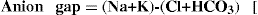1.5 Disorders of acid–base balance
Metabolic acidosis
Specific causes of metabolic acidosis are discussed in greater detail in the relevant cases in Part 2.
Metabolic alkalosis
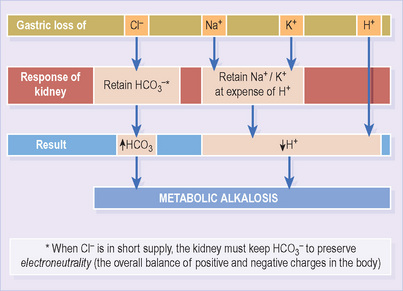
Loss of H+ ions may initiate the process but the kidneys have huge scope to correct threatened alkalosis by increasing HCO3 excretion. Factors that impair this response are therefore also necessary. The usual suspects are depletion of chloride (Cl−), potassium (K+) and sodium (Na+) ions – in most cases due to either sustained vomiting (Figure 17) or diuretic drugs.
Summary of metabolic acid–base disorders
Box 1.5.1 Metabolic acidosis (low HCO3)