormones.
For most purposes, the unbound T4 level is sufficient to confirm thyrotoxicosis, but 2–5% of patients have only an elevated T3 level (T3 toxicosis). Thus, unbound T3 levels should be measured in patients with a suppressed TSH but normal unbound T4 levels.
There are several clinical conditions in which the use of TSH as a screening test may be misleading, particularly without simultaneous unbound T4 determinations. Any severe nonthyroidal illness can cause abnormal TSH levels (see below). Although hypothyroidism is the most common cause of an elevated TSH level, rare causes include a TSH-secreting pituitary tumor (Chap. 403), thyroid hormone resistance, and assay artifact. Conversely, a suppressed TSH level, particularly <0.01 mIU/L, usually indicates thyrotoxicosis. However, subnormal TSH levels between 0.01 and 0.1 mIU/L may be seen during the first trimester of pregnancy (due to hCG secretion), after treatment of hyperthyroidism (because TSH can remain suppressed for several months), and in response to certain medications (e.g., high doses of glucocorticoids or dopamine). Importantly, secondary hypothyroidism, caused by hypothalamic-pituitary disease, is associated with a variable (low to high-normal) TSH level, which is inappropriate for the low T4 level. Thus, TSH should not be used as an isolated laboratory test to assess thyroid function in patients with suspected or known pituitary disease.
Tests for the end-organ effects of thyroid hormone excess or depletion, such as estimation of basal metabolic rate, tendon reflex relaxation rates, or serum cholesterol, are not useful as clinical determinants of thyroid function.
Tests to Determine the Etiology of Thyroid Dysfunction Autoimmune thyroid disease is detected most easily by measuring circulating antibodies against TPO and Tg. Because antibodies to Tg alone are uncommon, it is reasonable to measure only TPO antibodies. About 5–15% of euthyroid women and up to 2% of euthyroid men have thyroid antibodies; such individuals are at increased risk of developing thyroid dysfunction. Almost all patients with autoimmune hypothyroidism, and up to 80% of those with Graves’ disease, have TPO antibodies, usually at high levels.
TSIs are antibodies that stimulate the TSH-R in Graves’ disease. They are most commonly measured by commercially available tracer displacement assays called TRAb (TSH receptor antibody) with the assumption that elevated levels in the setting of clinical hyperthyroidism reflect stimulatory effects on the TSH receptor. A bioassay is less commonly used. The main use of these assays is to predict neonatal thyrotoxicosis caused by high maternal levels of TRAb or TSI (>3× upper limit of normal) in the last trimester of pregnancy.
Serum Tg levels are increased in all types of thyrotoxicosis except thyrotoxicosis factitia caused by self-administration of thyroid hormone. Tg levels are particularly increased in thyroiditis, reflecting thyroid tissue destruction and release of Tg. The main role for Tg measurement, however, is in the follow-up of thyroid cancer patients. After total thyroidectomy and radioablation, Tg levels should be undetectable; in the absence of anti-Tg antibodies, measurable levels indicate incomplete ablation or recurrent cancer.
Radioiodine Uptake and Thyroid Scanning The thyroid gland selectively transports radioisotopes of iodine (123I, 125I, 131I) and 99mTc pertechnetate, allowing thyroid imaging and quantitation of radioactive tracer fractional uptake.
Nuclear imaging of Graves’ disease is characterized by an enlarged gland and increased tracer uptake that is distributed homogeneously. Toxic adenomas appear as focal areas of increased uptake, with suppressed tracer uptake in the remainder of the gland. In toxic MNG, the gland is enlarged—often with distorted architecture—and there are multiple areas of relatively increased (functioning nodules) or decreased tracer uptake (suppressed thyroid parenchyma or nonfunctioning nodules). Subacute, viral, and postpartum thyroiditis are associated with very low uptake because of follicular cell damage and TSH suppression. Thyrotoxicosis factitia is also associated with low uptake. In addition, if there is excessive circulating exogenous iodine (e.g., from dietary sources of iodinated contrast dye), the radionuclide uptake is low even in the presence of increased thyroid hormone production.
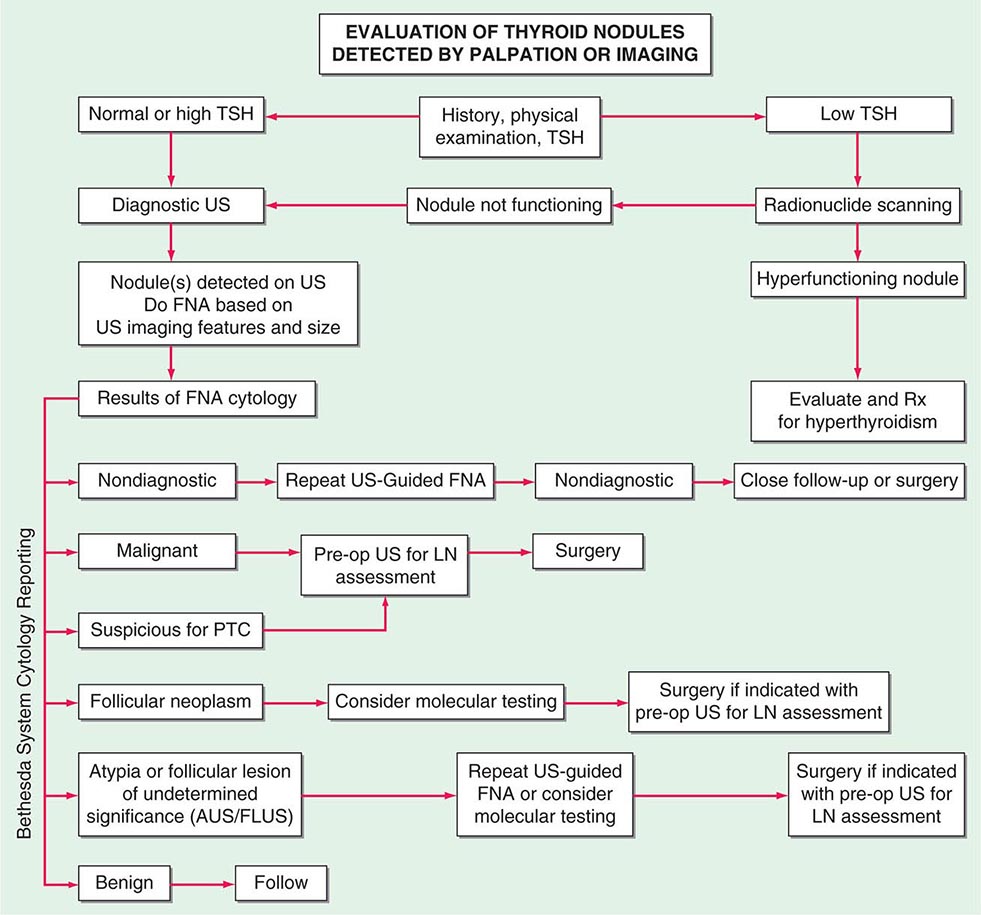
Thyroid scintigraphy is not used in the routine evaluation of patients with thyroid nodules, but should be performed if the serum TSH level is subnormal to determine if functioning thyroid nodules are present. Functioning or “hot” nodules are almost never malignant, and fine-needle aspiration (FNA) biopsy is not indicated. The vast majority of thyroid nodules do not produce thyroid hormone (“cold” nodules), and these are more likely to be malignant (~5–10%). Whole-body and thyroid scanning is also used in the treatment and surveillance of thyroid cancer. After thyroidectomy for thyroid cancer, the TSH level is raised by either using a thyroid hormone withdrawal protocol or recombinant human TSH injection (see below). Administration of 131I allows whole-body scanning (WBS) to confirm remnant ablation and to detect any functioning metastases. In addition, WBS may be helpful in surveillance of patients at risk for recurrence.
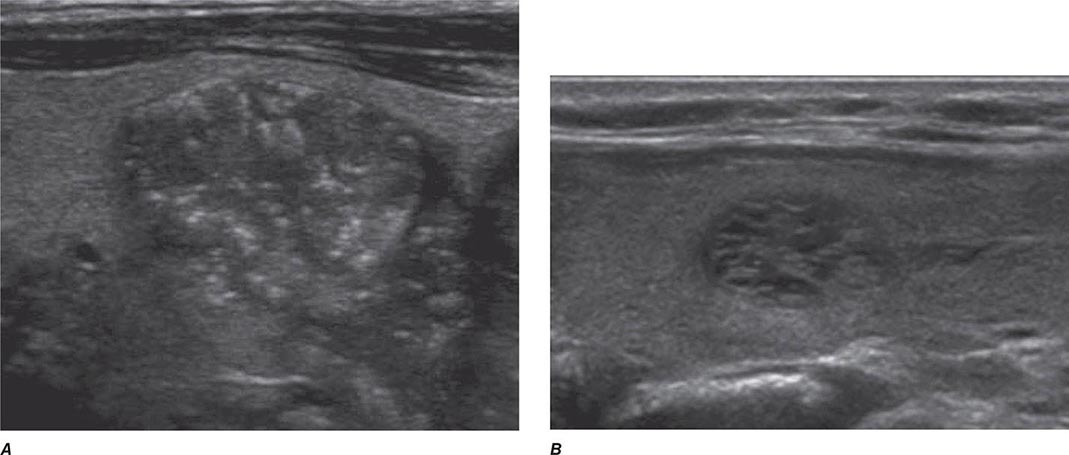
Thyroid Ultrasound Ultrasonography is valuable for the diagnosis and evaluation of patients with nodular thyroid disease (Table 405-4). Evidence-based guidelines recommend thyroid ultrasonography for all patients suspected of having thyroid nodules by either physical examination or another imaging study. Using 10- to 12-MHz linear transducers, resolution and image quality are excellent, allowing the characterization of nodules and cysts >3 mm. Certain sonographic patterns are highly suggestive of malignancy (e.g., hypoechoic solid nodules with infiltrative borders and microcalcifications), whereas other features correlate with benignity (e.g., spongiform nodules defined as those with multiple small internal cystic areas) (Fig. 405-5). In addition to evaluating thyroid nodules, ultrasound is useful for monitoring nodule size and for the aspiration of nodules or cystic lesions. Ultrasound-guided FNA biopsy of thyroid lesions lowers the rate of inadequate sampling and decreases sample error, thereby reducing the false-negative rate of FNA cytology. Ultrasonography of the central and lateral cervical lymph node compartments is indispensable in the evaluation thyroid cancer patients, preoperatively and during follow-up.
|
GRAYSCALE SONOGRAPHIC FEATURES ASSOCIATED WITH THYROID CANCER |
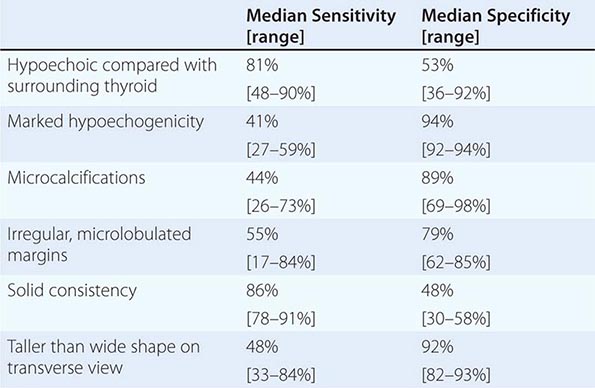
FIGURE 405-5 Sonographic patterns of thyroid nodules. A. High suspicion ultrasound pattern for thyroid malignancy (hypoechoic solid nodule with irregular borders and microcalcifications). B. Very low suspicion ultrasound pattern for thyroid malignancy (spongiform nodule with microcystic areas comprises over >50% of nodule volume).
HYPOTHYROIDISM
Iodine deficiency remains a common cause of hypothyroidism worldwide. In areas of iodine sufficiency, autoimmune disease (Hashimoto’s thyroiditis) and iatrogenic causes (treatment of hyperthyroidism) are most common (Table 405-5).
|
CAUSES OF HYPOTHYROIDISM |
Abbreviations: TSH, thyroid-stimulating hormone; TSH-R, TSH receptor.
CONGENITAL HYPOTHYROIDISM
Prevalence Hypothyroidism occurs in about 1 in 4000 newborns. It may be transient, especially if the mother has TSH-R blocking antibodies or has received antithyroid drugs, but permanent hypothyroidism occurs in the majority. Neonatal hypothyroidism is due to thyroid gland dysgenesis in 80–85%, to inborn errors of thyroid hormone synthesis in 10–15%, and is TSH-R antibody-mediated in 5% of affected newborns. The developmental abnormalities are twice as common in girls. Mutations that cause congenital hypothyroidism are being increasingly identified, but most remain idiopathic (Table 405-1).
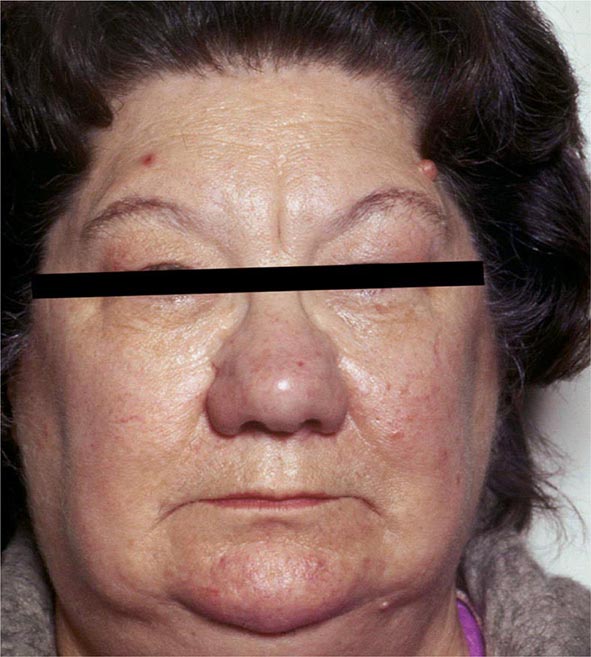
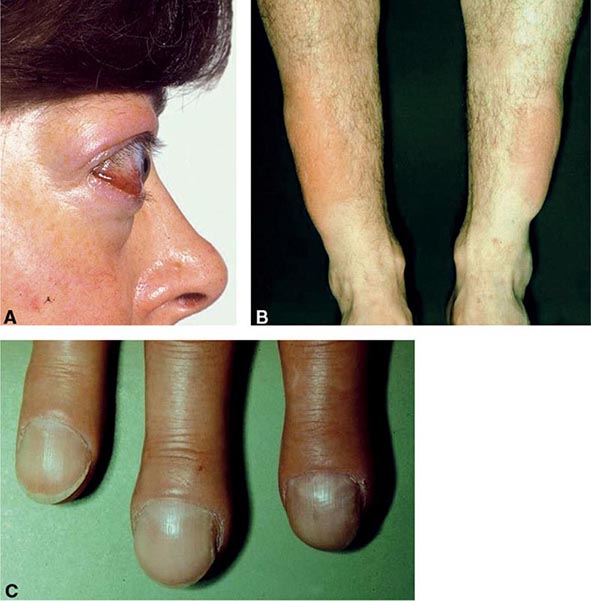
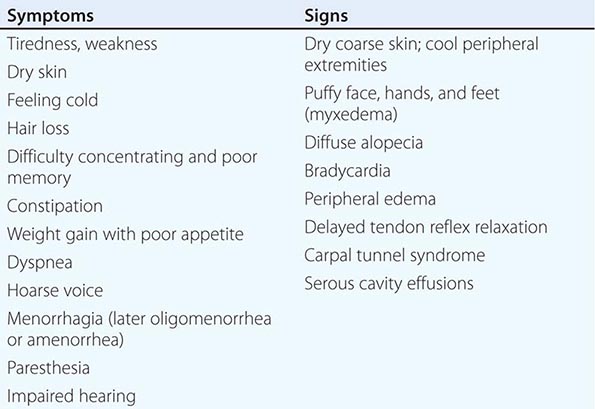
Clinical Manifestations The majority of infants appear normal at birth, and <10% are diagnosed based on clinical features, which include prolonged jaundice, feeding problems, hypotonia, enlarged tongue, delayed bone maturation, and umbilical hernia. Importantly, permanent neurologic damage results if treatment is delayed. Typical features of adult hypothyroidism may also be present (Table 405-6). Other congenital malformations, especially cardiac, are four times more common in congenital hypothyroidism.
|
SIGNS AND SYMPTOMS OF HYPOTHYROIDISM (DESCENDING ORDER OF FREQUENCY) |
Diagnosis and Treatment Because of the severe neurologic consequences of untreated congenital hypothyroidism, neonatal screening programs have been established. These are generally based on measurement of TSH or T4 levels in heel-prick blood specimens. When the diagnosis is confirmed, T4 is instituted at a dose of 10–15 μg/kg per day, and the dose is adjusted by close monitoring of TSH levels. T4 requirements are relatively great during the first year of life, and a high circulating T4 level is usually needed to normalize TSH. Early treatment with T4 results in normal IQ levels, but subtle neurodevelopmental abnormalities may occur in those with the most severe hypothyroidism at diagnosis or when treatment is delayed or suboptimal.
AUTOIMMUNE HYPOTHYROIDISM
Classification Autoimmune hypothyroidism may be associated with a goiter (Hashimoto’s, or goitrous thyroiditis) or, at the later stages of the disease, minimal residual thyroid tissue (atrophic thyroiditis). Because the autoimmune process gradually reduces thyroid function, there is a phase of compensation when normal thyroid hormone levels are maintained by a rise in TSH. Although some patients may have minor symptoms, this state is called subclinical hypothyroidism. Later, unbound T4 levels fall and TSH levels rise further; symptoms become more readily apparent at this stage (usually TSH >10 mIU/L), which is referred to as clinical hypothyroidism or overt hypothyroidism.
Prevalence The mean annual incidence rate of autoimmune hypothyroidism is up to 4 per 1000 women and 1 per 1000 men. It is more common in certain populations, such as the Japanese, probably because of genetic factors and chronic exposure to a high-iodine diet. The mean age at diagnosis is 60 years, and the prevalence of overt hypothyroidism increases with age. Subclinical hypothyroidism is found in 6–8% of women (10% over the age of 60) and 3% of men. The annual risk of developing clinical hypothyroidism is about 4% when subclinical hypothyroidism is associated with positive TPO antibodies.
Pathogenesis In Hashimoto’s thyroiditis, there is a marked lymphocytic infiltration of the thyroid with germinal center formation, atrophy of the thyroid follicles accompanied by oxyphil metaplasia, absence of colloid, and mild to moderate fibrosis. In atrophic thyroiditis, the fibrosis is much more extensive, lymphocyte infiltration is less pronounced, and thyroid follicles are almost completely absent. Atrophic thyroiditis likely represents the end stage of Hashimoto’s thyroiditis rather than a distinct disorder.
As with most autoimmune disorders, susceptibility to autoimmune hypothyroidism is determined by a combination of genetic and environmental factors, and the risk of either autoimmune hypothyroidism or Graves’ disease is increased among siblings. HLA-DR polymorphisms are the best documented genetic risk factors for autoimmune hypothyroidism, especially HLA-DR3, -DR4, and -DR5 in Caucasians. A weak association also exists between polymorphisms in CTLA-4, a T cell–regulatory gene, and autoimmune hypothyroidism. Both of these genetic associations are shared by other autoimmune diseases, which may explain the relationship between autoimmune hypothyroidism and other autoimmune diseases, especially type 1 diabetes mellitus, Addison’s disease, pernicious anemia, and vitiligo. HLA-DR and CTLA-4 polymorphisms account for approximately half of the genetic susceptibility to autoimmune hypothyroidism. Other contributory loci remain to be identified. A gene on chromosome 21 may be responsible for the association between autoimmune hypothyroidism and Down’s syndrome. The female preponderance of thyroid autoimmunity is most likely due to sex steroid effects on the immune response, but an × chromosome–related genetic factor is also possible and may account for the high frequency of autoimmune hypothyroidism in Turner’s syndrome. Environmental susceptibility factors are poorly defined at present. A high iodine intake and decreased exposure to microorganisms in childhood increase the risk of autoimmune hypothyroidism. These factors may account for the increase in prevalence over the last two to three decades.
The thyroid lymphocytic infiltrate in autoimmune hypothyroidism is composed of activated CD4+ and CD8+ T cells as well as B cells. Thyroid cell destruction is primarily mediated by the CD8+ cytotoxic T cells, which destroy their targets by either perforin-induced cell necrosis or granzyme B–induced apoptosis. In addition, local T cell production of cytokines, such as tumor necrosis factor (TNF), IL-1, and interferon γ (IFN-γ), may render thyroid cells more susceptible to apoptosis mediated by death receptors, such as Fas, which are activated by their respective ligands on T cells. These cytokines also impair thyroid cell function directly and induce the expression of other proinflammatory molecules by the thyroid cells themselves, such as cytokines, HLA class I and class II molecules, adhesion molecules, CD40, and nitric oxide. Administration of high concentrations of cytokines for therapeutic purposes (especially IFN-α) is associated with increased autoimmune thyroid disease, possibly through mechanisms similar to those in sporadic disease.
Antibodies to TPO and Tg are clinically useful markers of thyroid autoimmunity, but any pathogenic effect is restricted to a secondary role in amplifying an ongoing autoimmune response. TPO antibodies fix complement, and complement membrane-attack complexes are present in the thyroid in autoimmune hypothyroidism. However, transplacental passage of Tg or TPO antibodies has no effect on the fetal thyroid, which suggests that T cell–mediated injury is required to initiate autoimmune damage to the thyroid.
Up to 20% of patients with autoimmune hypothyroidism have antibodies against the TSH-R, which, in contrast to TSI, do not stimulate the receptor but prevent the binding of TSH. These TSH-R-blocking antibodies, therefore, cause hypothyroidism and, especially in Asian patients, thyroid atrophy. Their transplacental passage may induce transient neonatal hypothyroidism. Rarely, patients have a mixture of TSI and TSH-R-blocking antibodies, and thyroid function can oscillate between hyperthyroidism and hypothyroidism as one or the other antibody becomes dominant. Predicting the course of disease in such individuals is difficult, and they require close monitoring of thyroid function. Bioassays can be used to document that TSH-R-blocking antibodies reduce the cyclic AMP–inducing effect of TSH on cultured TSH-R-expressing cells, but these assays are difficult to perform. Thyrotropin-binding inhibitory immunoglobulin (TBII) assays that measure the binding of antibodies to the receptor by competition with radiolabeled TSH do not distinguish between TSI- and TSH-R-blocking antibodies, but a positive result in a patient with spontaneous hypothyroidism is strong evidence for the presence of blocking antibodies. The use of these assays does not generally alter clinical management, although it may be useful to confirm the cause of transient neonatal hypothyroidism.
Clinical Manifestations The main clinical features of hypothyroidism are summarized in Table 405-6. The onset is usually insidious, and the patient may become aware of symptoms only when euthyroidism is restored. Patients with Hashimoto’s thyroiditis may present because of goiter rather than symptoms of hypothyroidism. The goiter may not be large, but it is usually irregular and firm in consistency. It is often possible to palpate a pyramidal lobe, normally a vestigial remnant of the thyroglossal duct. Rarely is uncomplicated Hashimoto’s thyroiditis associated with pain.
Patients with atrophic thyroiditis or the late stage of Hashimoto’s thyroiditis present with symptoms and signs of hypothyroidism. The skin is dry, and there is decreased sweating, thinning of the epidermis, and hyperkeratosis of the stratum corneum. Increased dermal glycosaminoglycan content traps water, giving rise to skin thickening without pitting (myxedema). Typical features include a puffy face with edematous eyelids and nonpitting pretibial edema (Fig. 405-6). There is pallor, often with a yellow tinge to the skin due to carotene accumulation. Nail growth is retarded, and hair is dry, brittle, difficult to manage, and falls out easily. In addition to diffuse alopecia, there is thinning of the outer third of the eyebrows, although this is not a specific sign of hypothyroidism.
FIGURE 405-6 Facial appearance in hypothyroidism. Note puffy eyes and thickened skin.
Other common features include constipation and weight gain (despite a poor appetite). In contrast to popular perception, the weight gain is usually modest and due mainly to fluid retention in the myxedematous tissues. Libido is decreased in both sexes, and there may be oligomenorrhea or amenorrhea in long-standing disease, but menorrhagia is also common. Fertility is reduced, and the incidence of miscarriage is increased. Prolactin levels are often modestly increased (Chap. 403) and may contribute to alterations in libido and fertility and cause galactorrhea.
Myocardial contractility and pulse rate are reduced, leading to a reduced stroke volume and bradycardia. Increased peripheral resistance may be accompanied by hypertension, particularly diastolic. Blood flow is diverted from the skin, producing cool extremities. Pericardial effusions occur in up to 30% of patients but rarely compromise cardiac function. Although alterations in myosin heavy chain isoform expression have been documented, cardiomyopathy is unusual. Fluid may also accumulate in other serous cavities and in the middle ear, giving rise to conductive deafness. Pulmonary function is generally normal, but dyspnea may be caused by pleural effusion, impaired respiratory muscle function, diminished ventilatory drive, or sleep apnea.
Carpal tunnel and other entrapment syndromes are common, as is impairment of muscle function with stiffness, cramps, and pain. On examination, there may be slow relaxation of tendon reflexes and pseudomyotonia. Memory and concentration are impaired. Experimentally, positron emission tomography (PET) scans examining glucose metabolism in hypothyroid subjects show lower regional activity in the amygdala, hippocampus, and perigenual anterior cingulated cortex, among other regions, and this activity corrects after thyroxine replacement. Rare neurologic problems include reversible cerebellar ataxia, dementia, psychosis, and myxedema coma. Hashimoto’s encephalopathy has been defined as a steroid-responsive syndrome associated with TPO antibodies, myoclonus, and slow-wave activity on electroencephalography, but the relationship with thyroid autoimmunity or hypothyroidism is not established. The hoarse voice and occasionally clumsy speech of hypothyroidism reflect fluid accumulation in the vocal cords and tongue.
The features described above are the consequence of thyroid hormone deficiency. However, autoimmune hypothyroidism may be associated with signs or symptoms of other autoimmune diseases, particularly vitiligo, pernicious anemia, Addison’s disease, alopecia areata, and type 1 diabetes mellitus. Less common associations include celiac disease, dermatitis herpetiformis, chronic active hepatitis, rheumatoid arthritis, systemic lupus erythematosus (SLE), myasthenia gravis, and Sjögren’s syndrome. Thyroid-associated ophthalmopathy, which usually occurs in Graves’ disease (see below), occurs in about 5% of patients with autoimmune hypothyroidism.
Autoimmune hypothyroidism is uncommon in children and usually presents with slow growth and delayed facial maturation. The appearance of permanent teeth is also delayed. Myopathy, with muscle swelling, is more common in children than in adults. In most cases, puberty is delayed, but precocious puberty sometimes occurs. There may be intellectual impairment if the onset is before 3 years and the hormone deficiency is severe.
Laboratory Evaluation A summary of the investigations used to determine the existence and cause of hypothyroidism is provided in Fig. 405-7. A normal TSH level excludes primary (but not secondary) hypothyroidism. If the TSH is elevated, an unbound T4 level is needed to confirm the presence of clinical hypothyroidism, but T4 is inferior to TSH when used as a screening test, because it will not detect subclinical hypothyroidism. Circulating unbound T3 levels are normal in about 25% of patients, reflecting adaptive deiodinase responses to hypothyroidism. T3 measurements are, therefore, not indicated.
FIGURE 405-7 Evaluation of hypothyroidism. TPOAb+, thyroid peroxidase antibodies present; TPOAb–, thyroid peroxidase antibodies not present; TSH, thyroid-stimulating hormone.
Once clinical or subclinical hypothyroidism is confirmed, the etiology is usually easily established by demonstrating the presence of TPO antibodies, which are present in >90% of patients with autoimmune hypothyroidism. TBII can be found in 10–20% of patients, but measurement is not needed routinely. If there is any doubt about the cause of a goiter associated with hypothyroidism, FNA biopsy can be used to confirm the presence of autoimmune thyroiditis. Other abnormal laboratory findings in hypothyroidism may include increased creatine phosphokinase, elevated cholesterol and triglycerides, and anemia (usually normocytic or macrocytic). Except when accompanied by iron deficiency, the anemia and other abnormalities gradually resolve with thyroxine replacement.
Differential Diagnosis An asymmetric goiter in Hashimoto’s thyroiditis may be confused with a MNG or thyroid carcinoma, in which thyroid antibodies may also be present. Ultrasound can be used to show the presence of a solitary lesion or an MNG rather than the heterogeneous thyroid enlargement typical of Hashimoto’s thyroiditis. FNA biopsy is useful in the investigation of focal nodules. Other causes of hypothyroidism are discussed below and in Table 405-5 but rarely cause diagnostic confusion.
OTHER CAUSES OF HYPOTHYROIDISM
Iatrogenic hypothyroidism is a common cause of hypothyroidism and can often be detected by screening before symptoms develop. In the first 3–4 months after radioiodine treatment, transient hypothyroidism may occur due to reversible radiation damage. Low-dose thyroxine treatment can be withdrawn if recovery occurs. Because TSH levels are suppressed by hyperthyroidism, unbound T4 levels are a better measure of thyroid function than TSH in the months following radioiodine treatment. Mild hypothyroidism after subtotal thyroidectomy may also resolve after several months, as the gland remnant is stimulated by increased TSH levels.
Iodine deficiency is responsible for endemic goiter and cretinism but is an uncommon cause of adult hypothyroidism unless the iodine intake is very low or there are complicating factors, such as the consumption of thiocyanates in cassava or selenium deficiency. Although hypothyroidism due to iodine deficiency can be treated with thyroxine, public health measures to improve iodine intake should be advocated to eliminate this problem. Iodized salt or bread or a single bolus of oral or intramuscular iodized oil have all been used successfully.
Paradoxically, chronic iodine excess can also induce goiter and hypothyroidism. The intracellular events that account for this effect are unclear, but individuals with autoimmune thyroiditis are especially susceptible. Iodine excess is responsible for the hypothyroidism that occurs in up to 13% of patients treated with amiodarone (see below). Other drugs, particularly lithium, may also cause hypothyroidism. Transient hypothyroidism caused by thyroiditis is discussed below.
Secondary hypothyroidism is usually diagnosed in the context of other anterior pituitary hormone deficiencies; isolated TSH deficiency is very rare (Chap. 402). TSH levels may be low, normal, or even slightly increased in secondary hypothyroidism; the latter is due to secretion of immunoactive but bioinactive forms of TSH. The diagnosis is confirmed by detecting a low unbound T4 level. The goal of treatment is to maintain T4 levels in the upper half of the reference range, because TSH levels cannot be used to monitor therapy.
|
TREATMENT |
HYPOTHYROIDISM |
CLINICAL HYPOTHYROIDISM
If there is no residual thyroid function, the daily replacement dose of levothyroxine is usually 1.6 μg/kg body weight (typically 100–150 μg), ideally taken at least 30 min before breakfast. In many patients, however, lower doses suffice until residual thyroid tissue is destroyed. In patients who develop hypothyroidism after the treatment of Graves’ disease, there is often underlying autonomous function, necessitating lower replacement doses (typically 75–125 μg/d).
Adult patients under 60 years old without evidence of heart disease may be started on 50–100 μg levothyroxine (T4) daily. The dose is adjusted on the basis of TSH levels, with the goal of treatment being a normal TSH, ideally in the lower half of the reference range. TSH responses are gradual and should be measured about 2 months after instituting treatment or after any subsequent change in levothyroxine dosage. The clinical effects of levothyroxine replacement are slow to appear. Patients may not experience full relief from symptoms until 3–6 months after normal TSH levels are restored. Adjustment of levothyroxine dosage is made in 12.5- or 25-μg increments if the TSH is high; decrements of the same magnitude should be made if the TSH is suppressed. Patients with a suppressed TSH of any cause, including T4 overtreatment, have an increased risk of atrial fibrillation and reduced bone density.
Although desiccated animal thyroid preparations (thyroid extract USP) are available, they are not recommended because the ratio of T3 to T4 is nonphysiologic. The use of levothyroxine combined with liothyronine (triiodothyronine, T3) has been investigated, but benefit has not been confirmed in prospective studies. There is no place for liothyronine alone as long-term replacement, because the short half-life necessitates three or four daily doses and is associated with fluctuating T3 levels.
Once full replacement is achieved and TSH levels are stable, follow-up measurement of TSH is recommended at annual intervals and may be extended to every 2–3 years if a normal TSH is maintained over several years. It is important to ensure ongoing adherence, however, as patients do not feel any symptomatic difference after missing a few doses of levothyroxine, and this sometimes leads to self-discontinuation.
In patients of normal body weight who are taking ≥200 μg of levothyroxine per day, an elevated TSH level is often a sign of poor adherence to treatment. This is also the likely explanation for fluctuating TSH levels, despite a constant levothyroxine dosage. Such patients often have normal or high unbound T4 levels, despite an elevated TSH, because they remember to take medication for a few days before testing; this is sufficient to normalize T4, but not TSH levels. It is important to consider variable adherence, because this pattern of thyroid function tests is otherwise suggestive of disorders associated with inappropriate TSH secretion (Table 405-3). Because T4 has a long half-life (7 days), patients who miss a dose can be advised to take two doses of the skipped tablets at once. Other causes of increased levothyroxine requirements must be excluded, particularly malabsorption (e.g., celiac disease, small-bowel surgery), estrogen or selective estrogen receptor modulator therapy, ingestion with a meal, and drugs that interfere with T4 absorption or metabolism such as cholestyramine, ferrous sulfate, calcium supplements, proton pump inhibitors, lovastatin, aluminum hydroxide, rifampicin, amiodarone, carbamazepine, phenytoin, and tyrosine kinase inhibitors.
SUBCLINICAL HYPOTHYROIDISM
By definition, subclinical hypothyroidism refers to biochemical evidence of thyroid hormone deficiency in patients who have few or no apparent clinical features of hypothyroidism. There are no universally accepted recommendations for the management of subclinical hypothyroidism, but levothyroxine is recommended if the patient is a woman who wishes to conceive or is pregnant, or when TSH levels are above 10 mIU/L. When TSH levels are below 10 mIU/L, treatment should be considered when patients have suggestive symptoms of hypothyroidism, positive TPO antibodies, or any evidence of heart disease. It is important to confirm that any elevation of TSH is sustained over a 3-month period before treatment is given. As long as excessive treatment is avoided, there is no risk in correcting a slightly increased TSH. Treatment is administered by starting with a low dose of levothyroxine (25–50 μg/d) with the goal of normalizing TSH. If levothyroxine is not given, thyroid function should be evaluated annually.
SPECIAL TREATMENT CONSIDERATIONS
Rarely, levothyroxine replacement is associated with pseudotumor cerebri in children. Presentation appears to be idiosyncratic and occurs months after treatment has begun.
Women with a history or high risk of hypothyroidism should ensure that they are euthyroid prior to conception and during early pregnancy because maternal hypothyroidism may adversely affect fetal neural development and cause preterm delivery. The presence of thyroid autoantibodies alone, in a euthyroid patient, is also associated with miscarriage and preterm delivery; it is unclear if levothyroxine therapy improves outcomes. Thyroid function should be evaluated immediately after pregnancy is confirmed and every 4 weeks during the first half of the pregnancy, with less frequent testing after 20 weeks’ gestation (every 6–8 weeks depending on whether levothyroxine dose adjustment is ongoing). The levothyroxine dose may need to be increased by up to 50% during pregnancy, with a goal TSH of less than 2.5 mIU/L during the first trimester and less than 3.0 mIU/L during the second and third trimesters. After delivery, thyroxine doses typically return to prepregnancy levels. Pregnant women should be counseled to separate ingestion of prenatal vitamins and iron supplements from levothyroxine by at least 4 h.
Elderly patients may require 20% less thyroxine than younger patients. In the elderly, especially patients with known coronary artery disease, the starting dose of levothyroxine is 12.5–25 μg/d with similar increments every 2–3 months until TSH is normalized. In some patients, it may be impossible to achieve full replacement despite optimal antianginal treatment. Emergency surgery is generally safe in patients with untreated hypothyroidism, although routine surgery in a hypothyroid patient should be deferred until euthyroidism is achieved.
Myxedema coma still has a 20–40% mortality rate, despite intensive treatment, and outcomes are independent of the T4 and TSH levels. Clinical manifestations include reduced level of consciousness, sometimes associated with seizures, as well as the other features of hypothyroidism (Table 405-6). Hypothermia can reach 23°C (74°F). There may be a history of treated hypothyroidism with poor compliance, or the patient may be previously undiagnosed. Myxedema coma almost always occurs in the elderly and is usually precipitated by factors that impair respiration, such as drugs (especially sedatives, anesthetics, and antidepressants), pneumonia, congestive heart failure, myocardial infarction, gastrointestinal bleeding, or cerebrovascular accidents. Sepsis should also be suspected. Exposure to cold may also be a risk factor. Hypoventilation, leading to hypoxia and hypercapnia, plays a major role in pathogenesis; hypoglycemia and dilutional hyponatremia also contribute to the development of myxedema coma.
Levothyroxine can initially be administered as a single IV bolus of 500 μg, which serves as a loading dose. Although further levothyroxine is not strictly necessary for several days, it is usually continued at a dose of 50–100 μg/d. If suitable IV preparation is not available, the same initial dose of levothyroxine can be given by nasogastric tube (although absorption may be impaired in myxedema). An alternative is to give liothyronine (T3) intravenously or via nasogastric tube, in doses ranging from 10 to 25 μg every 8–12 h. This treatment has been advocated because T4 → T3 conversion is impaired in myxedema coma. However, excess liothyronine has the potential to provoke arrhythmias. Another option is to combine levothyroxine (200 μg) and liothyronine (25 μg) as a single, initial IV bolus followed by daily treatment with levothyroxine (50–100 μg/d) and liothyronine (10 μg every 8 h).
Supportive therapy should be provided to correct any associated metabolic disturbances. External warming is indicated only if the temperature is <30°C, as it can result in cardiovascular collapse (Chap. 478e). Space blankets should be used to prevent further heat loss. Parenteral hydrocortisone (50 mg every 6 h) should be administered, because there is impaired adrenal reserve in profound hypothyroidism. Any precipitating factors should be treated, including the early use of broad-spectrum antibiotics, pending the exclusion of infection. Ventilatory support with regular blood gas analysis is usually needed during the first 48 h. Hypertonic saline or IV glucose may be needed if there is severe hyponatremia or hypoglycemia; hypotonic IV fluids should be avoided because they may exacerbate water retention secondary to reduced renal perfusion and inappropriate vasopressin secretion. The metabolism of most medications is impaired, and sedatives should be avoided if possible or used in reduced doses. Medication blood levels should be monitored, when available, to guide dosage.
THYROTOXICOSIS
Thyrotoxicosis is defined as the state of thyroid hormone excess and is not synonymous with hyperthyroidism, which is the result of excessive thyroid function. However, the major etiologies of thyrotoxicosis are hyperthyroidism caused by Graves’ disease, toxic MNG, and toxic adenomas. Other causes are listed in Table 405-7.
|
CAUSES OF THYROTOXICOSIS |
aCirculating TSH levels are low in these forms of secondary hyperthyroidism.
Abbreviations: TSH, thyroid-stimulating hormone.
GRAVES’ DISEASE
Epidemiology Graves’ disease accounts for 60–80% of thyrotoxicosis. The prevalence varies among populations, reflecting genetic factors and iodine intake (high iodine intake is associated with an increased prevalence of Graves’ disease). Graves’ disease occurs in up to 2% of women but is one-tenth as frequent in men. The disorder rarely begins before adolescence and typically occurs between 20 and 50 years of age; it also occurs in the elderly.
Pathogenesis As in autoimmune hypothyroidism, a combination of environmental and genetic factors, including polymorphisms in HLA-DR, the immunoregulatory genes CTLA-4, CD25, PTPN22, FCRL3, and CD226, as well as the TSH-R, contribute to Graves’ disease susceptibility. The concordance for Graves’ disease in monozygotic twins is 20–30%, compared to <5% in dizygotic twins. Indirect evidence suggests that stress is an important environmental factor, presumably operating through neuroendocrine effects on the immune system. Smoking is a minor risk factor for Graves’ disease and a major risk factor for the development of ophthalmopathy. Sudden increases in iodine intake may precipitate Graves’ disease, and there is a threefold increase in the occurrence of Graves’ disease in the postpartum period. Graves’ disease may occur during the immune reconstitution phase after highly active antiretroviral therapy (HAART) or alemtuzumab treatment.
The hyperthyroidism of Graves’ disease is caused by TSI that are synthesized in the thyroid gland as well as in bone marrow and lymph nodes. Such antibodies can be detected by bioassays or by using the more widely available TBII assays. The presence of TBII in a patient with thyrotoxicosis implies the existence of TSI, and these assays are useful in monitoring pregnant Graves’ patients in whom high levels of TSI can cross the placenta and cause neonatal thyrotoxicosis. Other thyroid autoimmune responses, similar to those in autoimmune hypothyroidism (see above), occur concurrently in patients with Graves’ disease. In particular, TPO antibodies occur in up to 80% of cases and serve as a readily measurable marker of autoimmunity. Because the coexisting thyroiditis can also affect thyroid function, there is no direct correlation between the level of TSI and thyroid hormone levels in Graves’ disease. In the long term, spontaneous autoimmune hypothyroidism may develop in up to 15% of patients with Graves’ disease.
Cytokines appear to play a major role in thyroid-associated ophthalmopathy. There is infiltration of the extraocular muscles by activated T cells; the release of cytokines such as IFN-γ, TNF, and IL-1 results in fibroblast activation and increased synthesis of glycosaminoglycans that trap water, thereby leading to characteristic muscle swelling. Late in the disease, there is irreversible fibrosis of the muscles. Orbital fibroblasts may be particularly sensitive to cytokines, perhaps explaining the anatomic localization of the immune response. Though the pathogenesis of thyroid-associated ophthalmopathy remains unclear, there is mounting evidence that the TSH-R may be a shared autoantigen that is expressed in the orbit; this would explain the close association with autoimmune thyroid disease. Increased fat is an additional cause of retrobulbar tissue expansion. The increase in intraorbital pressure can lead to proptosis, diplopia, and optic neuropathy.
Clinical Manifestations Signs and symptoms include features that are common to any cause of thyrotoxicosis (Table 405-8) as well as those specific for Graves’ disease. The clinical presentation depends on the severity of thyrotoxicosis, the duration of disease, individual susceptibility to excess thyroid hormone, and the patient’s age. In the elderly, features of thyrotoxicosis may be subtle or masked, and patients may present mainly with fatigue and weight loss, a condition known as apathetic thyrotoxicosis.
|
SIGNS AND SYMPTOMS OF THYROTOXICOSIS (DESCENDING ORDER OF FREQUENCY) |
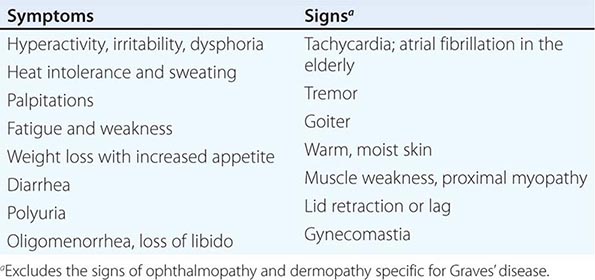
Thyrotoxicosis may cause unexplained weight loss, despite an enhanced appetite, due to the increased metabolic rate. Weight gain occurs in 5% of patients, however, because of increased food intake. Other prominent features include hyperactivity, nervousness, and irritability, ultimately leading to a sense of easy fatigability in some patients. Insomnia and impaired concentration are common; apathetic thyrotoxicosis may be mistaken for depression in the elderly. Fine tremor is a frequent finding, best elicited by having patients stretch out their fingers while feeling the fingertips with the palm. Common neurologic manifestations include hyperreflexia, muscle wasting, and proximal myopathy without fasciculation. Chorea is rare. Thyrotoxicosis is sometimes associated with a form of hypokalemic periodic paralysis; this disorder is particularly common in Asian males with thyrotoxicosis, but it occurs in other ethnic groups as well.
The most common cardiovascular manifestation is sinus tachycardia, often associated with palpitations, occasionally caused by supraventricular tachycardia. The high cardiac output produces a bounding pulse, widened pulse pressure, and an aortic systolic murmur and can lead to worsening of angina or heart failure in the elderly or those with preexisting heart disease. Atrial fibrillation is more common in patients >50 years of age. Treatment of the thyrotoxic state alone converts atrial fibrillation to normal sinus rhythm in about half of patients, suggesting the existence of an underlying cardiac problem in the remainder.
The skin is usually warm and moist, and the patient may complain of sweating and heat intolerance, particularly during warm weather. Palmar erythema, onycholysis, and, less commonly, pruritus, urticaria, and diffuse hyperpigmentation may be evident. Hair texture may become fine, and a diffuse alopecia occurs in up to 40% of patients, persisting for months after restoration of euthyroidism. Gastrointestinal transit time is decreased, leading to increased stool frequency, often with diarrhea and occasionally mild steatorrhea. Women frequently experience oligomenorrhea or amenorrhea; in men, there may be impaired sexual function and, rarely, gynecomastia. The direct effect of thyroid hormones on bone resorption leads to osteopenia in long-standing thyrotoxicosis; mild hypercalcemia occurs in up to 20% of patients, but hypercalciuria is more common. There is a small increase in fracture rate in patients with a previous history of thyrotoxicosis.
In Graves’ disease, the thyroid is usually diffusely enlarged to two to three times its normal size. The consistency is firm, but not nodular. There may be a thrill or bruit, best detected at the inferolateral margins of the thyroid lobes, due to the increased vascularity of the gland and the hyperdynamic circulation.
Lid retraction, causing a staring appearance, can occur in any form of thyrotoxicosis and is the result of sympathetic overactivity. However, Graves’ disease is associated with specific eye signs that comprise Graves’ ophthalmopathy (Fig. 405-8A). This condition is also called thyroid-associated ophthalmopathy, because it occurs in the absence of hyperthyroidism in 10% of patients. Most of these individuals have autoimmune hypothyroidism or thyroid antibodies. The onset of Graves’ ophthalmopathy occurs within the year before or after the diagnosis of thyrotoxicosis in 75% of patients but can sometimes precede or follow thyrotoxicosis by several years, accounting for some cases of euthyroid ophthalmopathy.
FIGURE 405-8 Features of Graves’ disease. A. Ophthalmopathy in Graves’ disease; lid retraction, periorbital edema, conjunctival injection, and proptosis are marked. B. Thyroid dermopathy over the lateral aspects of the shins. C. Thyroid acropachy.
Some patients with Graves’ disease have little clinical evidence of ophthalmopathy. However, the enlarged extraocular muscles typical of the disease, and other subtle features, can be detected in almost all patients when investigated by ultrasound or computed tomography (CT) imaging of the orbits. Unilateral signs are found in up to 10% of patients. The earliest manifestations of ophthalmopathy are usually a sensation of grittiness, eye discomfort, and excess tearing. About one-third of patients have proptosis, best detected by visualization of the sclera between the lower border of the iris and the lower eyelid, with the eyes in the primary position. Proptosis can be measured using an exophthalmometer. In severe cases, proptosis may cause corneal exposure and damage, especially if the lids fail to close during sleep. Periorbital edema, scleral injection, and chemosis are also frequent. In 5–10% of patients, the muscle swelling is so severe that diplopia results, typically, but not exclusively, when the patient looks up and laterally. The most serious manifestation is compression of the optic nerve at the apex of the orbit, leading to papilledema; peripheral field defects; and, if left untreated, permanent loss of vision.
The “NO SPECS” scoring system to evaluate ophthalmopathy is an acronym derived from the following changes:
0 = No signs or symptoms
1 = Only signs (lid retraction or lag), no symptoms
2 = Soft tissue involvement (periorbital edema)
3 = Proptosis (>22 mm)
4 = Extraocular muscle involvement (diplopia)
5 = Corneal involvement
6 = Sight loss
Although useful as a mnemonic, the NO SPECS scheme is inadequate to describe the eye disease fully, and patients do not necessarily progress from one class to another; alternative scoring systems that assess disease activity are preferable for monitoring purposes. When Graves’ eye disease is active and severe, referral to an ophthalmologist is indicated and objective measurements are needed, such as lid-fissure width; corneal staining with fluorescein; and evaluation of extraocular muscle function (e.g., Hess chart), intraocular pressure and visual fields, acuity, and color vision.
Thyroid dermopathy occurs in <5% of patients with Graves’ disease (Fig. 405-8B), almost always in the presence of moderate or severe ophthalmopathy. Although most frequent over the anterior and lateral aspects of the lower leg (hence the term pretibial myxedema), skin changes can occur at other sites, particularly after trauma. The typical lesion is a noninflamed, indurated plaque with a deep pink or purple color and an “orange skin” appearance. Nodular involvement can occur, and the condition can rarely extend over the whole lower leg and foot, mimicking elephantiasis. Thyroid acropachy refers to a form of clubbing found in <1% of patients with Graves’ disease (Fig. 405-8C). It is so strongly associated with thyroid dermopathy that an alternative cause of clubbing should be sought in a Graves’ patient without coincident skin and orbital involvement.
Laboratory Evaluation Investigations used to determine the existence and cause of thyrotoxicosis are summarized in Fig. 405-9. In Graves’ disease, the TSH level is suppressed, and total and unbound thyroid hormone levels are increased. In 2–5% of patients (and more in areas of borderline iodine intake), only T3 is increased (T3 toxicosis). The converse state of T4 toxicosis, with elevated total and unbound T4 and normal T3 levels, is occasionally seen when hyperthyroidism is induced by excess iodine, providing surplus substrate for thyroid hormone synthesis. Measurement of TPO antibodies or TRAb may be useful if the diagnosis is unclear clinically but is not needed routinely. Associated abnormalities that may cause diagnostic confusion in thyrotoxicosis include elevation of bilirubin, liver enzymes, and ferritin. Microcytic anemia and thrombocytopenia may occur.
FIGURE 405-9 Evaluation of thyrotoxicosis. aDiffuse goiter, positive TPO antibodies or TRAb, ophthalmopathy, dermopathy. bCan be confirmed by radionuclide scan. TSH, thyroid-stimulating hormone.
Differential Diagnosis Diagnosis of Graves’ disease is straightforward in a patient with biochemically confirmed thyrotoxicosis, diffuse goiter on palpation, ophthalmopathy, and often a personal or family history of autoimmune disorders. For patients with thyrotoxicosis who lack these features, the diagnosis is generally established by a radionuclide (99mTc, 123I, or 131I) scan and uptake of the thyroid, which will distinguish the diffuse, high uptake of Graves’ disease from destructive thyroiditis, ectopic thyroid tissue, and factitious thyrotoxicosis. Scintigraphy is the preferred diagnostic test; however, TRAb measurement can be used to assess autoimmune activity. In secondary hyperthyroidism due to a TSH-secreting pituitary tumor, there is also a diffuse goiter. The presence of a nonsuppressed TSH level and the finding of a pituitary tumor on CT or magnetic resonance scan (MRI) scan suggest this diagnosis.
Clinical features of thyrotoxicosis can mimic certain aspects of other disorders, including panic attacks, mania, pheochromocytoma, and weight loss associated with malignancy. The diagnosis of thyrotoxicosis can be easily excluded if the TSH and unbound T4 and T3 levels are normal. A normal TSH also excludes Graves’ disease as a cause of diffuse goiter.
Clinical Course Clinical features generally worsen without treatment; mortality was 10–30% before the introduction of satisfactory therapy. Some patients with mild Graves’ disease experience spontaneous relapses and remissions. Rarely, there may be fluctuation between hypo- and hyperthyroidism due to changes in the functional activity of TSH-R antibodies. About 15% of patients who enter remission after treatment develop hypothyroidism 10–15 years later as a result of the destructive autoimmune process.
The clinical course of ophthalmopathy does not follow that of the thyroid disease. Ophthalmopathy typically worsens over the initial 3–6 months, followed by a plateau phase over the next 12–18 months, with spontaneous improvement, particularly in the soft tissue changes. However, the course is more fulminant in up to 5% of patients, requiring intervention in the acute phase if there is optic nerve compression or corneal ulceration. Diplopia may appear late in the disease due to fibrosis of the extraocular muscles. Radioiodine treatment for hyperthyroidism worsens the eye disease in a small proportion of patients (especially smokers). Antithyroid drugs or surgery have no adverse effects on the clinical course of ophthalmopathy. Thyroid dermopathy, when it occurs, usually appears 1–2 years after the development of Graves’ hyperthyroidism; it may improve spontaneously.
|
TREATMENT |
GRAVES’ DISEASE |
The hyperthyroidism of Graves’ disease is treated by reducing thyroid hormone synthesis, using antithyroid drugs, or reducing the amount of thyroid tissue with radioiodine (131I) treatment or by thyroidectomy. Antithyroid drugs are the predominant therapy in many centers in Europe and Japan, whereas radioiodine is more often the first line of treatment in North America. These differences reflect the fact that no single approach is optimal and that patients may require multiple treatments to achieve remission.
The main antithyroid drugs are the thionamides, such as propylthiouracil, carbimazole (not available in the United States), and the active metabolite of the latter, methimazole. All inhibit the function of TPO, reducing oxidation and organification of iodide. These drugs also reduce thyroid antibody levels by mechanisms that remain unclear, and they appear to enhance rates of remission. Propylthiouracil inhibits deiodination of T4 → T3. However, this effect is of minor benefit, except in the most severe thyrotoxicosis, and is offset by the much shorter half-life of this drug (90 min) compared to methimazole (6 h). Due to the hepatotoxicity of propylthiouracil, the U.S. Food and Drug Administration (FDA) has limited indications for its use to the first trimester of pregnancy, the treatment of thyroid storm, and patients with minor adverse reactions to methimazole. If propylthiouracil is used, monitoring of liver function tests is recommended.
There are many variations of antithyroid drug regimens. The initial dose of carbimazole or methimazole is usually 10–20 mg every 8 or 12 h, but once-daily dosing is possible after euthyroidism is restored. Propylthiouracil is given at a dose of 100–200 mg every 6–8 h, and divided doses are usually given throughout the course. Lower doses of each drug may suffice in areas of low iodine intake. The starting dose of antithyroid drugs can be gradually reduced (titration regimen) as thyrotoxicosis improves. Alternatively, high doses may be given combined with levothyroxine supplementation (block-replace regimen) to avoid drug-induced hypothyroidism. The titration regimen is preferred to minimize the dose of antithyroid drug and provide an index of treatment response.
Thyroid function tests and clinical manifestations are reviewed 4–6 weeks after starting treatment, and the dose is titrated based on unbound T4 levels. Most patients do not achieve euthyroidism until 6–8 weeks after treatment is initiated. TSH levels often remain suppressed for several months and therefore do not provide a sensitive index of treatment response. The usual daily maintenance doses of antithyroid drugs in the titration regimen are 2.5–10 mg of carbimazole or methimazole and 50–100 mg of propylthiouracil. In the block-replace regimen, the initial dose of antithyroid drug is held constant, and the dose of levothyroxine is adjusted to maintain normal unbound T4 levels. When TSH suppression is alleviated, TSH levels can also be used to monitor therapy.
Maximum remission rates (up to 30–60% in some populations) are achieved by 12–18 months for the titration regimen and by 6 months for the block-replace regimen. For unclear reasons, remission rates appear to vary in different geographic regions. Younger patients, males, smokers, and patients with severe hyperthyroidism and large goiters are most likely to relapse when treatment stops, but outcomes are difficult to predict. All patients should be followed closely for relapse during the first year after treatment and at least annually thereafter.
The common minor side effects of antithyroid drugs are rash, urticaria, fever, and arthralgia (1–5% of patients). These may resolve spontaneously or after substituting an alternative antithyroid drug. Rare but major side effects include hepatitis (propylthiouracil; avoid use in children) and cholestasis (methimazole and carbimazole); an SLE-like syndrome; and, most important, agranulocytosis (<1%). It is essential that antithyroid drugs are stopped and not restarted if a patient develops major side effects. Written instructions should be provided regarding the symptoms of possible agranulocytosis (e.g., sore throat, fever, mouth ulcers) and the need to stop treatment pending an urgent complete blood count to confirm that agranulocytosis is not present. Management of agranulocytosis is described in Chap. 130. It is not useful to monitor blood counts prospectively, because the onset of agranulocytosis is idiosyncratic and abrupt.
Propranolol (20–40 mg every 6 h) or longer-acting selective β1 receptor blockers such as atenolol may be helpful to control adrenergic symptoms, especially in the early stages before antithyroid drugs take effect. Beta blockers are also useful in patients with thyrotoxic periodic paralysis, pending correction of thyrotoxicosis. In consultation with a cardiologist, anticoagulation with warfarin should be considered in all patients with atrial fibrillation who often spontaneously revert to sinus rhythm with control of hyperthyroidism. Decreased warfarin doses are required when patients are thyrotoxic. If digoxin is used, increased doses are often needed in the thyrotoxic state.
Radioiodine causes progressive destruction of thyroid cells and can be used as initial treatment or for relapses after a trial of antithyroid drugs. There is a small risk of thyrotoxic crisis (see below) after radioiodine, which can be minimized by pretreatment with antithyroid drugs for at least a month before treatment. Antecedent treatment with antithyroid drugs should be considered for all elderly patients or for those with cardiac problems to deplete thyroid hormone stores before administration of radioiodine. Carbimazole or methimazole must be stopped 3–5 days before radioiodine administration to achieve optimum iodine uptake. Propylthiouracil appears to have a prolonged radioprotective effect and should be stopped for a longer period before radioiodine is given, or a larger dose of radioiodine will be necessary.
Efforts to calculate an optimal dose of radioiodine that achieves euthyroidism without a high incidence of relapse or progression to hypothyroidism have not been successful. Some patients inevitably relapse after a single dose because the biologic effects of radiation vary between individuals, and hypothyroidism cannot be uniformly avoided even using accurate dosimetry. A practical strategy is to give a fixed dose based on clinical features, such as the severity of thyrotoxicosis, the size of the goiter (increases the dose needed), and the level of radioiodine uptake (decreases the dose needed). 131I dosage generally ranges between 370 MBq (10 mCi) and 555 MBq (15 mCi). Most authorities favor an approach aimed at thyroid ablation (as opposed to euthyroidism), given that levothyroxine replacement is straightforward and most patients ultimately progress to hypothyroidism over 5–10 years, frequently with some delay in the diagnosis of hypothyroidism.
Certain radiation safety precautions are necessary in the first few days after radioiodine treatment, but the exact guidelines vary depending on local protocols. In general, patients need to avoid close, prolonged contact with children and pregnant women for 5–7 days because of possible transmission of residual isotope and exposure to radiation emanating from the gland. Rarely, there may be mild pain due to radiation thyroiditis 1–2 weeks after treatment. Hyperthyroidism can persist for 2–3 months before radioiodine takes full effect. For this reason, β-adrenergic blockers or antithyroid drugs can be used to control symptoms during this interval. Persistent hyperthyroidism can be treated with a second dose of radioiodine, usually 6 months after the first dose. The risk of hypothyroidism after radioiodine depends on the dosage but is at least 10–20% in the first year and 5% per year thereafter. Patients should be informed of this possibility before treatment and require close follow-up during the first year followed by annual thyroid function testing.
Pregnancy and breast-feeding are absolute contraindications to radioiodine treatment, but patients can conceive safely 6 months after treatment. The presence of severe ophthalmopathy requires caution, and some authorities advocate the use of prednisone, 40 mg/d, at the time of radioiodine treatment, tapered over 6–12 weeks to prevent exacerbation of ophthalmopathy. The overall risk of cancer after radioiodine treatment in adults is not increased. Although many physicians avoid radioiodine in children and adolescents because of the theoretical risks of malignancy, emerging evidence suggests that radioiodine can be used safely in older children.
Subtotal or near-total thyroidectomy is an option for patients who relapse after antithyroid drugs and prefer this treatment to radioiodine. Some experts recommend surgery in young individuals, particularly when the goiter is very large. Careful control of thyrotoxicosis with antithyroid drugs, followed by potassium iodide (3 drops SSKI orally tid), is needed prior to surgery to avoid thyrotoxic crisis and to reduce the vascularity of the gland. The major complications of surgery—bleeding, laryngeal edema, hypoparathyroidism, and damage to the recurrent laryngeal nerves—are unusual when the procedure is performed by highly experienced surgeons. Recurrence rates in the best series are <2%, but the rate of hypothyroidism is only slightly less than that following radioiodine treatment.
The titration regimen of antithyroid drugs should be used to manage Graves’ disease in pregnancy because transplacental passage of these drugs may produce fetal hypothyroidism and goiter if the maternal dose is excessive. If available, propylthiouracil should be used in early gestation because of the association of rare cases of fetal aplasia cutis and other defects, such as choanal atresia with carbimazole and methimazole. As noted above, because of its rare association with hepatotoxicity, propylthiouracil should be limited to the first trimester and then maternal therapy should be converted to methimazole (or carbimazole) at a ratio of 15–20 mg of propylthiouracil to 1 mg of methimazole The lowest effective antithyroid drug dose should be used throughout gestation to maintain the maternal serum free T4 level at the upper limit of the nonpregnant normal reference range. It is often possible to stop treatment in the last trimester because TSIs tend to decline in pregnancy. Nonetheless, the transplacental transfer of these antibodies rarely causes fetal or neonatal thyrotoxicosis. Poor intrauterine growth, a fetal heart rate of >160 beats/min, and high levels of maternal TSI in the last trimester may herald this complication. Antithyroid drugs given to the mother can be used to treat the fetus and may be needed for 1–3 months after delivery, until the maternal antibodies disappear from the baby’s circulation. The postpartum period is a time of major risk for relapse of Graves’ disease. Breast-feeding is safe with low doses of antithyroid drugs. Graves’ disease in children is usually managed with methimazole or carbimazole (avoid propylthiouracil), often given as a prolonged course of the titration regimen. Surgery or radioiodine may be indicated for severe disease.
Thyrotoxic crisis, or thyroid storm, is rare and presents as a life-threatening exacerbation of hyperthyroidism, accompanied by fever, delirium, seizures, coma, vomiting, diarrhea, and jaundice. The mortality rate due to cardiac failure, arrhythmia, or hyperthermia is as high as 30%, even with treatment. Thyrotoxic crisis is usually precipitated by acute illness (e.g., stroke, infection, trauma, diabetic ketoacidosis), surgery (especially on the thyroid), or radioiodine treatment of a patient with partially treated or untreated hyperthyroidism. Management requires intensive monitoring and supportive care, identification and treatment of the precipitating cause, and measures that reduce thyroid hormone synthesis. Large doses of propylthiouracil (500–1000 mg loading dose and 250 mg every 4 h) should be given orally or by nasogastric tube or per rectum; the drug’s inhibitory action on T4 → T3 conversion makes it the antithyroid drug of choice. If not available, methimazole can be used in doses up to 30 mg every 12 h. One hour after the first dose of propylthiouracil, stable iodide is given to block thyroid hormone synthesis via the Wolff-Chaikoff effect (the delay allows the antithyroid drug to prevent the excess iodine from being incorporated into new hormone). A saturated solution of potassium iodide (5 drops SSKI every 6 h) or, where available, ipodate or iopanoic acid (500 mg per 12 h) may be given orally. Sodium iodide, 0.25 g IV every 6 h, is an alternative but is not generally available. Propranolol should also be given to reduce tachycardia and other adrenergic manifestations (60–80 mg PO every 4 h; or 2 mg IV every 4 h). Although other β-adrenergic blockers can be used, high doses of propranolol decrease T4 → T3 conversion, and the doses can be easily adjusted. Caution is needed to avoid acute negative inotropic effects, but controlling the heart rate is important, as some patients develop a form of high-output heart failure. Short-acting IV esmolol can be used to decrease heart rate while monitoring for signs of heart failure. Additional therapeutic measures include glucocorticoids (e.g., hydrocortisone 300 mg IV bolus, then 100 mg every 8 h), antibiotics if infection is present, cooling, oxygen, and IV fluids.
Ophthalmopathy requires no active treatment when it is mild or moderate, because there is usually spontaneous improvement. General measures include meticulous control of thyroid hormone levels, cessation of smoking, and an explanation of the natural history of ophthalmopathy. Discomfort can be relieved with artificial tears (e.g., 1% methylcellulose), eye ointment, and the use of dark glasses with side frames. Periorbital edema may respond to a more upright sleeping position or a diuretic. Corneal exposure during sleep can be avoided by using patches or taping the eyelids shut. Minor degrees of diplopia improve with prisms fitted to spectacles. Severe ophthalmopathy, with optic nerve involvement or chemosis resulting in corneal damage, is an emergency requiring joint management with an ophthalmologist. Pulse therapy with IV methylprednisolone (e.g., 500 mg of methylprednisolone once weekly for 6 weeks, then 250 mg once weekly for 6 weeks) is preferable to oral glucocorticoids, which are used for moderately active disease. When glucocorticoids are ineffective, orbital decompression can be achieved by removing bone from any wall of the orbit, thereby allowing displacement of fat and swollen extraocular muscles. The transantral route is used most often because it requires no external incision. Proptosis recedes an average of 5 mm, but there may be residual or even worsened diplopia. Once the eye disease has stabilized, surgery may be indicated for relief of diplopia and correction of the appearance. External beam radiotherapy of the orbits has been used for many years, but the efficacy of this therapy remains unclear, and it is best reserved for those with moderately active disease who have failed or are not candidates for glucocorticoid therapy. Other immunosuppressive agents such as rituximab have shown some benefit, but their role is yet to be established.
Thyroid dermopathy does not usually require treatment, but it can cause cosmetic problems or interfere with the fit of shoes. Surgical removal is not indicated. If necessary, treatment consists of topical, high-potency glucocorticoid ointment under an occlusive dressing. Octreotide may be beneficial in some cases.
OTHER CAUSES OF THYROTOXICOSIS
Destructive thyroiditis (subacute or silent thyroiditis) typically presents with a short thyrotoxic phase due to the release of preformed thyroid hormones and catabolism of Tg (see “Subacute Thyroiditis,” below). True hyperthyroidism is absent, as demonstrated by a low radionuclide uptake. Circulating Tg levels are usually increased. Other causes of thyrotoxicosis with low or absent thyroid radionuclide uptake include thyrotoxicosis factitia, iodine excess, and, rarely, ectopic thyroid tissue, particularly teratomas of the ovary (struma ovarii) and functional metastatic follicular carcinoma. Whole-body radionuclide studies can demonstrate ectopic thyroid tissue, and thyrotoxicosis factitia can be distinguished from destructive thyroiditis by the clinical features and low levels of Tg. Amiodarone treatment is associated with thyrotoxicosis in up to 10% of patients, particularly in areas of low iodine intake (see below).
TSH-secreting pituitary adenoma is a rare cause of thyrotoxicosis. It is characterized by the presence of an inappropriately normal or increased TSH level in a patient with hyperthyroidism, diffuse goiter, and elevated T4 and T3 levels (Chap. 403). Elevated levels of the α-subunit of TSH, released by the TSH-secreting adenoma, support this diagnosis, which can be confirmed by demonstrating the pituitary tumor on MRI or CT scan. A combination of transsphenoidal surgery, sella irradiation, and octreotide may be required to normalize TSH, because many of these tumors are large and locally invasive at the time of diagnosis. Radioiodine or antithyroid drugs can be used to control thyrotoxicosis.
Thyrotoxicosis caused by toxic MNG and hyperfunctioning solitary nodules is discussed below.
THYROIDITIS
A clinically useful classification of thyroiditis is based on the onset and duration of disease (Table 405-9).
|
CAUSES OF THYROIDITIS |
ACUTE THYROIDITIS
Acute thyroiditis is rare and due to suppurative infection of the thyroid. In children and young adults, the most common cause is the presence of a piriform sinus, a remnant of the fourth branchial pouch that connects the oropharynx with the thyroid. Such sinuses are predominantly left-sided. A long-standing goiter and degeneration in a thyroid malignancy are risk factors in the elderly. The patient presents with thyroid pain, often referred to the throat or ears, and a small, tender goiter that may be asymmetric. Fever, dysphagia, and erythema over the thyroid are common, as are systemic symptoms of a febrile illness and lymphadenopathy.
The differential diagnosis of thyroid pain includes subacute or, rarely, chronic thyroiditis; hemorrhage into a cyst; malignancy including lymphoma; and, rarely, amiodarone-induced thyroiditis or amyloidosis. However, the abrupt presentation and clinical features of acute thyroiditis rarely cause confusion. The erythrocyte sedimentation rate (ESR) and white cell count are usually increased, but thyroid function is normal. FNA biopsy shows infiltration by polymorphonuclear leukocytes; culture of the sample can identify the organism. Caution is needed in immunocompromised patients as fungal, mycobacterial, or Pneumocystis thyroiditis can occur in this setting. Antibiotic treatment is guided initially by Gram stain and, subsequently, by cultures of the FNA biopsy. Surgery may be needed to drain an abscess, which can be localized by CT scan or ultrasound. Tracheal obstruction, septicemia, retropharyngeal abscess, mediastinitis, and jugular venous thrombosis may complicate acute thyroiditis but are uncommon with prompt use of antibiotics.
SUBACUTE THYROIDITIS
This is also termed de Quervain’s thyroiditis, granulomatous thyroiditis, or viral thyroiditis. Many viruses have been implicated, including mumps, coxsackie, influenza, adenoviruses, and echoviruses, but attempts to identify the virus in an individual patient are often unsuccessful and do not influence management. The diagnosis of subacute thyroiditis is often overlooked because the symptoms can mimic pharyngitis. The peak incidence occurs at 30–50 years, and women are affected three times more frequently than men.
Pathophysiology The thyroid shows a characteristic patchy inflammatory infiltrate with disruption of the thyroid follicles and multinucleated giant cells within some follicles. The follicular changes progress to granulomas accompanied by fibrosis. Finally, the thyroid returns to normal, usually several months after onset. During the initial phase of follicular destruction, there is release of Tg and thyroid hormones, leading to increased circulating T4 and T3 and suppression of TSH (Fig. 405-10). During this destructive phase, radioactive iodine uptake is low or undetectable. After several weeks, the thyroid is depleted of stored thyroid hormone and a phase of hypothyroidism typically occurs, with low unbound T4 (and sometimes T3) and moderately increased TSH levels. Radioactive iodine uptake returns to normal or is even increased as a result of the rise in TSH. Finally, thyroid hormone and TSH levels return to normal as the disease subsides.
FIGURE 405-10 Clinical course of subacute thyroiditis. The release of thyroid hormones is initially associated with a thyrotoxic phase and suppressed thyroid-stimulating hormone (TSH). A hypothyroid phase then ensues, with low T4 and TSH levels that are initially low but gradually increase. During the recovery phase, increased TSH levels combined with resolution of thyroid follicular injury lead to normalization of thyroid function, often several months after the beginning of the illness. ESR, erythrocyte sedimentation rate; UT4, free or unbound T4.
Clinical Manifestations The patient usually presents with a painful and enlarged thyroid, sometimes accompanied by fever. There may be features of thyrotoxicosis or hypothyroidism, depending on the phase of the illness. Malaise and symptoms of an upper respiratory tract infection may precede the thyroid-related features by several weeks. In other patients, the onset is acute, severe, and without obvious antecedent. The patient typically complains of a sore throat, and examination reveals a small goiter that is exquisitely tender. Pain is often referred to the jaw or ear. Complete resolution is the usual outcome, but late-onset permanent hypothyroidism occurs in 15% of cases, particularly in those with coincidental thyroid autoimmunity. A prolonged course over many months, with one or more relapses, occurs in a small percentage of patients.
Laboratory Evaluation As depicted in Fig. 405-10, thyroid function tests characteristically evolve through three distinct phases over about 6 months: (1) thyrotoxic phase, (2) hypothyroid phase, and (3) recovery phase. In the thyrotoxic phase, T4 and T3 levels are increased, reflecting their discharge from the damaged thyroid cells, and TSH is suppressed. The T4/T3 ratio is greater than in Graves’ disease or thyroid autonomy, in which T3 is often disproportionately increased. The diagnosis is confirmed by a high ESR and low uptake of radioiodine (<5%) or 99mTc pertechnetate (as compared to salivary gland pertechnetate concentration). The white blood cell count may be increased, and thyroid antibodies are negative. If the diagnosis is in doubt, FNA biopsy may be useful, particularly to distinguish unilateral involvement from bleeding into a cyst or neoplasm.
|
TREATMENT |
SUBACUTE THYROIDITIS |
Relatively large doses of aspirin (e.g., 600 mg every 4–6 h) or NSAIDs are sufficient to control symptoms in many cases. If this treatment is inadequate, or if the patient has marked local or systemic symptoms, glucocorticoids should be given. The usual starting dose is 40–60 mg of prednisone, depending on severity. The dose is gradually tapered over 6–8 weeks, in response to improvement in symptoms and the ESR. If a relapse occurs during glucocorticoid withdrawal, treatment should be started again and withdrawn more gradually. In these patients, it is useful to wait until the radioactive iodine uptake normalizes before stopping treatment. Thyroid function should be monitored every 2–4 weeks using TSH and unbound T4 levels. Symptoms of thyrotoxicosis improve spontaneously but may be ameliorated by β-adrenergic blockers; antithyroid drugs play no role in treatment of the thyrotoxic phase. Levothyroxine replacement may be needed if the hypothyroid phase is prolonged, but doses should be low enough (50–100 μg daily) to allow TSH-mediated recovery.
SILENT THYROIDITIS
Painless thyroiditis, or “silent” thyroiditis, occurs in patients with underlying autoimmune thyroid disease and has a clinical course similar to that of subacute thyroiditis. The condition occurs in up to 5% of women 3–6 months after pregnancy and is then termed postpartum thyroiditis. Typically, patients have a brief phase of thyrotoxicosis lasting 2–4 weeks, followed by hypothyroidism for 4–12 weeks, and then resolution; often, however, only one phase is apparent. The condition is associated with the presence of TPO antibodies antepartum, and it is three times more common in women with type 1 diabetes mellitus. As in subacute thyroiditis, the uptake of 99mTc pertechnetate or radioactive iodine is initially suppressed. In addition to the painless goiter, silent thyroiditis can be distinguished from subacute thyroiditis by a normal ESR and the presence of TPO antibodies. Glucocorticoid treatment is not indicated for silent thyroiditis. Severe thyrotoxic symptoms can be managed with a brief course of propranolol, 20–40 mg three or four times daily. Thyroxine replacement may be needed for the hypothyroid phase but should be withdrawn after 6–9 months, as recovery is the rule. Annual follow-up thereafter is recommended, because a proportion of these individuals develop permanent hypothyroidism. The condition may recur in subsequent pregnancies.
DRUG-INDUCED THYROIDITIS
Patients receiving cytokines such as IFN-α or IL-2 may develop painless thyroiditis. IFN-α, which is used to treat chronic hepatitis B or C and hematologic and skin malignancies, causes thyroid dysfunction in up to 5% of treated patients. It has been associated with painless thyroiditis, hypothyroidism, and Graves’ disease, and is most common in women with TPO antibodies prior to treatment. For discussion of amiodarone, see “Amiodarone Effects on Thyroid Function,” below.
CHRONIC THYROIDITIS
Focal thyroiditis is present in 20–40% of euthyroid autopsy cases and is associated with serologic evidence of autoimmunity, particularly the presence of TPO antibodies. The most common clinically apparent cause of chronic thyroiditis is Hashimoto’s thyroiditis, an autoimmune disorder that often presents as a firm or hard goiter of variable size (see above). Riedel’s thyroiditis is a rare disorder that typically occurs in middle-aged women. It presents with an insidious, painless goiter with local symptoms due to compression of the esophagus, trachea, neck veins, or recurrent laryngeal nerves. Dense fibrosis disrupts normal gland architecture and can extend outside the thyroid capsule. Despite these extensive histologic changes, thyroid dysfunction is uncommon. The goiter is hard, nontender, often asymmetric, and fixed, leading to suspicion of a malignancy. Diagnosis requires open biopsy as FNA biopsy is usually inadequate. Treatment is directed to surgical relief of compressive symptoms. Tamoxifen may also be beneficial. There is an association between Riedel’s thyroiditis and IgG4-related systemic disease causing idiopathic fibrosis at other sites (retroperitoneum, mediastinum, biliary tree, lung, and orbit).
SICK EUTHYROID SYNDROME (NONTHYROIDAL ILLNESS)
Any acute, severe illness can cause abnormalities of circulating TSH or thyroid hormone levels in the absence of underlying thyroid disease, making these measurements potentially misleading. The major cause of these hormonal changes is the release of cytokines such as IL-6. Unless a thyroid disorder is strongly suspected, the routine testing of thyroid function should be avoided in acutely ill patients.
The most common hormone pattern in sick euthyroid syndrome (SES) is a decrease in total and unbound T3 levels (low T3 syndrome) with normal levels of T4 and TSH. The magnitude of the fall in T3 correlates with the severity of the illness. T4 conversion to T3 via peripheral 5′ (outer ring) deiodination is impaired, leading to increased reverse T3 (rT3). Since rT3 is metabolized by 5′ deiodination, its clearance is also reduced. Thus, decreased clearance rather than increased production is the major basis for increased rT3. Also, T4 is alternately metabolized to the hormonally inactive T3 sulfate. It is generally assumed that this low T3 state is adaptive, because it can be induced in normal individuals by fasting. Teleologically, the fall in T3 may limit catabolism in starved or ill patients.
Very sick patients may exhibit a dramatic fall in total T4 and T3 levels (low T4 syndrome). With decreased tissue perfusion, muscle and liver expression of the type 3 deiodinase leads to accelerated T4 and T3 metabolism. This state has a poor prognosis. Another key factor in the fall in T4 levels is altered binding to TBG. The commonly used free T4 assays are subject to artifact when serum binding proteins are low and underestimate the true free T4 level. Fluctuation in TSH levels also creates challenges in the interpretation of thyroid function in sick patients. TSH levels may range from <0.1 mIU/L in very ill patients, especially with dopamine or glucocorticoid therapy, to >20 mIU/L during the recovery phase of SES. The exact mechanisms underlying the subnormal TSH seen in 10% of sick patients and the increased TSH seen in 5% remain unclear but may be mediated by cytokines including IL-12 and IL-18.
Any severe illness can induce changes in thyroid hormone levels, but certain disorders exhibit a distinctive pattern of abnormalities. Acute liver disease is associated with an initial rise in total (but not unbound) T3 and T4 levels due to TBG release; these levels become subnormal with progression to liver failure. A transient increase in total and unbound T4 levels, usually with a normal T3 level, is seen in 5–30% of acutely ill psychiatric patients. TSH values may be transiently low, normal, or high in these patients. In the early stage of HIV infection, T3 and T4 levels rise, even if there is weight loss. T3 levels fall with progression to AIDS, but TSH usually remains normal. Renal disease is often accompanied by low T3 concentrations, but with normal rather than increased rT3 levels, due to an unknown factor that increases uptake of rT3 into the liver.
The diagnosis of SES is challenging. Historic information may be limited, and patients often have multiple metabolic derangements. Useful features to consider include previous history of thyroid disease and thyroid function tests, evaluation of the severity and time course of the patient’s acute illness, documentation of medications that may affect thyroid function or thyroid hormone levels, and measurements of rT3 together with unbound thyroid hormones and TSH. The diagnosis of SES is frequently presumptive, given the clinical context and pattern of laboratory values; only resolution of the test results with clinical recovery can clearly establish this disorder. Treatment of SES with thyroid hormone (T4 and/or T3) is controversial, but most authorities recommend monitoring the patient’s thyroid function tests during recovery, without administering thyroid hormone, unless there is historic or clinical evidence suggestive of hypothyroidism. Sufficiently large randomized controlled trials using thyroid hormone are unlikely to resolve this therapeutic controversy in the near future, because clinical presentations and outcomes are highly variable.
AMIODARONE EFFECTS ON THYROID FUNCTION
Amiodarone is a commonly used type III antiarrhythmic agent (Chap. 277). It is structurally related to thyroid hormone and contains 39% iodine by weight. Thus, typical doses of amiodarone (200 mg/d) are associated with very high iodine intake, leading to greater than fortyfold increases in plasma and urinary iodine levels. Moreover, because amiodarone is stored in adipose tissue, high iodine levels persist for >6 months after discontinuation of the drug. Amiodarone inhibits deiodinase activity, and its metabolites function as weak antagonists of thyroid hormone action. Amiodarone has the following effects on thyroid function: (1) acute, transient suppression of thyroid function; (2) hypothyroidism in patients susceptible to the inhibitory effects of a high iodine load; and (3) thyrotoxicosis that may be caused by either a Jod-Basedow effect from the iodine load, in the setting of MNG or incipient Graves’ disease, or a thyroiditis-like condition.
The initiation of amiodarone treatment is associated with a transient decrease of T4 levels, reflecting the inhibitory effect of iodine on T4 release. Soon thereafter, most individuals escape from iodide-dependent suppression of the thyroid (Wolff-Chaikoff effect), and the inhibitory effects on deiodinase activity and thyroid hormone receptor action become predominant. These events lead to the following pattern of thyroid function tests: increased T4, decreased T3, increased rT3, and a transient TSH increase (up to 20 mIU/L). TSH levels normalize or are slightly suppressed within 1–3 months.
The incidence of hypothyroidism from amiodarone varies geographically, apparently correlating with iodine intake. Hypothyroidism occurs in up to 13% of amiodarone-treated patients in iodine-replete countries, such as the United States, but is less common (<6% incidence) in areas of lower iodine intake, such as Italy or Spain. The pathogenesis appears to involve an inability of the thyroid gland to escape from the Wolff-Chaikoff effect in autoimmune thyroiditis. Consequently, amiodarone-associated hypothyroidism is more common in women and individuals with positive TPO antibodies. It is usually unnecessary to discontinue amiodarone for this side effect, because levothyroxine can be used to normalize thyroid function. TSH levels should be monitored, because T4 levels are often increased for the reasons described above.
The management of amiodarone-induced thyrotoxicosis (AIT) is complicated by the fact that there are different causes of thyrotoxicosis and because the increased thyroid hormone levels exacerbate underlying arrhythmias and coronary artery disease. Amiodarone treatment causes thyrotoxicosis in 10% of patients living in areas of low iodine intake and in 2% of patients in regions of high iodine intake. There are two major forms of AIT, although some patients have features of both. Type 1 AIT is associated with an underlying thyroid abnormality (preclinical Graves’ disease or nodular goiter). Thyroid hormone synthesis becomes excessive as a result of increased iodine exposure (Jod-Basedow phenomenon). Type 2 AIT occurs in individuals with no intrinsic thyroid abnormalities and is the result of drug-induced lysosomal activation leading to destructive thyroiditis with histiocyte accumulation in the thyroid; the incidence rises as cumulative amiodarone dosage increases. Mild forms of type 2 AIT can resolve spontaneously or can occasionally lead to hypothyroidism. Color-flow Doppler thyroid scanning shows increased vascularity in type 1 AIT but decreased vascularity in type 2 AIT. Thyroid scintiscans are difficult to interpret in this setting because the high endogenous iodine levels diminish tracer uptake. However, the presence of normal or rarely increased uptake favors type 1 AIT.
In AIT, the drug should be stopped, if possible, although this is often impractical because of the underlying cardiac disorder. Discontinuation of amiodarone will not have an acute effect because of its storage and prolonged half-life. High doses of antithyroid drugs can be used in type 1 AIT but are often ineffective. In type 2 AIT, oral contrast agents, such as sodium ipodate (500 mg/d) or sodium tyropanoate (500 mg, 1–2 doses/d), rapidly reduce T4 and T3 levels, decrease T4 → T3 conversion, and may block tissue uptake of thyroid hormones. Potassium perchlorate, 200 mg every 6 h, has been used to reduce thyroidal iodide content. Perchlorate treatment has been associated with agranulocytosis, although the risk appears relatively low with short-term use. Glucocorticoids, as administered for subacute thyroiditis, have modest benefit in type 2 AIT. Lithium blocks thyroid hormone release and can also provide some benefit. Near-total thyroidectomy rapidly decreases thyroid hormone levels and may be the most effective long-term solution if the patient can undergo the procedure safely.
THYROID FUNCTION IN PREGNANCY
Five factors alter thyroid function in pregnancy: (1) the transient increase in hCG during the first trimester, which stimulates the TSH-R; (2) the estrogen-induced rise in TBG during the first trimester, which is sustained during pregnancy; (3) alterations in the immune system, leading to the onset, exacerbation, or amelioration of an underlying autoimmune thyroid disease (see above); (4) increased thyroid hormone metabolism by the placenta; and (5) increased urinary iodide excretion, which can cause impaired thyroid hormone production in areas of marginal iodine sufficiency. Women with a precarious iodine intake (<50 μg/d) are most at risk of developing a goiter during pregnancy or giving birth to an infant with a goiter and hypothyroidism. The World Health Organization recommends a daily iodine intake of 250 μg during pregnancy and prenatal vitamins should contain 150 μg per tablet.
The rise in circulating hCG levels during the first trimester is accompanied by a reciprocal fall in TSH that persists into the middle of pregnancy. This reflects the weak binding of hCG, which is present at very high levels, to the TSH-R. Rare individuals have been described with variant TSH-R sequences that enhance hCG binding and TSH-R activation. hCG-induced changes in thyroid function can result in transient gestational hyperthyroidism that may be associated with hyperemesis gravidarum, a condition characterized by severe nausea and vomiting and risk of volume depletion. However, since the hyperthyroidism is not causal, antithyroid drugs are not indicated unless concomitant Graves’ disease is suspected. Parenteral fluid replacement usually suffices until the condition resolves.
During pregnancy, subclinical hypothyroidism occurs in 2% of women, but overt hypothyroidism is present in only 1 in 500. Prospective randomized controlled trials have not shown a benefit for universal thyroid disease screening in pregnancy. Targeted TSH testing for hypothyroidism is recommended for women planning a pregnancy if they have a strong family history of autoimmune thyroid disease, other autoimmune disorders (e.g., type 1 diabetes), prior preterm delivery or recurrent miscarriage, or signs or symptoms of thyroid disease. Thyroid hormone requirements are increased by up to 50% during pregnancy in levothyroxine-treated hypothyroid women (see above section on treatment of hypothyroidism).
GOITER AND NODULAR THYROID DISEASE
Goiter refers to an enlarged thyroid gland. Biosynthetic defects, iodine deficiency, autoimmune disease, and nodular diseases can each lead to goiter, although by different mechanisms. Biosynthetic defects and iodine deficiency are associated with reduced efficiency of thyroid hormone synthesis, leading to increased TSH, which stimulates thyroid growth as a compensatory mechanism to overcome the block in hormone synthesis. Graves’ disease and Hashimoto’s thyroiditis are also associated with goiter. In Graves’ disease, the goiter results mainly from the TSH-R–mediated effects of TSI. The goitrous form of Hashimoto’s thyroiditis occurs because of acquired defects in hormone synthesis, leading to elevated levels of TSH and its consequent growth effects. Lymphocytic infiltration and immune system–induced growth factors also contribute to thyroid enlargement in Hashimoto’s thyroiditis. Nodular disease is characterized by the disordered growth of thyroid cells, often combined with the gradual development of fibrosis. Because the management of goiter depends on the etiology, the detection of thyroid enlargement on physical examination should prompt further evaluation to identify its cause.
Nodular thyroid disease is common, occurring in about 3–7% of adults when assessed by physical examination. Using ultrasound, nodules are present in up to 50% of adults, with the majority being <1 cm in diameter. Thyroid nodules may be solitary or multiple, and they may be functional or nonfunctional.
DIFFUSE NONTOXIC (SIMPLE) GOITER
Etiology and Pathogenesis When diffuse enlargement of the thyroid occurs in the absence of nodules and hyperthyroidism, it is referred to as a diffuse nontoxic goiter. This is sometimes called simple goiter, because of the absence of nodules, or colloid goiter, because of the presence of uniform follicles that are filled with colloid. Worldwide, diffuse goiter is most commonly caused by iodine deficiency and is termed endemic goiter when it affects >5% of the population. In nonendemic regions, sporadic goiter occurs, and the cause is usually unknown. Thyroid enlargement in teenagers is sometimes referred to as juvenile goiter. In general, goiter is more common in women than men, probably because of the greater prevalence of underlying autoimmune disease and the increased iodine demands associated with pregnancy.
In iodine-deficient areas, thyroid enlargement reflects a compensatory effort to trap iodide and produce sufficient hormone under conditions in which hormone synthesis is relatively inefficient. Somewhat surprisingly, TSH levels are usually normal or only slightly increased, suggesting increased sensitivity to TSH or activation of other pathways that lead to thyroid growth. Iodide appears to have direct actions on thyroid vasculature and may indirectly affect growth through vasoactive substances such as endothelins and nitric oxide. Endemic goiter is also caused by exposure to environmental goitrogens such as cassava root, which contains a thiocyanate; vegetables of the Cruciferae family (known as cruciferous vegetables) (e.g., Brussels sprouts, cabbage, and cauliflower); and milk from regions where goitrogens are present in grass. Although relatively rare, inherited defects in thyroid hormone synthesis lead to a diffuse nontoxic goiter. Abnormalities at each step in hormone synthesis, including iodide transport (NIS), Tg synthesis, organification and coupling (TPO), and the regeneration of iodide (dehalogenase), have been described.
CLINICAL MANIFESTATIONS AND DIAGNOSIS
If thyroid function is preserved, most goiters are asymptomatic. Examination of a diffuse goiter reveals a symmetrically enlarged, nontender, generally soft gland without palpable nodules. Goiter is defined, somewhat arbitrarily, as a lateral lobe with a volume greater than the thumb of the individual being examined. If the thyroid is markedly enlarged, it can cause tracheal or esophageal compression. These features are unusual, however, in the absence of nodular disease and fibrosis. Substernal goiter may obstruct the thoracic inlet. Pemberton’s sign refers to symptoms of faintness with evidence of facial congestion and external jugular venous obstruction when the arms are raised above the head, a maneuver that draws the thyroid into the thoracic inlet. Respiratory flow measurements and CT or MRI should be used to evaluate substernal goiter in patients with obstructive signs or symptoms.
Thyroid function tests should be performed in all patients with goiter to exclude thyrotoxicosis or hypothyroidism. It is not unusual, particularly in iodine deficiency, to find a low total T4, with normal T3 and TSH, reflecting enhanced T4 → T3 conversion. A low TSH with a normal free T3 and free T4, particularly in older patients, suggests the possibility of thyroid autonomy or undiagnosed Graves’ disease, and is termed subclinical thyrotoxicosis. The benefit of treatment (typically with radioiodine) in subclinical thyrotoxicosis, versus follow-up and implementing treatment if free T3 or free T4 levels become abnormal, is unclear, but treatment is increasingly recommended in the elderly to reduce the risk of atrial fibrillation and bone loss. TPO antibodies may be useful to identify patients at increased risk of autoimmune thyroid disease. Low urinary iodine levels (<50 μg/L) support a diagnosis of iodine deficiency. Thyroid scanning is not generally necessary but will reveal increased uptake in iodine deficiency and most cases of dyshormonogenesis. Ultrasound is not generally indicated in the evaluation of diffuse goiter unless a nodule is palpable on physical examination.
|
TREATMENT |
DIFFUSE NONTOXIC (SIMPLE) GOITER |
Iodine replacement induces variable regression of goiter in iodine deficiency, depending on how long it has been present and the degree of fibrosis that has developed. Surgery is rarely indicated for diffuse goiter. Exceptions include documented evidence of tracheal compression or obstruction of the thoracic inlet, which are more likely to be associated with substernal MNGs (see below). Subtotal or near-total thyroidectomy for these or cosmetic reasons should be performed by an experienced surgeon to minimize complication rates. Surgery should be followed by replacement with levothyroxine, with the aim of keeping the TSH level at the lower end of the reference range to prevent regrowth of the goiter.
NONTOXIC MULTINODULAR GOITER
Etiology and Pathogenesis Depending on the population studied, MNG or nodular enlargement of the thyroid occurs in up to 12% of adults. MNG is more common in women than men and increases in prevalence with age. It is more common in iodine-deficient regions but also occurs in regions of iodine sufficiency, reflecting multiple genetic, autoimmune, and environmental influences on the pathogenesis.
There is typically wide variation in nodule size. Histology reveals a spectrum of morphologies ranging from hypercellular regions to cystic areas filled with colloid. Fibrosis is often extensive, and areas of hemorrhage or lymphocytic infiltration may be seen. Using molecular techniques, most nodules within an MNG are polyclonal in origin, suggesting a hyperplastic response to locally produced growth factors and cytokines. TSH, which is usually not elevated, may play a permissive or contributory role. Monoclonal lesions also occur within an MNG, reflecting mutations in genes that confer a selective growth advantage to the progenitor cell.
Clinical Manifestations Most patients with nontoxic MNG are asymptomatic and euthyroid. MNG typically develops over many years and is detected on routine physical examination, when an individual notices an enlargement in the neck, or as an incidental finding on imaging. If the goiter is large enough, it can ultimately lead to compressive symptoms including difficulty swallowing, respiratory distress (tracheal compression), or plethora (venous congestion), but these symptoms are uncommon. Symptomatic MNGs are usually extraordinarily large and/or develop fibrotic areas that cause compression. Sudden pain in an MNG is usually caused by hemorrhage into a nodule but should raise the possibility of invasive malignancy. Hoarseness, reflecting laryngeal nerve involvement, also suggests malignancy.
Diagnosis On examination, thyroid architecture is distorted, and multiple nodules of varying size can be appreciated. Because many nodules are deeply embedded in thyroid tissue or reside in posterior or substernal locations, it is not possible to palpate all nodules. Pemberton’s sign, characterized by facial suffusion when the patient’s arms are elevated above the head, suggests that the goiter has increased pressure in the thoracic inlet. A TSH level should be measured to exclude subclinical hyper- or hypothyroidism, but thyroid function is usually normal. Tracheal deviation is common, but compression must usually exceed 70% of the tracheal diameter before there is significant airway compromise. Pulmonary function testing can be used to assess the functional effects of compression, which characteristically causes inspiratory stridor. CT or MRI can be used to evaluate the anatomy of the goiter and the extent of substernal extension or tracheal narrowing. A barium swallow may reveal the extent of esophageal compression. The risk of malignancy in MNG is similar to that in solitary nodules. Ultrasonography can be used to identify which nodules should be biopsied based on sonographic features (see section above on ultrasound) and size. For nodules with more suspicious imaging characteristics (e.g., hypoechogenicity, microcalcifications, irregular margins), biopsy is recommended when ≥1 cm.
|
TREATMENT |
NONTOXIC MULTINODULAR GOITER |
Most nontoxic MNGs can be managed conservatively. T4 suppression is rarely effective for reducing goiter size and introduces the risk of subclinical or overt thyrotoxicosis, particularly if there is underlying autonomy or if it develops during treatment. If levothyroxine is used, it should be started at low doses (50 μg daily) and advanced gradually while monitoring the TSH level to avoid excessive suppression. Contrast agents and other iodine-containing substances should be avoided because of the risk of inducing the Jod-Basedow effect, characterized by enhanced thyroid hormone production by autonomous nodules. Radioiodine is used with increasing frequency in areas where large goiters are more prevalent because it can decrease goiter size and may selectively ablate regions of autonomy. Dosage of 131I depends on the size of the goiter and radioiodine uptake but is usually about 3.7 MBq (0.1 mCi) per gram of tissue, corrected for uptake (typical dose 370–1070 MBq [10 to 29 mCi]). Repeat treatment may be needed and effectiveness may be increased by concurrent administration of low-dose recombinant TSH (0.1 mg IM). It is possible to achieve a 40–50% reduction in goiter size in most patients. Earlier concerns about radiation-induced thyroid swelling and tracheal compression have diminished, as studies have shown this complication to be rare. When acute compression occurs, glucocorticoid treatment or surgery may be needed. Radiation-induced hypothyroidism is less common than after treatment for Graves’ disease. However, posttreatment autoimmune thyrotoxicosis may occur in up to 5% of patients treated for nontoxic MNG. Surgery remains highly effective but is not without risk, particularly in older patients with underlying cardiopulmonary disease.
TOXIC MULTINODULAR GOITER
The pathogenesis of toxic MNG appears to be similar to that of nontoxic MNG; the major difference is the presence of functional autonomy in toxic MNG. The molecular basis for autonomy in toxic MNG remains unknown. As in nontoxic goiters, many nodules are polyclonal, whereas others are monoclonal and vary in their clonal origins. Genetic abnormalities known to confer functional autonomy, such as activating TSH-R or Gsα mutations (see below), are not usually found in the autonomous regions of toxic MNG goiter.
In addition to features of goiter, the clinical presentation of toxic MNG includes subclinical hyperthyroidism or mild thyrotoxicosis. The patient is usually elderly and may present with atrial fibrillation or palpitations, tachycardia, nervousness, tremor, or weight loss. Recent exposure to iodine, from contrast dyes or other sources, may precipitate or exacerbate thyrotoxicosis. The TSH level is low. The uncombined T4 level may be normal or minimally increased; T3 is often elevated to a greater degree than T4. Thyroid scan shows heterogeneous uptake with multiple regions of increased and decreased uptake; 24-h uptake of radioiodine may not be increased but is usually in the upper normal range.
Prior to definitive treatment of the hyperthyroidism, ultrasound imaging should be performed to assess the presence of discrete nodules corresponding to areas of decreased uptake (“cold” nodules). If present, FNA may be indicated based on sonographic features and size cutoffs. The cytology results, if indeterminate or suspicious, may direct the therapy to surgery.
|
TREATMENT |
TOXIC MULTINODULAR GOITER |
Antithyroid drugs normalize thyroid function and are particularly useful in the elderly or ill patients with limited lifespan. In contrast to Graves’ disease, spontaneous remission does not occur and so treatment is long-term. Radioiodine is generally the treatment of choice; it treats areas of autonomy as well as decreasing the mass of the goiter. Sometimes, however, a degree of autonomy remains, presumably because multiple autonomous regions emerge as soon as others are treated, and further radioiodine treatment may be necessary. Surgery provides definitive treatment of underlying thyrotoxicosis as well as goiter. Patients should be rendered euthyroid using an antithyroid drug before operation.
HYPERFUNCTIONING SOLITARY NODULE
A solitary, autonomously functioning thyroid nodule is referred to as toxic adenoma. The pathogenesis of this disorder has been unraveled by demonstrating the functional effects of mutations that stimulate the TSH-R signaling pathway. Most patients with solitary hyperfunctioning nodules have acquired somatic, activating mutations in the TSH-R (Fig. 405-11). These mutations, located primarily in the receptor transmembrane domain, induce constitutive receptor coupling to GSα, increasing cyclic AMP levels and leading to enhanced thyroid follicular cell proliferation and function. Less commonly, somatic mutations are identified in GSα. These mutations, which are similar to those seen in McCune-Albright syndrome (Chap. 412) or in a subset of somatotrope adenomas (Chap. 403), impair guanosine triphosphate (GTP) hydrolysis, causing constitutive activation of the cyclic AMP signaling pathway. In most series, activating mutations in either the TSH-R or the GSα subunit genes are identified in >90% of patients with solitary hyperfunctioning nodules.
FIGURE 405-11 Activating mutations of the thyroid-stimulating hormone receptor (TSH-R). Mutations (*) that activate TSH-R reside mainly in transmembrane 5 and intracellular loop 3, although mutations have occurred in a variety of different locations. The effect of these mutations is to induce conformational changes that mimic TSH binding, thereby leading to coupling to stimulatory G protein (GSα) and activation of adenylate cyclase (AC), an enzyme that generates cyclic AMP.
Thyrotoxicosis is usually mild. The disorder is suggested by a subnormal TSH level; the presence of the thyroid nodule, which is generally large enough to be palpable; and the absence of clinical features suggestive of Graves’ disease or other causes of thyrotoxicosis. A thyroid scan provides a definitive diagnostic test, demonstrating focal uptake in the hyperfunctioning nodule and diminished uptake in the remainder of the gland, as activity of the normal thyroid is suppressed.
|
TREATMENT |
HYPERFUNCTIONING SOLITARY NODULE |
Radioiodine ablation is usually the treatment of choice. Because normal thyroid function is suppressed, 131I is concentrated in the hyperfunctioning nodule with minimal uptake and damage to normal thyroid tissue. Relatively large radioiodine doses (e.g., 370–1110 MBq [10–29.9 mCi] 131I) have been shown to correct thyrotoxicosis in about 75% of patients within 3 months. Hypothyroidism occurs in <10% of those patients over the next 5 years. Surgical resection is also effective and is usually limited to enucleation of the adenoma or lobectomy, thereby preserving thyroid function and minimizing risk of hypoparathyroidism or damage to the recurrent laryngeal nerves. Medical therapy using antithyroid drugs and beta blockers can normalize thyroid function but is not an optimal long-term treatment. Using ultrasound guidance, repeated ethanol injections and percutaneous radiofrequency thermal ablation have been used successfully in some centers to ablate hyperfunctioning nodules, and these techniques have also been used to reduce the size of nonfunctioning thyroid nodules.
BENIGN NEOPLASMS
The various types of benign thyroid nodules are listed in Table 405-10. These lesions are common (5–10% adults), particularly when assessed by sensitive techniques such as ultrasound. The risk of malignancy is very low for macrofollicular adenomas and normofollicular adenomas. Microfollicular, trabecular, and Hürthle cell variants raise greater concern, and the histology is more difficult to interpret. Many are mixed cystic/solid lesions on ultrasound and may appear spongiform reflecting the pathology of macrofollicular structure. However, the majority of solid nodules (whether hypo-, iso-, or hyperechoic) are also benign. FNA, usually performed with ultrasound guidance, is the diagnostic procedure of choice to evaluate thyroid nodules (see the “Approach to the Patient” section on thyroid nodules). Pure thyroid cysts, <2% of all thyroid growths, consist of colloid and are benign as well. Cysts frequently recur, even after repeated aspiration, and may require surgical excision if they are large. Ethanol ablation to sclerose the cyst has been used successfully for patients who are symptomatic.
|
CLASSIFICATION OF THYROID NEOPLASMS |
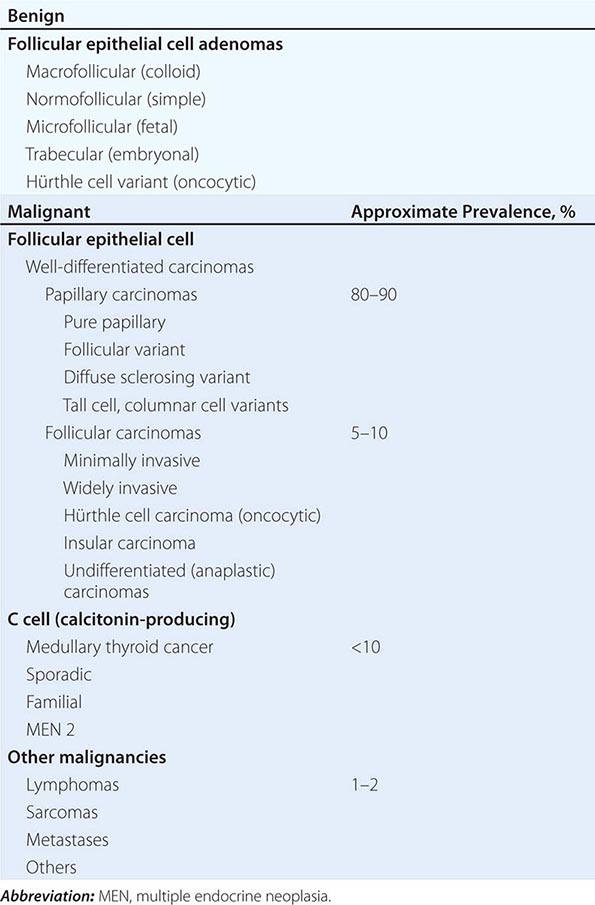
TSH suppression with levothyroxine therapy does not decrease thyroid nodule size in iodine-sufficient populations. However, if there is relative iodine deficiency, both iodine and levothyroxine therapy may decrease nodule volume. If levothyroxine is administered in this situation and the nodule has not decreased in size after 6–12 months of suppressive therapy, treatment should be discontinued because little benefit is likely to accrue from long-term treatment; the risk of iatrogenic subclinical thyrotoxicosis should also be considered.
THYROID CANCER
Thyroid carcinoma is the most common malignancy of the endocrine system. Malignant tumors derived from the follicular epithelium are classified according to histologic features. Differentiated tumors, such as papillary thyroid cancer (PTC) or follicular thyroid cancer (FTC), are often curable, and the prognosis is good for patients identified with early-stage disease. In contrast, anaplastic thyroid cancer (ATC) is aggressive, responds poorly to treatment, and is associated with a bleak prognosis.
The incidence of thyroid cancer is ~12/100,000 per year in the United States and increases with age. Prognosis is worse in older persons (>65 years). Thyroid cancer is twice as common in women as men, but male gender is associated with a worse prognosis. Additional important risk factors include a history of childhood head or neck irradiation, large nodule size (≥4 cm), evidence for local tumor fixation or invasion into lymph nodes, and the presence of metastases (Table 405-11). Several unique features of thyroid cancer facilitate its management: (1) thyroid nodules are amenable to biopsy by FNA; (2) iodine radioisotopes can be used to diagnose (123I) and treat (131I) differentiated thyroid cancer, reflecting the unique uptake of this anion by the thyroid gland; and (3) serum markers allow the detection of residual or recurrent disease, including the use of Tg levels for PTC and FTC, and calcitonin for medullary thyroid cancer (MTC).
|
RISK FACTORS FOR THYROID CARCINOMA IN PATIENTS WITH THYROID NODULE |
Abbreviation: MEN, multiple endocrine neoplasia.
CLASSIFICATION
Thyroid neoplasms can arise in each of the cell types that populate the gland, including thyroid follicular cells, calcitonin-producing C cells, lymphocytes, and stromal and vascular elements, as well as metastases from other sites (Table 405-10). The American Joint Committee on Cancer (AJCC) has designated a staging system using the tumor, node, metastasis (TNM) classification (Table 405-12). Several other classification and staging systems are also widely used, some of which place greater emphasis on histologic features or risk factors such as age or gender.
|
THYROID CANCER CLASSIFICATIONa |
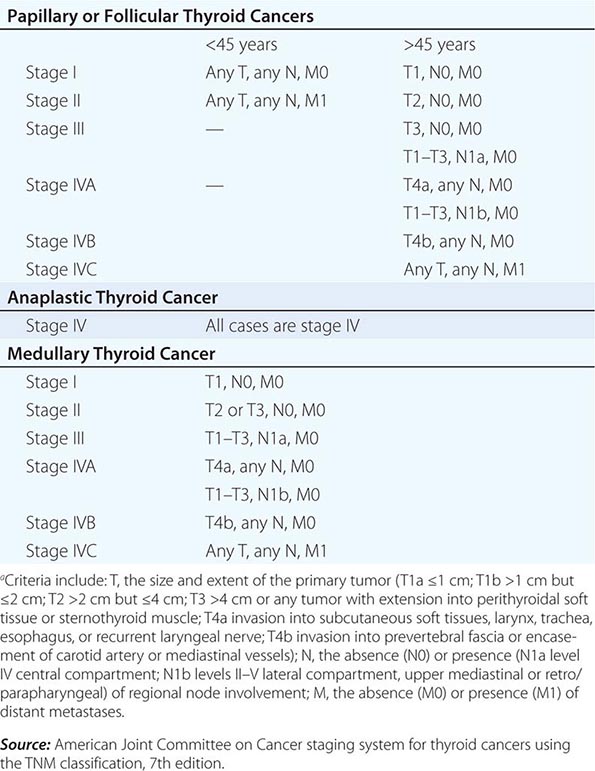
PATHOGENESIS AND GENETIC BASIS
Radiation Early studies of the pathogenesis of thyroid cancer focused on the role of external radiation, which predisposes to chromosomal breaks, leading to genetic rearrangements and loss of tumor-suppressor genes. External radiation of the mediastinum, face, head, and neck region was administered in the past to treat an array of conditions, including acne and enlargement of the thymus, tonsils, and adenoids. Radiation exposure increases the risk of benign and malignant thyroid nodules, is associated with multicentric cancers, and shifts the incidence of thyroid cancer to an earlier age group. Radiation from nuclear fallout also increases the risk of thyroid cancer. Children seem more predisposed to the effects of radiation than adults. Of note, radiation derived from 131I therapy appears to contribute minimal increased risk of thyroid cancer.
TSH and Growth Factors Many differentiated thyroid cancers express TSH receptors and, therefore, remain responsive to TSH. Higher serum TSH levels, even within normal range, are associated with increased thyroid cancer risk in patients with thyroid nodules. These observations provide the rationale for T4 suppression of TSH in patients with thyroid cancer. Residual expression of TSH receptors also allows TSH-stimulated uptake of 131I therapy (see below).
Oncogenes and Tumor-Suppressor Genes Thyroid cancers are monoclonal in origin, consistent with the idea that they originate as a consequence of mutations that confer a growth advantage to a single cell. In addition to increased rates of proliferation, some thyroid cancers exhibit impaired apoptosis and features that enhance invasion, angiogenesis, and metastasis. Thyroid neoplasms have been analyzed for a variety of genetic alterations, but without clear evidence of an ordered acquisition of somatic mutations as they progress from the benign to the malignant state. On the other hand, certain mutations are relatively specific for thyroid neoplasia, some of which correlate with histologic classification (Table 405-13).
|
GENETIC ALTERATIONS IN THYROID NEOPLASIA |
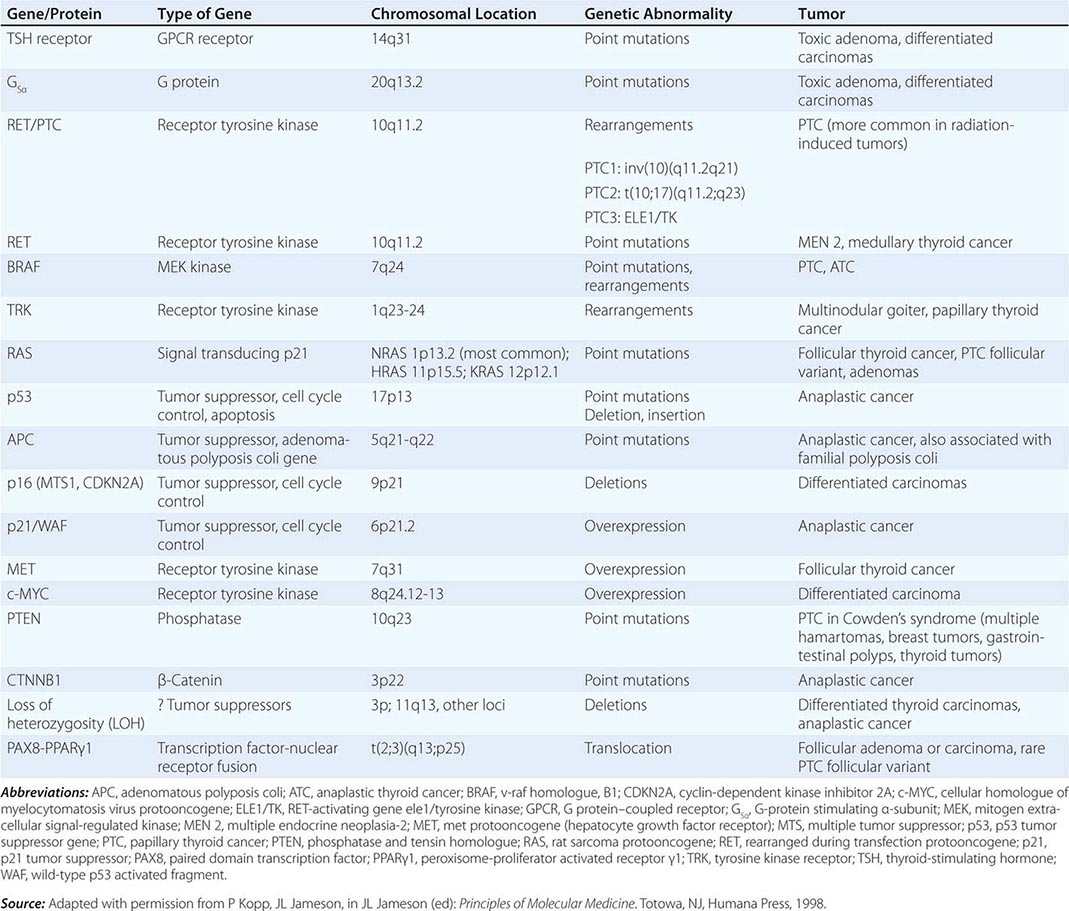
As described above, activating mutations of the TSH-R and the GSα subunit are associated with autonomously functioning nodules. Although these mutations induce thyroid cell growth, this type of nodule is almost always benign.
Activation of the RET-RAS-BRAF signaling pathway is seen in up to 70% of PTCs, although the types of mutations are heterogeneous. A variety of rearrangements involving the RET gene on chromosome 10 bring this receptor tyrosine kinase under the control of other promoters, leading to receptor overexpression. RET rearrangements occur in 20–40% of PTCs in different series and were observed with increased frequency in tumors developing after the Chernobyl radiation accident. Rearrangements in PTC have also been observed for another tyrosine kinase gene, TRK1, which is located on chromosome 1. To date, the identification of PTC with RET or TRK1 rearrangements has not proven useful for predicting prognosis or treatment responses. BRAF V600E mutations appear to be the most common genetic alteration in PTC. These mutations activate the kinase, which stimulates the mitogen-activated protein MAP kinase (MAPK) cascade. RAS mutations, which also stimulate the MAPK cascade, are found in about 20–30% of thyroid neoplasms (NRAS > HRAS > KRAS), including both PTC and FTC. Of note, simultaneous RET, BRAF, and RAS mutations rarely occur in the same tumor, suggesting that activation of the MAPK cascade is critical for tumor development, independent of the step that initiates the cascade.
RAS mutations also occur in FTCs. In addition, a rearrangement of the thyroid developmental transcription factor PAX8 with the nuclear receptor PPARγ is identified in a significant fraction of FTCs. Overall, about 70% of follicular cancers have mutations or genetic rearrangements. Loss of heterozygosity of 3p or 11q, consistent with deletions of tumor-suppressor genes, is also common in FTCs.
Most of the mutations seen in differentiated thyroid cancers have also been detected in ATCs. BRAF mutations are seen in up to 50% of ATCs. Mutations in CTNNB1, which encodes β-catenin, occur in about two-thirds of ATCs, but not in PTC or FTC. Mutations of the tumor-suppressor P53 also play an important role in the development of ATC. Because P53 plays a role in cell cycle surveillance, DNA repair, and apoptosis, its loss may contribute to the rapid acquisition of genetic instability as well as poor treatment responses (Chap. 102e) (Table 405-13).
The role of molecular diagnostics in the clinical management of thyroid cancer is under investigation. In principle, analyses of specific mutations might aid in classification, prognosis, or choice of treatment. Although BRAF V600E mutations are associated with loss of iodine uptake by tumor cells, there is no clear evidence to date that this information alters clinical decision making. Higher recurrence rates have been variably reported in patients with BRAF-positive PTC, but the impact on survival rates is unclear. Sequencing of thyroid cancers as part of the Cancer Genome Atlas (TCGA) is likely to lead to new classification schemes based on molecular abnormalities in tumors.
MTC, when associated with multiple endocrine neoplasia (MEN) type 2, harbors an inherited mutation of the RET gene. Unlike the rearrangements of RET seen in PTC, the mutations in MEN 2 are point mutations that induce constitutive activity of the tyrosine kinase (Chap. 408). MTC is preceded by hyperplasia of the C cells, raising the likelihood that as-yet-unidentified “second hits” lead to cellular transformation. A subset of sporadic MTC contains somatic mutations that activate RET.
WELL-DIFFERENTIATED THYROID CANCER
Papillary PTC is the most common type of thyroid cancer, accounting for 70–90% of well-differentiated thyroid malignancies. Microscopic PTC is present in up to 25% of thyroid glands at autopsy, but most of these lesions are very small (several millimeters) and are not clinically significant. Characteristic cytologic features of PTC help make the diagnosis by FNA or after surgical resection; these include psammoma bodies, cleaved nuclei with an “orphan-Annie” appearance caused by large nucleoli, and the formation of papillary structures.
PTC tends to be multifocal and to invade locally within the thyroid gland as well as through the thyroid capsule and into adjacent structures in the neck. It has a propensity to spread via the lymphatic system but can metastasize hematogenously as well, particularly to bone and lung. Because of the relatively slow growth of the tumor, a significant burden of pulmonary metastases may accumulate, sometimes with remarkably few symptoms. The prognostic implication of lymph node spread is debated. Lymph node involvement by thyroid cancer can be well tolerated but appears to increase the risk of recurrence and mortality, particularly in older patients. The staging of PTC by the TNM system is outlined in Table 405-12. Most papillary cancers are identified in the early stages (>80% stages I or II) and have an excellent prognosis, with survival curves similar to expected survival (Fig. 405-12). Mortality is markedly increased in stage IV disease, especially in the presence of distant metastases (stage IVC), but this group comprises only about 1% of patients. The treatment of PTC is described below.
FIGURE 405-12 Survival rates of patients with different stages of papillary cancer. (Adapted with permission from Edge SB, Byrd DR: Thyroid, in Compton CC, Fritz AB, Greene FL, Trotti A [eds]: AJCC Cancer Staging Manual, 7th ed. New York, Springer, 2010, pp 87–92.)
Follicular The incidence of FTC varies widely in different parts of the world; it is more common in iodine-deficient regions. Currently, FTC accounts for only about 5% of all thyroid cancers diagnosed in the United States. FTC is difficult to diagnose by FNA because the distinction between benign and malignant follicular neoplasms rests largely on evidence of invasion into vessels, nerves, or adjacent structures. FTC tends to spread by hematogenous routes leading to bone, lung, and central nervous system metastases. Mortality rates associated with FTC are less favorable than for PTC, in part because a larger proportion of patients present with stage IV disease. Poor prognostic features include distant metastases, age >50 years, primary tumor size >4 cm, Hürthle cell histology, and the presence of marked vascular invasion.
|
WELL-DIFFERENTIATED THYROID CANCER |
SURGERY
All well-differentiated thyroid cancers should be surgically excised. In addition to removing the primary lesion, surgery allows accurate histologic diagnosis and staging, and multicentric disease is commonly found in the contralateral thyroid lobe. Preoperative sonography should be performed in all patients to assess the central and lateral cervical lymph node compartments for suspicious adenopathy, which if present, can undergo FNA and then be removed at surgery. Bilateral, near-total thyroidectomy has been shown to reduce recurrence rates in all patients except those with T1a tumors (≤1 cm). If cytology is diagnostic for thyroid cancer, bilateral surgery should be done. If malignancy is identified pathologically after lobectomy, completion surgery is recommended unless the tumor is T1a or is a minimally invasive follicular cancer. Bilateral surgery for patients at higher risk allows monitoring of serum Tg levels and administration of radioiodine for remnant ablation and potential treatment of iodine-avid metastases, if indicated. Therefore, near-total thyroidectomy is preferable in almost all patients; complication rates are acceptably low if the surgeon is highly experienced in the procedure.
TSH SUPPRESSION THERAPY
Because most tumors are still TSH-responsive, levothyroxine suppression of TSH is a mainstay of thyroid cancer treatment. Although TSH suppression clearly provides therapeutic benefit, there are no prospective studies that define the optimal level of TSH suppression. The degree of TSH suppression should be individualized based on a patient’s risk of recurrence. It should be adjusted over time as surveillance blood tests and imaging confirm absence of disease or, alternatively, indicate possible residual/recurrent cancer. For patients at low risk of recurrence, TSH should be suppressed into the low but detectable range (0.1–0.5 mIU/L). If subsequent surveillance testing indicates no evidence of disease, the TSH target may rise to the lower half of the normal range. For patients at high risk of recurrence or with known metastatic disease, TSH levels should be kept to <0.1 mIU/L if there are no strong contraindications to mild thyrotoxicosis. In this instance, unbound T4 must also be monitored to avoid excessive treatment.
RADIOIODINE TREATMENT
After near-total thyroidectomy, substantial thyroid tissue often remains, particularly in the thyroid bed and surrounding the parathyroid glands. Postsurgical radioablation of the remnant thyroid eliminates residual normal thyroid, facilitating the use of Tg determinations and radioiodine scanning for long-term follow-up. In addition, well-differentiated thyroid cancer often incorporates radioiodine, although less efficiently than normal thyroid follicular cells. Radioiodine uptake is determined primarily by expression of the NIS and is stimulated by TSH, requiring expression of the TSH-R. The retention time for radioactivity is influenced by the extent to which the tumor retains differentiated functions such as iodide trapping and organification. Consequently, for patients at risk of recurrence and for those with known distant metastatic disease, 131I ablation may also potentially treat residual tumor cells.
Indications Not all patients benefit from radioiodine therapy. Neither recurrence nor survival rates are improved in stage I patients with T1 tumors (≤2 cm) confined to the thyroid. However, in higher risk patients (larger tumors, more aggressive variants of papillary cancer, tumor vascular invasion, presence of large-volume lymph node metastases), radioiodine reduces recurrence and may increase survival.
131I Thyroid Ablation and Treatment As noted above, the decision to use 131I for thyroid ablation should be coordinated with the surgical approach, because radioablation is much more effective when there is minimal remaining normal thyroid tissue. Radioiodine is administered after iodine depletion (patient follows a low-iodine diet for 1≤2 weeks) and in the presence of elevated serum TSH levels to stimulate uptake of the isotope into both the remnant and potentially any residual tumor. To achieve high serum TSH levels, there are two approaches. A patient may be withdrawn from thyroid hormone so that endogenous TSH is secreted and, ideally, the serum TSH level is >25 mIU/L at the time of 131I therapy. A typical strategy is to treat the patient for several weeks postoperatively with liothyronine (25 μg qd or bid), followed by thyroid hormone withdrawal for 2 weeks. Alternatively, recombinant human TSH (rhTSH) is administered as two daily consecutive injections (0.9 mg) with administration of 131I 24 h after the second injection. The patient can continue to take levothyroxine and remains euthyroid. Both approaches have equal success in achieving remnant ablation.
A pretreatment scanning dose of 131I (usually 111–185 MBq [3–5 mCi]) or 123I (74 MBq [2 mCi]) can reveal the amount of residual tissue and provides guidance about the dose needed to accomplish ablation. However, because of concerns about radioactive “stunning” that impairs subsequent treatment, there is a trend to avoid pretreatment scanning with 131I and use either 123I or proceed directly to ablation, unless there is suspicion that the amount of residual tissue will alter therapy or that there is distant metastatic disease. In the United States, outpatient doses of up to 6475 MBq (175 mCi) can be given at most centers. The administered dose depends on the indication for therapy with lower doses of 1850–2775 MBq (50–75 mCi) given for remnant ablation but higher doses of 3700–5500 MBq (100–150 mCi) used as adjuvant therapy when residual disease may be present. A WBS following radioiodine treatment is used to confirm the 131I uptake in the remnant and to identify possible metastatic disease.
Follow-Up Whole-Body Thyroid Scanning and Thyroglobulin Determinations Serum thyroglobulin is a sensitive marker of residual/recurrent thyroid cancer after ablation of the residual postsurgical thyroid tissue. However, newer Tg assays have functional sensitivities as low as 0.1 ng/mL, as opposed to older assays with functional sensitivities of 1 ng/mL, reducing the number of patients with truly undetectable serum Tg levels. Because the vast majority of papillary thyroid cancer recurrences are in cervical lymph nodes, a neck ultrasound should be performed about 6 months after thyroid ablation; ultrasound has been shown to be more sensitive than WBS in this scenario.
In low-risk patients who have no clinical evidence of residual disease after ablation and a basal Tg <1 ng/mL on levothyroxine, an rhTSH-stimulated Tg level should be obtained 6–12 months after ablation, without WBS. If stimulated Tg levels are low (<1 ng/mL) and, ideally, undetectable, the risk of recurrence is <5% at 5 years. Newer data indicate that rhTSH stimulation may not be required for patients with undetectable basal Tg levels in sensitive assays, if there is documented absence of Tg antibodies. These patients can be followed with unstimulated Tg every 6–12 months and neck ultrasound as indicated. Levothyroxine dosing may then be titrated to a higher TSH level of 0.5–1.5 mIU/L.
The use of WBS is reserved for patients with known iodine-avid metastases or those with elevated serum thyroglobulin levels and negative imaging with ultrasound, chest CT, and neck cross-sectional imaging who may require additional 131I therapy.
In addition, most authorities advocate radioiodine treatment for scan-negative, Tg-positive (Tg >5–10 ng/mL) patients, as many derive therapeutic benefit from a large dose of 131I. For such patients, rhTSH preparation is not FDA approved for the treatment of metastatic disease, and the traditional approach of thyroid hormone withdrawal should be followed. This involves switching patients from levothyroxine (T4) to the more rapidly cleared hormone liothyronine (T3), thereby allowing TSH to increase more quickly. Whenever 131I is administered, posttherapy WBS is the gold standard to assess iodine-avid metastases.
In addition to radioiodine, external beam radiotherapy is also used to treat specific metastatic lesions, particularly when they cause bone pain or threaten neurologic injury (e.g., vertebral metastases).
New Potential Therapies Kinase inhibitors are being explored as a means to target pathways known to be active in thyroid cancer, including the RAS, BRAF, EGFR, VEGFR, and angiogenesis pathways. A multicenter randomized controlled trial of the multikinase inhibitor sorafenib in 417 patients with progressive metastatic thyroid cancer reported a doubling of progression-free survival to 10.8 months in the treatment group compared with the placebo group. Ongoing trials are exploring whether differentiation protocols with kinase inhibitors or other approaches might enhance radioiodine uptake and efficacy.
ANAPLASTIC AND OTHER FORMS OF THYROID CANCER
Anaplastic Thyroid Cancer As noted above, ATC is a poorly differentiated and aggressive cancer. The prognosis is poor, and most patients die within 6 months of diagnosis. Because of the undifferentiated state of these tumors, the uptake of radioiodine is usually negligible, but it can be used therapeutically if there is residual uptake. Chemotherapy has been attempted with multiple agents, including anthracyclines and paclitaxel, but it is usually ineffective. External beam radiation therapy can be attempted and continued if tumors are responsive.
Thyroid Lymphoma Lymphoma in the thyroid gland often arises in the background of Hashimoto’s thyroiditis. A rapidly expanding thyroid mass suggests the possibility of this diagnosis. Diffuse large-cell lymphoma is the most common type in the thyroid. Biopsies reveal sheets of lymphoid cells that can be difficult to distinguish from small-cell lung cancer or ATC. These tumors are often highly sensitive to external radiation. Surgical resection should be avoided as initial therapy because it may spread disease that is otherwise localized to the thyroid. If staging indicates disease outside of the thyroid, treatment should follow guidelines used for other forms of lymphoma (Chap. 134).
MEDULLARY THYROID CARCINOMA
MTC can be sporadic or familial and accounts for about 5% of thyroid cancers. There are three familial forms of MTC: MEN 2A, MEN 2B, and familial MTC without other features of MEN (Chap. 408). In general, MTC is more aggressive in MEN 2B than in MEN 2A, and familial MTC is more aggressive than sporadic MTC. Elevated serum calcitonin provides a marker of residual or recurrent disease. All patients with MTC should be tested for RET mutations, because genetic counseling and testing of family members can be offered to those individuals who test positive for mutations.
The management of MTC is primarily surgical. Unlike tumors derived from thyroid follicular cells, these tumors do not take up radioiodine. External radiation treatment and chemotherapy may provide palliation in patients with advanced disease (Chap. 408).
406 |
Disorders of the Adrenal Cortex |
The adrenal cortex produces three classes of corticosteroid hormones: glucocorticoids (e.g., cortisol), mineralocorticoids (e.g., aldosterone), and adrenal androgen precursors (e.g., dehydroepiandrosterone [DHEA]) (Fig. 406-1). Glucocorticoids and mineralocorticoids act through specific nuclear receptors, regulating aspects of the physiologic stress response as well as blood pressure and electrolyte homeostasis. Adrenal androgen precursors are converted in the gonads and peripheral target cells to sex steroids that act via nuclear androgen and estrogen receptors.
FIGURE 406-1 Adrenal steroidogenesis. ADX, adrenodoxin; CYP11A1, side chain cleavage enzyme; CYP11B1, 11β-hydroxylase; CYP11B2, aldosterone synthase; CYP17A1, 17α-hydroxylase/17,20 lyase; CYP21A2, 21-hydroxylase; DHEA, dehydroepiandrosterone; DHEAS, dehydroepiandrosterone sulfate; H6PDH, hexose-6-phosphate dehydrogenase; HSD11B1, 11β-hydroxysteroid dehydrogenase type 1; HSD11B2, 11β-hydroxysteroid dehydrogenase type 2; HSD17B, 17β-hydroxysteroid dehydrogenase; HSD3B2, 3β-hydroxysteroid dehydrogenase type 2; PAPSS2, PAPS synthase type 2; POR, P450 oxidoreductase; SRD5A, 5α-reductase; SULT2A1, DHEA sulfotransferase.
Disorders of the adrenal cortex are characterized by deficiency or excess of one or several of the three major corticosteroid classes. Hormone deficiency can be caused by inherited glandular or enzymatic disorders or by destruction of the pituitary or adrenal gland by autoimmune disorders, infection, infarction, or iatrogenic events such as surgery or hormonal suppression. Hormone excess is usually the result of neoplasia, leading to increased production of adrenocorticotropic hormone (ACTH) by the pituitary or neuroendocrine cells (ectopic ACTH) or increased production of glucocorticoids, mineralocorticoids, or adrenal androgen precursors by adrenal nodules. Adrenal nodules are increasingly identified incidentally during abdominal imaging performed for other reasons.
ADRENAL ANATOMY AND DEVELOPMENT
The normal adrenal glands weigh 6–11 g each. They are located above the kidneys and have their own blood supply. Arterial blood flows initially to the subcapsular region and then meanders from the outer cortical zona glomerulosa through the intermediate zona fasciculata to the inner zona reticularis and eventually to the adrenal medulla. The right suprarenal vein drains directly into the vena cava, while the left suprarenal vein drains into the left renal vein.
During early embryonic development, the adrenals originate from the urogenital ridge and then separate from gonads and kidneys at about the sixth week of gestation. Concordant with the time of sexual differentiation (seventh to ninth week of gestation, Chap. 410), the adrenal cortex starts to produce cortisol and the adrenal sex steroid precursor DHEA. The orphan nuclear receptors SF1 (steroidogenic factor 1; encoded by the gene NR5A1) and DAX1 (dosage-sensitive sex reversal gene 1; encoded by the gene NR0B1), among others, play a crucial role during this period of development, as they regulate a multitude of adrenal genes involved in steroidogenesis.
REGULATORY CONTROL OF STEROIDOGENESIS
Production of glucocorticoids and adrenal androgens is under the control of the hypothalamic-pituitary-adrenal (HPA) axis, whereas mineralocorticoids are regulated by the renin-angiotensin-aldosterone (RAA) system.
Glucocorticoid synthesis is under inhibitory feedback control by the hypothalamus and the pituitary (Fig. 406-2). Hypothalamic release of corticotropin-releasing hormone (CRH) occurs in response to endogenous or exogenous stress. CRH stimulates the cleavage of the 241–amino acid polypeptide proopiomelanocortin (POMC) by pituitary-specific prohormone convertase 1 (PC1), yielding the 39–amino acid peptide ACTH. ACTH is released by the corticotrope cells of the anterior pituitary and acts as the pivotal regulator of adrenal cortisol synthesis, with additional short-term effects on mineralocorticoid and adrenal androgen synthesis. The release of CRH, and subsequently ACTH, occurs in a pulsatile fashion that follows a circadian rhythm under the control of the hypothalamus, specifically its suprachiasmatic nucleus (SCN), with additional regulation by a complex network of cell-specific clock genes. Reflecting the pattern of ACTH secretion, adrenal cortisol secretion exhibits a distinct circadian rhythm, starting to rise in the early morning hours prior to awakening, with peak levels in the morning and low levels in the evening (Fig. 406-3).
FIGURE 406-2 Regulation of the hypothalamic-pituitary-adrenal (HPA) axis. ACTH, adrenocorticotropic hormone; CRH, corticotropin-releasing hormone.
FIGURE 406-3 Physiologic cortisol circadian rhythm. Circulating cortisol concentrations (geometrical mean ± standard deviation values and fitted cosinor) drop under the rhythm-adjusted mean (MESOR) in the early evening hours, with nadir levels around midnight and a rise in the early morning hours; peak levels are observed ~8:30 AM (acrophase). (Modified after M Debono et al: Modified-release hydrocortisone to provide circadian cortisol profiles. J Clin Endocrinol Metab 94:1548, 2009.)
Diagnostic tests assessing the HPA axis make use of the fact that it is regulated by negative feedback. Glucocorticoid excess is diagnosed by employing a dexamethasone suppression test. Dexamethasone, a potent synthetic glucocorticoid, suppresses CRH/ACTH by binding hypothalamic-pituitary glucocorticoid receptors and, therefore, results in downregulation of endogenous cortisol synthesis. Various versions of the dexamethasone suppression test are described in detail in Chap. 403. If cortisol production is autonomous (e.g., adrenal nodule), ACTH is already suppressed and dexamethasone has little additional effect. If cortisol production is driven by an ACTH-producing pituitary adenoma, dexamethasone suppression is ineffective at low doses but usually induces suppression at high doses. If cortisol production is driven by an ectopic source of ACTH, the tumors are usually resistant to dexamethasone suppression. Thus, the dexamethasone suppression test is useful to establish the diagnosis of Cushing’s syndrome and to assist with the differential diagnosis of cortisol excess.
Conversely, to assess glucocorticoid deficiency, ACTH stimulation of cortisol production is used. The ACTH peptide contains 39 amino acids but the first 24 are sufficient to elicit a physiologic response. The standard ACTH stimulation test involves administration of cosyntropin (ACTH 1-24), 0.25 mg IM or IV, and collection of blood samples at 0, 30, and 60 min for cortisol. A normal response is defined as a cortisol level >20 μg/dL (>550 nmol/L) 30–60 min after cosyntropin stimulation. A low-dose (1 μg cosyntropin IV) version of this test has been advocated; however, it has no superior diagnostic value and is more cumbersome to carry out. Alternatively, an insulin tolerance test (ITT) can be used to assess adrenal function. It involves injection of insulin to induce hypoglycemia, which represents a strong stress signal that triggers hypothalamic CRH release and activation of the entire HPA axis. The ITT involves administration of regular insulin 0.1 U/kg IV (dose should be lower if hypopituitarism is likely) and collection of blood samples at 0, 30, 60, and 120 min for glucose, cortisol, and growth hormone (GH), if also assessing the GH axis. Oral or IV glucose is administered after the patient has achieved symptomatic hypoglycemia (usually glucose <40 mg/dL). A normal response is defined as a cortisol >20 μg/dL and GH >5.1 μg/L. The ITT requires careful clinical monitoring and sequential measurements of glucose. It is contraindicated in patients with coronary disease, cerebrovascular disease, or seizure disorders, which has made the short cosyntropin test the commonly accepted first-line test.
Mineralocorticoid production is controlled by the RAA regulatory cycle, which is initiated by the release of renin from the juxtaglomerular cells in the kidney, resulting in cleavage of angiotensinogen to angiotensin I in the liver (Fig. 406-4). Angiotensin-converting enzyme (ACE) cleaves angiotensin I to angiotensin II, which binds and activates the angiotensin II receptor type 1 (AT1 receptor [AT1R]), resulting in increased adrenal aldosterone production and vasoconstriction. Aldosterone enhances sodium retention and potassium excretion, and increases the arterial perfusion pressure, which in turn regulates renin release. Because mineralocorticoid synthesis is primarily under the control of the RAA system, hypothalamic-pituitary damage does not significantly impact the capacity of the adrenal to synthesize aldosterone.
FIGURE 406-4 Regulation of the renin-angiotensin-aldosterone (RAA) system.
Similar to the HPA axis, the assessment of the RAA system can be used for diagnostic purposes. If mineralocorticoid excess is present, there is a counter-regulatory downregulation of plasma renin (see below for testing). Conversely, in mineralocorticoid deficiency, plasma renin is markedly increased. Physiologically, oral or IV sodium loading results in suppression of aldosterone, a response that is attenuated or absent in patients with autonomous mineralocorticoid excess.
STEROID HORMONE SYNTHESIS, METABOLISM, AND ACTION
ACTH stimulation is required for the initiation of steroidogenesis. The ACTH receptor MC2R (melanocortin 2 receptor) interacts with the MC2R-accessory protein MRAP, and the complex is transported to the adrenocortical cell membrane, where it binds to ACTH (Fig. 406-5). ACTH stimulation generates cyclic AMP (cAMP), which upregulates the protein kinase A (PKA) signaling pathway. Inactive PKA is a tetramer of two regulatory and two catalytic subunits that is dissociated by cAMP into a dimer of two regulatory subunits bound to cAMP and two free and active catalytic subunits. PKA activation impacts steroidogenesis in three distinct ways: (1) increases the import of cholesterol esters; (2) increases the activity of hormone-sensitive lipase, which cleaves cholesterol esters to cholesterol for import into the mitochondrion; and (3) increases the availability and phosphorylation of CREB (cAMP response element binding), a transcription factor that enhances transcription of CYP11A1 and other enzymes required for glucocorticoid synthesis.
FIGURE 406-5 ACTH effects on adrenal steroidogenesis. ACTH, adrenocorticotropic hormone; binding protein; MRAP, MC2R-accessory protein; protein kinase A catalytic subunit (C; PRKACA), PKA regulatory subunit (R; PRKAR1A); StAR, steroidogenic acute regulatory (protein); TSPO, translocator protein.
Adrenal steroidogenesis occurs in a zone-specific fashion, with mineralocorticoid synthesis occurring in the outer zona glomerulosa, glucocorticoid synthesis in the zona fasciculata, and adrenal androgen synthesis in the inner zona reticularis (Fig. 406-1). All steroidogenic pathways require cholesterol import into the mitochondrion, a process initiated by the action of the steroidogenic acute regulatory (StAR) protein, which shuttles cholesterol from the outer to the inner mitochondrial membrane. The majority of steroidogenic enzymes are cytochrome P450 (CYP) enzymes, which are either located in the mitochondrion (side chain cleavage enzyme, CYP11A1; 11β-hydroxylase, CYP11B1; aldosterone synthase, CYP11B2) or in the endoplasmic reticulum membrane (17α-hydroxylase, CYP17A1; 21-hydroxylase, CYP21A2; aromatase, CYP19A1). These enzymes require electron donation via specific redox cofactor enzymes, P450 oxidoreductase (POR), and adrenodoxin/adrenodoxin reductase (ADX/ADR) for the microsomal and mitochondrial CYP enzymes, respectively. In addition, the short-chain dehydrogenase 3β-hydroxysteroid dehydrogenase type 2 (3β-HSD2), also termed Δ4,Δ5 isomerase, plays a major role in adrenal steroidogenesis.
The cholesterol side chain cleavage enzyme CYP11A1 generates pregnenolone. Glucocorticoid synthesis requires conversion of pregnenolone to progesterone by 3β-HSD2, followed by conversion to 17-hydroxyprogesterone by CYP17A1, further hydroxylation at carbon 21 by CYP21A2, and eventually, 11β-hydroxylation by CYP11B1 to generate active cortisol (Fig. 406-1). Mineralocorticoid synthesis also requires progesterone, which is first converted to deoxycorticosterone by CYP21A2 and then converted via corticosterone and 18-hydroxycorticosterone to aldosterone in three steps catalyzed by CYP11B2. For adrenal androgen synthesis, pregnenolone undergoes conversion by CYP17A1, which uniquely catalyzes two enzymatic reactions. Via its 17α-hydroxylase activity, CYP17A1 converts pregnenolone to 17-hydroxypregnenolone, followed by generation of the universal sex steroid precursor DHEA via CYP17A1 17,20 lyase activity. The majority of DHEA is secreted by the adrenal in the form of its sulfate ester, DHEAS, generated by DHEA sulfotransferase (SULT2A1).
Following its release from the adrenal, cortisol circulates in the bloodstream mainly bound to cortisol-binding globulin (CBG) and to a lesser extent to albumin, with only a minor fraction circulating as free, unbound hormone. Free cortisol is thought to enter cells directly, not requiring active transport. In addition, in a multitude of peripheral target tissues of glucocorticoid action, including adipose, liver, muscle, and brain, cortisol is generated from inactive cortisone within the cell by the enzyme 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) (Fig. 406-6). Thereby, 11β-HSD1 functions as a tissue-specific prereceptor regulator of glucocorticoid action. For the conversion of inactive cortisone to active cortisol, 11β-HSD1 requires nicotinamide adenine dinucleotide phosphate (NADPH [reduced form]), which is provided by the enzyme hexose-6-phosphate dehydrogenase (H6PDH). Like the catalytic domain of 11β-HSD1, H6PDH is located in the lumen of the endoplasmic reticulum, and converts glucose-6-phosphate (G6P) to 6-phosphogluconate (6PGL), thereby regenerating NADP+ to NADPH, which drives the activation of cortisol from cortisone by 11β-HSD1.
FIGURE 406-6 Prereceptor activation of cortisol and glucocorticoid receptor (GR) action. AP-1 activator protein-1; G6P, glucose-6-phosphate; GRE, glucocorticoid response elements; HSP, heat shock proteins; NADPH, nicotinamide adenine dinucleotide phosphate (reduced form); 6PGL, 6-phosphogluconate.
In the cytosol of target cells, cortisol binds and activates the glucocorticoid receptor (GR), which results in dissociation of heat shock proteins (HSP) from the receptor and subsequent dimerization (Fig. 406-6). Cortisol-bound GR dimers translocate to the nucleus and activate glucocorticoid response elements (GRE) in the DNA sequence, thereby enhancing transcription of glucocorticoid-regulated genes (GR transactivation). However, cortisol-bound GR can also form heterodimers with transcription factors such as AP-1 or NF-κB, resulting in transrepression of proinflammatory genes, a mechanism of major importance for the anti-inflammatory action of glucocorticoids. It is important to note that corticosterone also exerts glucocorticoid activity, albeit much weaker than cortisol itself. However, in rodents, corticosterone is the major glucocorticoid, and in patients with 17-hydroxylase deficiency, lack of cortisol can be compensated for by higher concentrations of corticosterone that accumulates as a consequence of the enzymatic block.
Cortisol is inactivated to cortisone by the microsomal enzyme 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2) (Fig. 406-7), mainly in the kidney, but also in the colon, salivary glands, and other target tissues. Cortisol and aldosterone bind the mineralocorticoid receptor (MR) with equal affinity; however, cortisol circulates in the bloodstream at about a thousandfold higher concentration. Thus, only rapid inactivation of cortisol to cortisone by 11β-HSD2 prevents MR activation by excess cortisol, thereby acting as a tissue-specific modulator of the MR pathway. In addition to cortisol and aldosterone, deoxycorticosterone (DOC) (Fig. 406-1) also exerts mineralocorticoid activity. DOC accumulation due to 11β-hydroxylase deficiency or due to tumor-related excess production can result in mineralocorticoid excess.
FIGURE 406-7 Prereceptor inactivation of cortisol and mineralocorticoid receptor action. ENaC, epithelial sodium channel; HRE, hormone response element; NADH, nicotinamide adenine dinucleotide; SGK-1, serum glucocorticoid-inducible kinase-1.
Aldosterone synthesis in the adrenal zona glomerulosa cells is driven by the enzyme aldosterone synthase (CYP11B2). The binding of angiotensin II to the AT1 receptor causes glomerulosa cell membrane depolarization by increasing intracellular sodium through inhibition of sodium potassium (Na+/K+) ATPase enzymes as well as potassium channels. This drives an increase in intracellular calcium by opening of voltage-dependent calcium channels or inhibition of calcium (Ca2+) ATPase enzymes. Consequently, the calcium signaling pathway is triggered, resulting in upregulation of CYP11B2 transcription (Fig. 406-8).
FIGURE 406-8 Regulation of adrenal aldosterone synthesis. AngII, angiotensin II; AT1R, angiotensin II receptor type 1; CYP11B2, aldosterone synthase. (Modified after F Beuschlein: Regulation of aldosterone secretion: from physiology to disease. Eur J Endocrinol 168:R85, 2013.)
Analogous to cortisol action via the GR, aldosterone (or cortisol) binding to the MR in the kidney tubule cell dissociates the HSP–receptor complex, allowing homodimerization of the MR, and translocation of the hormone-bound MR dimer to the nucleus (Fig. 406-7). The activated MR enhances transcription of the epithelial sodium channel (ENaC) and serum glucocorticoid-inducible kinase 1 (SGK-1). In the cytosol, interaction of ENaC with Nedd4 prevents cell surface expression of ENaC. However, SGK-1 phosphorylates serine residues within the Nedd4 protein, reduces the interaction between Nedd4 and ENaC, and consequently, enhances the trafficking of ENaC to the cell surface, where it mediates sodium retention.
CUSHING’S SYNDROME
(See also Chap. 403) Cushing’s syndrome reflects a constellation of clinical features that result from chronic exposure to excess glucocorticoids of any etiology. The disorder can be ACTH-dependent (e.g., pituitary corticotrope adenoma, ectopic secretion of ACTH by nonpituitary tumor) or ACTH-independent (e.g., adrenocortical adenoma, adrenocortical carcinoma, nodular adrenal hyperplasia), as well as iatrogenic (e.g., administration of exogenous glucocorticoids to treat various inflammatory conditions). The term Cushing’s disease refers specifically to Cushing’s syndrome caused by a pituitary corticotrope adenoma.
Epidemiology Cushing’s syndrome is generally considered a rare disease. It occurs with an incidence of 1–2 per 100,000 population per year. However, it is debated whether mild cortisol excess may be more prevalent among patients with several features of Cushing’s such as centripetal obesity, type 2 diabetes, and osteoporotic vertebral fractures, recognizing that these are relatively nonspecific and common in the population.