1.3 Disorders of gas exchange
Hypoxia, hypoxaemia and impaired oxygenation
The above terms are often used interchangeably but mean different things.
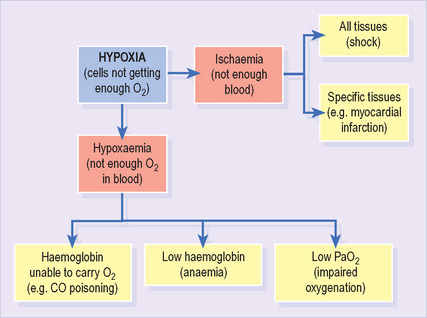
Hypoxia refers to any state in which tissues receive an inadequate supply of O2 to support normal aerobic metabolism1 (Figure 9). It may result from either hypoxaemia (see below) or impaired blood supply to tissues (ischaemia). It is often associated with lactic acidosis as cells resort to anaerobic metabolism.
Hypoxaemia refers to any state in which the O2 content of arterial blood is reduced. It may result from impaired oxygenation (see below), low haemoglobin (anaemia) or reduced affinity of haemoglobin for O2 (e.g. carbon monoxide).
Impaired oxygenation refers to hypoxaemia resulting from reduced transfer of O2 from lungs to the bloodstream. It is identified by a low PaO2 (<10.7 kPa; <80 mmHg).
It is important to note the distinction between impaired oxygenation (which results in hypoxaemia) and inadequate oxygenation (which results in hypoxia). Consider a patient with a PaO2 of 8.5 kPa. He has impaired oxygenation, suggesting the presence of important lung disease. Nevertheless, his PaO2 would usually result in an SaO2 > 90% and, provided the haemoglobin and cardiac output are normal, adequate O2 delivery to tissues.
Type 1 respiratory impairment
Type 1 respiratory impairment1 is defined as low PaO2 with normal or low PaCO2. This implies defective oxygenation despite adequate ventilation. V/Q mismatch is usually responsible and may result from a number of causes (Box 1.3.1). The PaCO2 is often low due to compensatory hyperventilation.
Box 1.3.1 Common causes of type 1 respiratory impairment*
| Pneumonia | Acute asthma |
| Pulmonary embolism | Acute respiratory distress syndrome |
| Pneumothorax | Fibrosing alveolitis |
| Pulmonary oedema | Chronic obstructive pulmonary disease |
* The usual mechanism is V/Q mismatch; however, some conditions (e.g. alveolitis) impair diffusion of gases across the alveolar capillary membrane.
If the ABG is drawn from a patient on supplemental O2, the PaO2 may not be below the normal range, but will be inappropriately low for the FiO2.
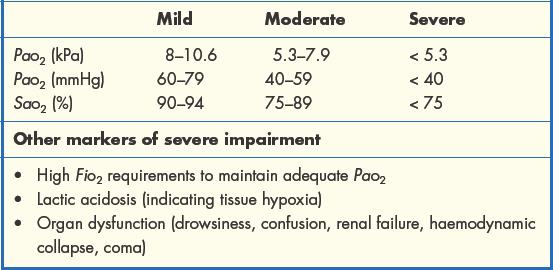
The severity of type 1 respiratory impairment is judged according to the scale of the resulting hypoxaemia and, ultimately, the presence of hypoxia (Table 1.3.1). Here it is important to remember the O2 dissociation curve. Reductions in PaO2 as far as 8 kPa have a relatively minor effect on SaO2 and are well tolerated. Beyond this threshold, we reach the ‘steep part’ of the curve and further reductions in PaO2 will lead to much greater falls in SaO2, significantly lowering the O2 content of arterial blood.
Initial treatment of type 1 respiratory impairment is aimed at achieving an adequate PaO2 and SaO2 with supplemental O2 while attempting to correct the underlying cause. In many cases pulse oximetry can be used as an alternative to repeated ABG sampling to monitor progress.
Type 2 respiratory impairment
Type 2 respiratory impairment is defined by a high PaCO2 (hypercapnia) and is due to inadequate alveolar ventilation. Since oxygenation also depends on ventilation, the PaO2 is usually low, but may be normal if the patient is on supplemental O2. It is important to note that any cause of type 1 impairment may lead to type 2 impairment if exhaustion supervenes.
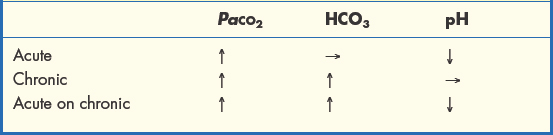
Acute rises in PaCO2 lead to dangerous accumulation of acid in the blood (see section 1.4) and must be reversed. Chronic hypercapnia is accompanied by a rise in bicarbonate (HCO3), which preserves acid–base balance. However, patients with chronic type 2 impairment who experience a further sharp decline in ventilation will also have a rapid rise in PaCO2 (acute on chronic), leading to acid accumulation and low blood pH (Table 1.3.2).
Supplemental O2 improves hypoxaemia but not hypercapnia, so treatment of type 2 respiratory impairment should also include measures to improve ventilation (e.g. reversal of sedation, relief of airways obstruction, assisted ventilation). The overzealous administration of supplemental O2 to some patients with chronic type 2 impairment may further depress ventilation by abolishing hypoxic drive (p. 7).
Pulse oximetry provides no information on PaCO2 so is not a suitable substitute for ABG monitoring in type 2 respiratory impairment.
Box 1.3.2 Common causes of type 2 respiratory impairment
| Chronic obstructive pulmonary disease* | Opiate/benzodiazepine toxicity |
| Exhaustion | Inhaled foreign body |
| Flail chest injury | Neuromuscular disorders |
| Kyphoscoliosis | Obstructive sleep apnoea |
* May lead to either type 1 or type 2 respiratory impairment
Box 1.3.3 Clinical signs of hypercapnia
| Confusion | Drowsiness |
| Flapping tremor | Bounding pulse |
| Warm extremities | Headache |
Hyperventilation
Hyperventilation leads to a low PaCO2 (hypocapnia) and a corresponding rise in blood pH (see section 1.4). In chronic cases it is accompanied by a rise in HCO3, which corrects blood pH. An increase in the rate and depth of breathing is usually apparent. A large drop in PaCO2 may lead to tingling around the mouth and extremities, light-headedness and even syncope.
Psychogenic hyperventilation often presents in a dramatic fashion, with patients complaining of severe breathlessness and an inability to take in enough air. It may be difficult to distinguish from respiratory disease. The ABG shows a low PaCO2 with a normal PaO2.
Hyperventilation also occurs as a compensatory response to metabolic acidosis (secondary hyperventilation), as described in section 1.4. Other causes are shown in Table 1.3.3.
Table 1.3.3 Common causes of hyperventilation
| Primary | Anxiety (psychogenic) | Pain or distress |
| Hypoxaemia | Fever | |
| Salicylate toxicity | Central nervous system disorders | |
| Hepatic cirrhosis | ||
| Secondary | Metabolic acidosis (of any aetiology) |
Summary of gas exchange abnormalities
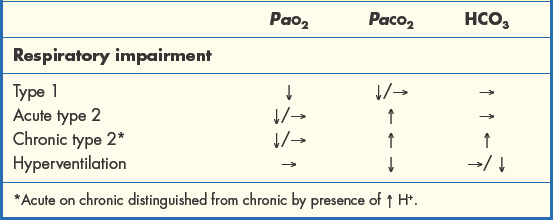
The four main patterns of ABG abnormality in disorders of gas exchange are summarised in Table 1.3.4.
The A–a gradient is the difference between the alveolar Po2 (averaged across all alveoli) and the Po2 in arterial blood. It tells us whether the PaO2 is appropriate for the level of alveolar ventilation and is therefore a measure of the degree of V/Q mismatch.
In practice its main uses lie in detecting subtle increases in V/Q mismatch where the PaO2 is still within the normal range (e.g. pulmonary embolism) and identifying the presence of V/Q mismatch in patients with type 2 respiratory impairment (this distinguishes pure type 2 respiratory impairment from mixed type 1 and type 2 impairment).
Calculation of the A–a gradient is not required for the scenarios in Part 2 of the book but, for those interested, a guide can be found in Appendix 1.