Chapter 58 Diseases of the Lymphatic Circulation
Anatomy of Lymphatic Circulation
Recognition of the lymphatic system and comprehension of its importance came relatively late to the medical and scientific communities. It was not until the 17th century that Gasparo Aselli recognized the lymphatics as a distinct anatomical entity.1 The gifted professor of anatomy and surgery from Milan was frequently asked by friends and colleagues to perform his elegant vivisections, and on July 23, 1622, he intended to demonstrate to them the action and innervation of the canine diaphragm.
“While I was attempting this, and for that purpose had opened the abdomen and was pulling down with my hand the intestines and stomach…I suddenly beheld a great number of cords, as it were, exceedingly thin and beautifully white, scattered over the whole of the mesentery and the intestine, and starting from almost innumerable beginnings…I noticed that the nerves belonging to the intestine were distinct from these cords, and wholly unlike them, and besides, were distributed quite separately from them. Wherefore struck by the novelty of the thing, I stood for some time silent…When I gathered my wits together for the sake of the experiment, having laid hold of a very sharp scalpel, I pricked one of these cords and indeed one of the largest of them. I had hardly touched it, when I saw a white liquid like milk or cream forthwith gush out. Seeing this, I could hardly restrain my delight.”1
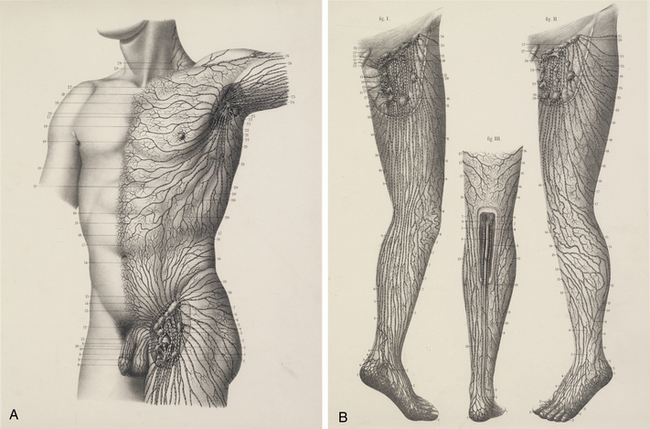
The chylous return from the intestine of the recently fed animal allowed Aselli to recognize the lymphatics; when he repeated the demonstration several days later, no vessels were to be seen. Aselli eventually realized the relation between feedings and the visibility of the mesenteric lymphatics and duplicated the work in several species (Fig. 58-1). Over the following half century, Aselli’s work was extended by Pecquet, Bartholinus, and Rudbeck, who defined the gross anatomy of the lymphatic system in toto.1 By the 18th century, smaller lymphatic channels were visualized by Anton Nuck using mercury injections. Using those techniques, Sappey observed and recorded the human lymphatic system in exquisite detail (Fig. 58-2). Even greater resolution of the anatomy was provided by von Recklinghausen in 1862 with his discovery that the lymphatic endothelium stained darkly with silver nitrate. Using that technique, he differentiated lymphatic capillaries from blood vessel capillaries. Most recently, substantial advances in the techniques of immunohistochemistry and transmission electron microscopy have enabled the certain identification of the lymphatic microcirculation and discrimination from blood vasculature.2,3
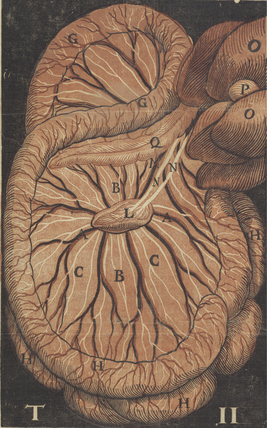
Figure 58-1 Original anatomical illustration of visceral lymphatics by Gasparo Aselli.
(From the monograph De Lactibus Sive Lacteis Venis, courtesy Harvard Medical Library, Francis A. Countway Library of Medicine.)
It is now well established that lymphatic capillaries are blind-ended tubes formed by a single layer of endothelial cells (ECs). The ECs of lymphatic capillaries closely resemble those of blood vessels and have a common embryonic origin.4 Like blood vascular endothelium, cultured lymphatic ECS form confluent “cobblestone” monolayers that “sprout” to form tubules. They demonstrate identical histological markers (von Willebrand factor (vWF), F-actin, fibronectin (FN), and Weibel-Palade bodies). Unlike systemic capillaries, the basement membrane of lymphatic capillaries is absent or widely fenestrated, allowing greater entry of interstitial proteins and particles.
The capillaries join to form larger vessels (100-200 μm) that are invested with smooth muscle and capable of vasomotion. Those vessels in turn merge to form larger conduits composed of three distinct layers: intima, media, and adventitia. Those conduits possess intraluminal valves located a few millimeters to centimeters apart that ensure lymph flow is directed centrally.5,6
Physiology of Lymphatic Circulation
In 1786, William Hunter and two of his pupils, William Cruikshank and William Hewson, published the results of their work, laying a foundation for the physiology of the lymphatic system.1 They correctly inferred from clinical observation that the lymphatics were involved in the response to infection, as well as in the absorption of interstitial fluid. A century later, their theories received experimental support from the physiological studies of Karl Ludwig and Ernest Starling. Ludwig cannulated lymph vessels, collected and analyzed the lymph, and proposed that it was a filtrate of plasma. Starling elucidated the forces governing fluid transfer from the blood capillaries to the interstitial space and offered evidence that the same forces apply to the lymphatic capillaries. He proposed that an imbalance in those forces could give rise to edema formation:
“In health, therefore, the two processes, lymph production and absorption are exactly proportional. Dropsy depends on a loss of balance between these two processes—on an excess of lymph-production over lymph-absorption. A scientific investigation of the causation of dropsy will therefore involve, in the first place, an examination of the factors which determine the extent of these two processes and, so far as is possible, the manner in which these processes are carried out.”7
As first enunciated by Starling, interstitial fluid is largely an ultrafiltrate of blood. Its rate of production reflects the balance between factors that favor filtration out of capillaries (capillary hydrostatic pressure and tissue oncotic pressure) and those that favor reabsorption (interstitial hydrostatic pressure and capillary oncotic pressure).7 Under normal conditions, filtration exceeds reabsorption at a rate sufficient to create 2 to 4 L of interstitial fluid per day. There is a net filtration of protein (primarily albumin) from the vasculature into the interstitium; approximately 100 g of circulating protein may escape into the interstitial space daily. The interstitial fluid also receives the waste products of cellular metabolism, as well as foreign matter or microbes that enter through breaks in the skin or by hematogenous routes.
Not surprisingly, the lymphatics require a complex interplay of specific anatomy and function to meet physiological requirements. Several forces drive fluid through the lymphatic system. The lymphatic vessels in skeletal muscle are compressed by extrinsic muscular contractions that propel the fluid centrally through the unidirectional valves. In other tissues, such as the splanchnic and cutaneous systems, it is primarily contractions of lymphatic smooth muscle that generate the driving force.8,9 These contractions are increased in frequency and amplitude by elevated filling pressure, sympathetic nerve activity, and shock, and they may be modulated by circulating hormones and prostanoids.10–12 Considerable force can be generated by those contractions; experimentally induced obstruction of the popliteal lymphatic system augments the strength and frequency of contraction, generating pressures of up to 50 mmHg.13 Other factors that may contribute to lymphatic flow include intermittent compression from arterial pulsations and gastrointestinal peristalsis. In addition, it has most recently been proposed that the initial lymphatics (small lymphatic capillaries that begin blindly in the tissues) most likely possess a two-valve system.14,15 In addition to the classically described secondary intralymphatic valves, the initial lymphatics are thought to possess a primary valve system at the level of the endothelium to ensure unidirectional flow at this level. Once lymph enters the thorax, negative intrathoracic pressure generated during inspiration aspirates fluid into the thoracic duct (the “respiratory pump”).13 Failure of adequate lymph transport promotes lymphedema and likely contributes to the pathological presentation of a wide variety of lymphatic vascular diseases.
Lymphatic Insufficiency (Lymphedema)
Pathogenesis of Edema
Edema develops when production of interstitial fluid (lymph) exceeds its transport through the lymphatic system. Thus, overproduction of lymph (enhanced lymphatic load) or decreased ability to remove fluid (defective transport) from the interstitium may result in edema. Conditions associated with overproduction of lymph include elevated venous pressures, increased capillary permeability, and hypoproteinemia. Elevated hydrostatic pressure in the veins results in increased filtration of plasma from venules and blood capillaries (as seen in right-sided congestive heart failure (CHF), tricuspid regurgitation, and deep vein thrombosis [DVT]). Conversely, local inflammation increases capillary permeability, accelerating loss of protein and fluid to the interstitium despite a normal capillary hydrostatic pressure. Lymph production may increase by 10- to 20-fold, exceeding lymphatic transport and resulting in marked edema.16 Hypoproteinemia also may lead to marked edema, in which case hydrostatic pressure and capillary permeability are normal, but capillary oncotic pressure is reduced, favoring osmotic flow to the interstitium. The edema that ensues in these conditions can, strictly speaking, be called lymphedema only when there is objective evidence of impaired lymphatic clearance or physical evidence of consequences of impaired lymphatic function in the skin or subcutaneous tissues.
Pathogenesis of Lymphedema
Primary lymphedema
Prevalence estimates for the heritable causes of lymphedema are difficult to ascertain and vary substantially. Primary lymphedema is thought to occur in approximately 1 of every 6 to 10,000 live births. Females are affected 2- to 10-fold more commonly than males.17,18 Primary lymphedema represents a heterogeneous group of disorders, and therefore its classification schemata are numerous. Affected individuals can be classified by age of onset, functional anatomical attributes, or clinical setting.
Age of Onset
When distinguished by age of clinical onset, primary lymphedema can typically be divided into the following categories19:
Anatomical Patterns
An alternative classification scheme relies on an anatomical description of the lymphatic vasculature20,21:
1. Aplasia: no collecting vessels identified.
2. Hypoplasia: a diminished number of vessels are seen.
3. Numerical hyperplasia (as defined by Kinmonth): an increased number of vessels are seen.
4. Hyperplasia: in addition to an increase in number, the vessels have valvular incompetence and display tortuosity and dilation (megalymphatics).
Approximately one third of all cases are secondary to agenesis, hypoplasia, or obstruction of the distal lymphatic vessels, with relatively normal proximal vessels.26,27 In those cases, swelling is usually bilateral and mild and affects females much more frequently than males. The prognosis in such cases is good. Generally after the first year of symptoms, there is little extension in the same limb or to uninvolved extremities. Although the extent of involvement is established early in the disease in about 40% of patients, the girth of the limb continues to increase.
In more than half of all cases, the defect primarily involves obstruction of the proximal lymphatics or nodes, with initial lack of involvement of distal lymphatic vessels. Pathological studies reveal intranodal fibrosis.15 In those cases, swelling tends to be unilateral and severe; there may be a slight predominance of females in this group. In patients with proximal involvement, the extent and degree of the abnormality is more likely to progress and require surgical intervention. Initially uninvolved distal lymphatic vessels may become obliterated with time.
Clinical Characteristics
As a third alternative, the primary lymphedemas can often be characterized by associated clinical anomalies or abnormal phenotype.21 Although sporadic instances of primary lymphedema are more common, the tendency for congenital lymphedema to cluster in families is significant. The syndrome of a familial predisposition to congenital lymphedema, ultimately determined to ensue from an autosomal dominant form of inheritance with variable penetrance, was first described by Milroy in 1892.22 He reported “hereditary edema” affecting 22 individuals of one family over six generations. Although Milroy ultimately came to consider not only congenital lymphedema but also praecox and tarda forms as variants of the syndrome that bears his name,23 the praecox form of primary lymphedema more often carries the eponym of Meige’s disease.24
In fact, a long list of disorders are associated with heritable forms of lymphedema. Increasingly these disorders have yielded to chromosomal mapping techniques. Lymphedema-cholestasis, or Aagenaes’ syndrome, has been mapped to chromosome 15q.25 In several family cohorts of Milroy’s disease, it has been determined that the disorder reflects missense inactivating mutations in the tyrosine kinase domain of vascular endothelial growth factor receptor 3 (VEGFR3),26,27 thus underscoring the likelihood that the pathogenesis of this condition likely reflects an inherited defect in lymphatic vasculogenesis. Several additional lymphedema syndromes have recently lent themselves to successful genetic mapping.21 Lymphedema-distichiasis, an autosomal dominant dysmorphic syndrome in which lymphedema presents in association with a supplementary row of eyelashes arising from the meibomian glands, has been linked to truncating mutations in the forkhead-related transcription factor FOXC228; mutations in FOXC2 have subsequently been associated with a broad variety of primary lymphedema presentations.29 Similarly, a more unusual form of congenital lymphedema, hypotrichosis-lymphedema-telangiectasia, has been ascribed to both recessive and dominant forms of inheritance of mutations in the transcription factor gene SOX18.30 Most recently, linkage analysis of three affected family cohorts has associated the occurrence of autosomal recessive congenital lymphatic dysplasia (Hennekam’s syndrome) to the gene CCBE1,31,32 also identified as critical to lymphangiogenesis in zebrafish.31 It is altogether plausible that further elucidation of the molecular pathogenesis of these diseases linked to FOXC2, SOX18, and CCBE1 mutations will lead to enhanced insights into mechanisms of normal and abnormal lymphatic development. Furthermore, mutational analysis of families expressing inherited forms of lymphedema have disclosed specific mutations in hepatocyte growth factor (HGF) (which encodes HGF) and MET (the HGF receptor).33 GJC2, the gene that encodes connexin-47 has also been implicated in the familial occurrence of lymphedema.34
In general, autosomal or sex-linked recessive forms of congenital lymphedema occur less commonly than the dominant forms of inheritance. Nevertheless, the list of heritable lymphedema-associated syndromes is long and growing (Box 58-1). Primary lymphedema has been described in association with various forms of chromosomal aneuploidy (e.g., Turner’s and Klinefelter’s syndromes), various dysmorphogenic genetic anomalies (e.g., Noonan’s syndrome, neurofibromatosis), and with various as yet unrelated disorders such as yellow nail syndrome, intestinal lymphangiectasia, lymphangiomyomatosis, and arteriovenous malformation (AVM).35 The association of lymphedema with vascular anomalies likely derives from the shared embryological origin of the lymphatic and venous vasculature.
![]() Box 58-1 Hereditary Conditions Associated with Lymphedema
Box 58-1 Hereditary Conditions Associated with Lymphedema
Dysmorphogenic-Genetic Disturbances
From Rockson SG: Syndromic lymphedema: keys to the kingdom of lymphatic structure and function? Lymph Res Biol 1:181, 2003.21
Secondary lymphedema
Infection
Recurrent bacterial lymphangitis leads to thrombosis and fibrosis of the lymphatic channels and is one of the most common causes of lymphedema.36 The responsible bacteria are almost always streptococci, which tend to enter through breaks in the skin or fissures induced by trichophytosis. Recurrent bacterial lymphangitis is also a frequent complicating factor of lymphedema from any cause.
The microfilaria are transmitted by a mosquito vector and induce recurrent lymphangitis and eventual fibrosis of lymph nodes. It is unclear whether filaria themselves produce the lymphangitis or simply predispose those afflicted to recurrent episodes of bacterial lymphangitis. The filaria also can be identified in blood specimens of tissue obtained by fine-needle biopsy of affected areas, and eosinophilia is a common local and systemic feature. Diethylcarbamazine remains the most popular drug for treating filariasis; although side effects are frequent, it is extremely efficacious.37 Ivermectin is a newer antifilarial agent that may replace diethylcarbamazine; it is less toxic, and a single oral dose (25 μg/kg) appears to be as efficacious as a 2-week course of diethylcarbamazine.38
Lymphatic Trauma
Within this category, by far the most common mechanism of lymphedema relates to surgical excision of lymph nodes.39 This occurs most commonly in the setting of cancer staging and therapeutics. Breast cancer–associated lymphedema is the most common form of lymphedema in the United States. Both axillary lymph node dissection and adjuvant radiation therapy, particularly to the breast and axilla, predispose to development of secondary lymphedema of the upper extremity.40 In clinical follow-up after periods of up to 13 years following invasive treatment of breast cancer, a late incidence of lymphedema of 14% can be expected in surgically treated patients with adjuvant postoperative irradiation.41 Even less radical surgery (e.g., lumpectomy) can occasionally be complicated by lymphedema. Despite improvements in surgical and radiotherapeutic techniques, lymphedema remains a potential complication.42,43 Similarly, edema of the leg may occur after surgery for pelvic or genital cancer, particularly when there has been inguinal and pelvic lymph node dissection or irradiation.44 Its reported frequency varies between 1% and 47%.45,46 Pelvic irradiation increases the frequency of leg lymphedema after cancer surgery47 for such conditions as malignant melanoma, prostate cancer, and gynecological malignancies.
Lymphedema can also ensue from other mechanisms of lymphatic trauma, among them burns, large or circumferential wounds of the extremity, or other iatrogenic causes.48
Other Causes
Other conditions leading to or associated with obstruction of lymphatic channels include tuberculosis, contact dermatitis, rheumatoid arthritis, and pregnancy.49 Autoimmune destruction of the lymphatics remains an interesting but unproven etiology.50 Factitious lymphedema (“oedema bleu”) also occurs. The condition usually affects the hand, arm, or both; is unilateral; and is induced by applications of tourniquets, self-inflicted cellulitis, or maintenance of the limb in an immobile and dependent state. Chronic subcutaneous injections of drugs (most notably, pentazocine hydrochloride) also may lead to lymphatic sclerosis and obstruction.
Pathology of Lymphedema
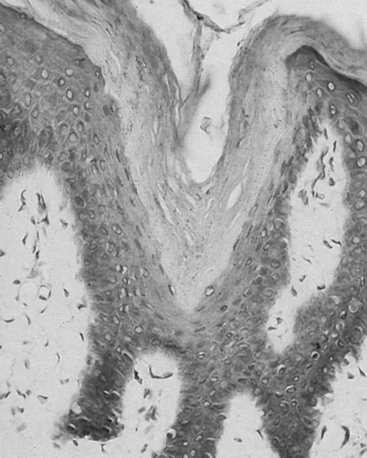
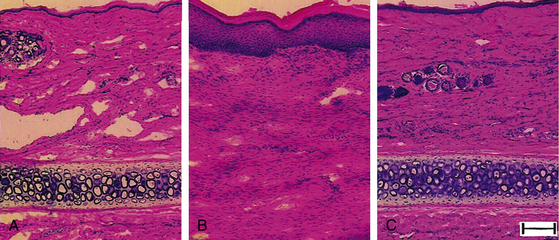
Early in the natural history of lymphedema, there is variable thickening of the epidermis; the skin becomes rough with hyperkeratosis and accentuation of skin folds. Organization and fibrosis may lead to development of papillomatosis. Early on, substantial edema of the dermis may occur. On cut section of gross specimens, the dermis is firm and gray, as is the deep fascia. Usually later in the course, but sometimes quite early, there may be expansion of the subcutaneous adipose tissue, often septated by prominent fibrous strands. In some specimens, compression causes lymph to exude from the dermis and subcutaneous tissue, although this is not a prominent feature. Microscopic examination reveals hyperkeratosis with prominent dermal fibrosis, as well as variable degrees of dermal edema (Fig. 58-3). Abundant subcutaneous fat with prominent fibrous septa is apparent in all cases. Often, perivascular inflammatory cells (lymphocytes, plasma cells, and occasionally eosinophils) can be seen. The lymphatic vessels often are difficult to visualize and may be obliterated or thrombosed by previous inflammatory episodes or may be congenitally absent or hypoplastic. Dilation of the lymphatics may be seen.
Clinical Presentation
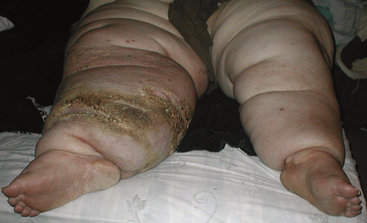
Clinical signs of lymphedema largely depend on duration and severity of the disease. Initially the interstitial space is expanded by an excess accumulation of relatively protein-rich fluid volume. The swelling produced by that fluid collection typically is soft, is easily displaced with pressure (“pitting edema”), and may substantially decrease with elevation of the limb. In the lower extremities, the edema typically extends to the distal aspects of the feet, resulting in the characteristic “square toes” seen in this condition. Over a period of years, the limb may take on a woody texture as the surrounding tissue becomes indurated and fibrotic (Fig. 58-4). In these later stages, pitting edema is no longer a major component, and limb elevation or external compression is much less successful at reducing the girth of the extremity.
Proliferation of subcutaneous connective and adipose tissue leads to thickening of the skin and loss of flexibility; the affected limb is grossly enlarged, and a mossy or cobblestone skin texture may develop. Correlates of these changes that can be sought on physical examination include the peau d’orange that reflects cutaneous and subcutaneous fibrosis, and the so-called Stemmer sign (inability to tent the skin at the base of the digits), which is considered to be pathognomonic of lymphedema when present in a swollen limb.51 In many cases of long-standing lymphedema, deposition of substantial amounts of subcutaneous adipose tissue has been described,52 hypothetically ascribable to abnormalities of adipogenesis or lipid accumulation that accompany chronic stagnation of lymph.53 However, the mechanisms of this consequence of chronic lymphatic circulatory insufficiency have not yet been delineated.
Natural History and Differential Diagnosis
The natural history of lymphedema is quite variable and often may encompass a substantial interval of subclinical asymptomatic disease. For example, even 3 years following modified radical mastectomy and axillary lymph node dissection, more than 80% of women remain free of any overt clinical evidence of lymphatic impairment despite extensive iatrogenic destruction of the lymphatic architecture in these patients. Similarly, in many forms of primary lymphedema, there may be a protracted phase of apparently adequate lymphatic function despite the inherited anatomical or functional pathology. Precipitating factors for the appearance of overt lymphedema are unknown. At the onset of clinical lymphedema, swelling of the involved extremity is typically described as puffy, and at times, the edematous changes may even be intermittent. With chronicity, the involved structures develop the characteristic features of induration and fibrosis.54 In many of these patients, the maximal volume increase of the involved limb is determined within the first year after clinical onset unless there are supervening complications like recurrent cellulitis. The propensity to recurrent soft-tissue infection is one of the most troublesome aspects of long-standing lymphedema. In addition to the proinfectious features of accumulated fluid and proteins, lymphatic dysfunction also impairs local immune responses.55,56 With recurrent infections, there is progressive damage of lymphatic capillaries.
In rare cases, long-standing chronic lymphedema may be complicated by local development of malignant tumors. Although it is unnecessary to burden patients about the possibility of malignancy, they should be alerted to report any changes in appearance of the limb. Neoplastic transformation of blood or lymph vessels can develop in long-standing lymphedema of any cause, including primary or secondary lymphedema.57,58 Angiosarcomas or lymphangiosarcomas are potentially devastating but fortunately uncommon complications of long-standing lymphedema, occurring in less than 1% of cases.59 Lymphangiosarcomas can be either sclerotic plaques or multicentric lesions with blue-tinged nodules or bullous changes. Early detection and amputation can be lifesaving, but recognition of the condition is often delayed by a lack of awareness on the part of the patient and physician. Other malignancies including lymphoma, Kaposi sarcoma, squamous cell cancer, and malignant melanoma have been reported in association with chronic lymphedema.
The hypertrophied limb with thickened skin seen in chronic lymphedema has little similarity to the edematous limb of deep venous insufficiency (see Chapter 55). In the latter case, a soft-pitting edema is prominent and seen in association with stasis dermatitis, hemosiderin deposition, and superficial venous varicosities. The history and examination easily differentiate chronic lymphedema from venous disorders and other causes of limb swelling (see Fig. 58-4). Earlier in the presentation, however, it may be more difficult to distinguish lymphedema from venous disease, reflex sympathetic dystrophy, or other causes of limb swelling.
Diagnostic Modalities
Lymphangiography
Human lymphatics were first visualized in vivo by Hudack and McMaster at the Rockefeller Institute in 1933.1 They used one another as subjects to demonstrate superficial lymphatic plexuses along the forearm and inner thigh, using subcutaneous injection of vital dyes. In 1948, Glenn cannulated a lymphatic vessel in the dog hindlimb and injected contrast media to produce a lymphangiogram in the canine leg and groin.1 Subsequently, Servelle and Deysson visualized the lymphatics in patients with elephantiasis, using retrograde injection.1 Visualization of the dilated lymph channels, however, depended on partial or complete valvular incompetence. Direct contrast lymphangiography was developed by Kinmonth and coworkers in 1952.60 The technique involves identification of a distal vessel made visible by an intradermal injection of a vital dye into the metatarsal web spaces. The vessel is isolated and cannulated, and iodinated contrast material is injected. Following the injection, the contrast material is visualized radiographically as it progresses proximally through lymphatic channels.
There are several drawbacks to the procedure, including frequent requirements for surgical exposure in the edematous limb, microsurgical techniques to achieve direct cannulation, and occasionally the need for general anesthesia.27 Of greater importance is the fact that the irritation caused by the contrast agent results in lymphangitis in one third of the studies and potentially can worsen the lymphedema.61 For these reasons, the use of lymphangiography as a diagnostic modality for the edematous limb has largely been abandoned and is contraindicated in patients with lymphedema.
Lymphoscintigraphy
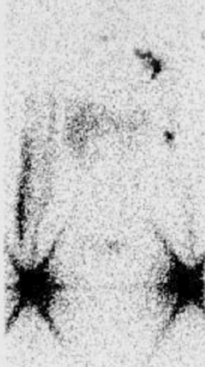
Lymphoscintigraphy involves injection of radiolabeled macromolecules, such as sulfur colloid or albumin, into the distal subcutaneous tissue of the affected extremity (e.g., dorsum of foot). The progress of the radionuclide through the lymphatic system is followed by a radioscintigraphic camera. In primary lymphedema, channels are obliterated or absent; in a small percentage of cases, they are ectatic and incompetent. In secondary lymphedema, often with dilation of the vascular channels, the level of obstruction can be determined.62 In lymphedema of any cause, the proximal progression of the radionuclide is delayed, and its accumulation distally in the dilated channels of the dermis is manifested as a “dermal backflow” pattern (Fig. 58-5).
Lymphoscintigraphy is easier to perform than lymphangiography and is not reported to cause lymphangitis. It lacks the spatial resolution of lymphangiography; resolution is maximized by reducing the swelling of the extremity as much as possible before the study (reducing dilution of the radionuclide in stagnant lymph). Effective use of lymphoscintigraphy to plan therapeutic interventions requires an understanding of the pathophysiology of lymphedema and the influence of technical factors, such as selection of the radiopharmaceutical, imaging times after injection, and patient activity after injection on the images.63
Computed tomography
Computed tomography (CT) scans of a lymphedematous limb are characterized by a honeycomb pattern in the affected area.64 Computed tomography cannot directly localize the level of obstruction.65 This technique can, however, provide insight into volume changes within various compartments visualized on cross-sectional images of the affected limb.66 The greatest utility of CT is its ability to distinguish some of the causes of secondary lymphedema (e.g., lymphoma, pelvic tumor). Certainly, the typical honeycomb pattern of lymphedema can be sought. In addition, elements of the differential diagnosis (venous obstruction, obesity, hematoma, ruptured popliteal cyst) can be further delineated through CT images.
Magnetic resonance imaging
Magnetic resonance imaging (MRI) is an alternative, and most likely superior, technique to image the soft tissues in edema.67 Among the discernible attributes by MRI are: cutaneous thickening with a honeycomb pattern in the subdermis, dilated lymphatic channels (when present as a consequence of lymphangioma or lymph reflux), and dermal accumulation of free fluid with surrounding fibrosis. This technique has particular virtue in differentiating lymphedema from lipedema. In addition, more recently it has been demonstrated that nonenhanced three-dimensional (3D) heavily T2-weighted images obtained with two-dimensional (2D) prospective acquisition and correction has the capacity to visualize the thoracic duct, cisterna chyli, and lumbar lymphatics, at least in healthy volunteers.68
Treatment
Medical therapy
Successful treatment of lymphedema requires close collaboration between patient and physician. To that end, the physician should (1) carefully instruct the patient in the details of the medical program and (2) attend to the psychological impact of the disease. Associated emotional problems are not uncommon and are often neglected by physicians.24 The need to address the psychological aspects of long-term disfigurement, especially with adolescent patients, cannot be overemphasized. In discussing these issues with the patient, the physician should be realistic about the possibility of progression but should emphasize the patient’s ability to modulate the course of the disease by careful attention to the details of the medical program.
The physiotherapeutic approach to lymphedema has been termed decongestive lymphatic therapy. Meticulous attention to control of edema may reduce the likelihood of disease progression and limit the incidence of soft-tissue infections.69 The elements of this therapeutic approach have been designed to accomplish the initial reduction in edematous limb volume, maintain these therapeutic gains, and ensure optimal health and functional integrity of the skin.70,71 In addition to the acute reduction in limb volume that is attainable, a well-maintained therapeutic program has been demonstrated to accelerate lymph transport and enhance dispersal of accumulated protein. Decongestive lymphatic therapy integrates elements of meticulous skin care, massage, bandaging, exercise, and use of compressive elastic garments.
The specialized massage technique for these patients (so-called manual lymphatic drainage or therapy) is an empirically derived technique. Its goal is to enhance lymphatic contractility and to augment and redirect lymph flow through the unobstructed cutaneous lymphatics. Manual lymphatic drainage should not be confused with other forms of therapeutic massage that do not share this ability to augment lymphatic contractility and may in fact be detrimental to lymphatic function (e.g., athletic massages). The mild tissue compression during manual lymphatic drainage results in enhanced filling of the initial lymphatics and improves transport capacity through cutaneous lymphatic dilation and development of accessory lymph collectors.71 Typically, 8 to 15 consecutive daily sessions of manual lymphatic drainage are required to achieve optimal reduction of limb volume in a previously untreated patient.
During this acute approach to volume reduction, nonelastic compressive bandages should be applied in multiple layers after each session of manual lymphatic drainage (Fig. 58-6). These are worn during muscular exertion (which is encouraged) to prevent reaccumulation of fluid and promote lymph flow during exertion. Multilayer bandaging can also help reverse skin changes, soften subcutaneous tissues, and reduce the degree of lymphorrhea when present. In the maintenance phase of lymphedema care, use of multilayer bandages is most often supplanted by daily use of compressive elastic garments.
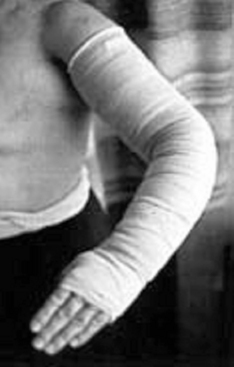
Figure 58-6 Short-stretch multilayer compression bandaging in secondary lymphedema of the upper extremity.
Elastic support hose should be fitted to the patient’s limb after edema in the extremity has been maximally reduced by compression and elevation.36 This is an important detail. The stocking or sleeve does not reduce the size of the limb but maintains the circumference to which it is fitted. However, if the limb is fitted for a stocking while in a swollen state, the limb will be maintained by the stocking in a swollen state. The prescription of compressive garments is a necessary adjunct to all other forms of maintenance lymphedema therapy. Relatively inelastic sleeves, stockings, and underwear that transmit high-grade compression (40-80 mmHg) will prevent reaccumulation of fluid after successful decongestive treatments. Garments must be fitted properly and replaced when they lose their elasticity (every 3-6 months). In addition to standard fitted garments for upper and lower extremities, various additional appliances are now available. They provide the capacity to maintain limb volume during sleep, when the sleeve or stocking is removed, and during various forms of activity.
Although the elements of decongestive lymphatic therapy were initially derived empirically, the efficacy of these interventions has now been demonstrated in numerous prospective observations.72–74 Long-term efficacy is particularly enhanced when there is attention to focused patient instruction in maintenance self-care.73
Various adjunctive treatment approaches have been investigated. Of these, perhaps the most useful is intermittent pneumatic compression (IPC). Multichamber pneumatic devices are available that intermittently compress the limb; techniques that employ sequential graduated compression (in which the cuffs are inflated sequentially from distal to proximal sites with a pressure gradient from the most distal cuff to the most proximal) are the most efficacious.75 Pneumatic compression techniques cannot, however, clear edema fluid from adjacent noncompressed sections of the limb. Consequently, as fluid shifts occur during pneumatic compression, the root of the limb must be decompressed with the aforementioned manual techniques.
Incorporation of IPC into a multidisciplinary therapeutic approach has long been advocated empirically by some physiotherapeutic schools.75 Individual reports of complications and lack of efficacy have reduced enthusiasm for the use of pneumatic compression as stand-alone therapy. More recently, it has been demonstrated that when IPC is used adjunctively with other established elements of decongestive lymphatic therapy, it enhances therapeutic response, both in the initial and maintenance approaches to the patient. Pneumatic compression is well tolerated and remarkably free of complications.76,77 However, it must be stressed that any form of compressive therapy requires sufficient arterial blood supply to the limb. In cases where severe peripheral artery disease (PAD) coexists, any form of sustained compression can further compromise arterial blood flow.
Other physical forms of therapy are under investigation. Low-level laser therapy may be effective in postmastectomy lymphedema; in one small series, subjective improvement accompanied an objective documentation of improved bioimpedance and reduced extracellular and intracellular fluid accumulation.78 In other hands, such techniques as local hyperthermia or intraarterial injection of autologous lymphocytes79 have independently produced favorable results. In the latter approach, it is postulated that regression of edema is linked to the expression of L-selectin, a lymphocyte-specific adhesion molecule.79 Observations in these pilot studies must be confirmed in larger controlled trials.
Other than antibiotic therapy where needed, pharmacotherapy has little role in the management of lymphedema. Diuretics, although widely prescribed for this chronic edematous condition, are rarely useful and may in fact be deleterious. On the other hand, in edema of mixed origin, diuretics may have a beneficial effect through their ability to reduce circulating blood volume and thereby reduce capillary filtration. An understanding of the mechanisms inducing the proliferation of subcutaneous connective tissue and lymphedema may lead to more definitive treatment. Agents might then be designed to alter the relationship between the deposition and lysis of collagen fibers such that lysis is favored, thereby reducing fibrosis.5
Benzopyrones (coumarin, hydroxyethylrutin) represent a class of agents that have been reported to reduce volume in affected limbs, purportedly by stimulating tissue macrophages, which in turn increase interstitial proteolysis. Although initial trials appeared favorable,80,81 subsequent evaluation suggests that the therapeutic gains are small82; furthermore, the utility of coumarin is significantly hampered by the risk of drug-related hepatotoxicity.83 An important question left unanswered is whether coumarin is additive in its effects to the usual compressive measures. The agent is not yet approved by the U.S. Food and Drug Administration (FDA) for use in the United States.
Surgical treatment
Direct microsurgical anastomotic procedures also have been used.84 Lymphovenous anastomoses can be made between lymphatic vessels distal to an obstruction in nearby small veins; these allow lymph from the obstructed region to flow directly into the venous system. Anastomoses also can be made from the lymph nodes to the adjacent vein. One of the latest techniques involves harvesting normal autogenous lymphatic vessels for use as bypass grafts around a lymphatic obstruction. All these microsurgical techniques require the presence of dilated lymphatic vessels distal to the obstruction. These operations obviously are of no value when the lymphatic obstruction is at the level of the smaller distal vessels. The argument has been made, however, that lymphatic bypass operations should be performed as soon as possible after the onset of obstruction to avoid the cutaneous changes of chronic lymphedema, as well as the gradual destruction of the distal lymphatic channels. An appropriate candidate for such surgery would be an individual with a recent onset of lymphedema secondary to trauma and with an otherwise normal lymphatic system proximal and distal to the area of obstruction. In a recently published large series of such appropriately selected patients, microsurgical lymphatic-venous anastomosis accomplished objective reduction of limb volume in 85% of cases.85
Currently, medical therapy is directed at preventing complications and retarding progression of the disorder, whereas surgery is palliative. Of interest are recent reports of therapeutic success of liposuction in advanced stable lymphedema. Surgical liposuction of chronic postmastectomy lymphedema has been reported to produce excellent results, with sustained reduction of excess volume. In one series, an average long-term reduction of edema volume of 106% was observed in 28 patients with an average edema volume of 1845 mL.52 Liposuction combined with long-term decongestive compression therapy reduces edema volume more successfully than compression therapy alone. However, the volume reduction is unsuccessful unless compression therapy is maintained after the surgical intervention.52
Prospects for molecular therapy
Among the mitogenic substances that initiate and regulate the growth of vascular structures, those in the VEGF family play a central role.86,87 VEGF-C and VEGF-D direct the development and growth of the lymphatic vasculature in embryonic and postnatal life through binding to VEGFR-3 receptors on lymphatic endothelia.88–90 Exogenous administration of VEGF-C upregulates the VEGFR-3 receptor, leading to a lymphangiogenic response,92 and in transgenic mice that overexpress VEGF-C, the lymphatic vessels demonstrate a hyperplastic proliferative response with secondary cutaneous changes.90
These molecular observations have shed light on the mechanisms that contribute to disease expression in the most common heritable form of lymphedema, the autosomal dominant condition known as Milroy’s disease. This disease has been linked to the FLT4 locus encoding vascular VEGFR-3.26 Disease-associated alleles contain missense mutations that produce an inactive tyrosine kinase, thereby preventing downstream gene activation. It is believed that the mutant form of the receptor is excessively stable as well as inactive, so the normal signaling mechanism is blunted, leading to hypoplastic development of the lymphatic vessels.93
The prospects for therapeutic lymphangiogenesis in human lymphedema have been underscored by the recent description of a mouse model of inherited limb edema based on mutations in the VEGFR-3 signaling mechanism and pathology that resembles human disease.91 In this model, therapeutic overexpression of VEGF-C using a viral vector induces the generation of new functional lymphatics and the amelioration of lymphedema. Similarly, in rodent models of acquired postsurgical lymphatic insufficiency (i.e., resembling postmastectomy lymphedema), exogenous administration of human recombinant VEGF-C restores lymphatic flow (as assessed by lymphoscintigraphy), increases lymphatic vascularity, and reverses the hypercellularity that characterizes the untreated lymphedematous condition94–97 (Fig. 58-7).
Diseases of the Lymphatic Vasculature
Complex Vascular Pathology with Lymphatic Anomalies
There is a broad constellation of developmental anomalies of the arteriovenous circulation that concurrently distort lymphatic anatomy, function, or both. These mixed vascular deformities are best characterized by the dominant vascular anomaly, whether angiomatous, venous, or arteriovenous.20 The reader is referred to Chapter 64 for more on vascular anomalies.
Maffucci’S syndrome
Maffucci’s syndrome is described as severe dyschondroplasia in association with multiple lymphangiomata (see later discussion). In this condition, lymphatic vasculature and nodes are typically hypoplastic.20
Proliferative Growth of Lymphatic Vascular Structures and Neoplasm
Lymphangiomatosis
Lymphangiomatosis is a rare developmental condition in which proliferation of lymphatic vascular structures involves dermis, soft tissue, bone, and parenchyma in a diffuse manner. The organs most typically affected are liver, spleen, lung, and pleura. Associated lymphangiectasia can be observed in numerous additional organs including liver, kidney, testes, lymph nodes, adrenals, and intestines. Involvement of the viscera typically confers a poor prognosis. When chylothorax is present, repeated thoracentesis and pleurodesis is often required. In one small series, all patients died within 6 to 33 months of clinical presentation.98
Lymphangiosarcoma
Lymphangiosarcomas, malignant angiosarcomas that develop in association with lymphedema, develop as multicentric lesions that have a high propensity for systemic metastasis. The vast majority of such lesions have been observed in lymphedema patients that are breast cancer survivors with chronic significant edema. It is seen only rarely in other forms of lymphedema. Whatever the clinical substrate, the prognosis for survival is poor, even following radical amputation. The reader is referred to Chapter 64 for more on vascular tumors.
Visceral Lymphatic Disorders
Protein-losing enteropathy
The presence of visceral lymphatic vascular disease can predispose to a life-threatening form of metabolic insufficiency called protein-losing enteropathy. When chyle refluxes back into the villi as a consequence of the effective blockade of its passage into the central lymphatics, this condition engenders weight loss, diarrhea, and steatorrhea as protein, fat, calcium, and fat-soluble vitamins are malabsorbed. In addition to the secondary forms of lymphatic obstruction (usually malignant), the primary hypoplastic and lymphangiectatic disorders can also predispose to enteropathy; in these cases, lymphedema of an extremity often precedes or accompanies the appearance of the enteropathy. As with other forms of reflux, the initial therapeutic strategy should entail medium-chain triglyceride supplementation, with restriction of total dietary fat intake. Where the response to conservative therapy is insufficient, it has been suggested that systemic treatment with octreotide may help alleviate the severity of the disorder, although the mechanism of benefit is not entirely understood.99–102
1 Kanter M.A. The lymphatic system: an historical perspective. Plast Reconstr Surg. 1987;79:131.
2 Rockson S.G. Preclinical models of lymphatic disease: the potential for growth factor and gene therapy. Ann N Y Acad Sci. 2002;979:64.
3 Shin W.S., Szuba A., Rockson S.G. Animal models for the study of lymphatic insufficiency. Lymphat Res Biol. 2003;1:159.
4 Oliver G. Lymphatic vasculature development. Nat Rev Immunol. 2004;4:35.
5 Baldwin M., Stacker S., Achen M. Molecular control of lymphangiogenesis. Bioessays. 2002;24:1030.
6 Gashev A. Physiologic aspects of lymphatic contractile function: current perspectives. Ann N Y Acad Sci. 2002;979:178.
7 Sparks H.V., Rooke T.W. Essentials of cardiovascular physiology. Minneapolis: University of Minnesota Press; 1987.
8 Cotton K.D., Hollywood M.A., McHale N.G., et al. Outward currents in smooth muscle cells isolated from sheep mesenteric lymphatics. J Physiol. 1997;503(pt 1):1.
9 von der Weid P.Y., Zawieja D.C. Lymphatic smooth muscle: the motor unit of lymph drainage. Int J Biochem Cell Biol. 2004;36:1147.
10 Gashev A.A., Zawieja D.C. Physiology of human lymphatic contractility: a historical perspective. Lymphology. 2001;34:124.
11 Muthuchamy M., Gashev A., Boswell N., et al. Molecular and functional analyses of the contractile apparatus in lymphatic muscle. FASEB J. 2003;17:920.
12 McHale N.G., Thornbury K.D., Hollywood M.A. 5-HT inhibits spontaneous contractility of isolated sheep mesenteric lymphatics via activation of 5-HT(4) receptors. Microvasc Res. 2000;60:261.
13 Hall J.L., Morris B., Wooley L. Intrinsic rhythmic propulsion of lymph in anesthetized sheep. J Physiol. 1965;180:336.
14 Trzewik J., Mallipattu S.K., Artmann G.M., et al. Evidence for a second valve system in lymphatics: endothelial microvalves. FASEB J. 2001;15:1711.
15 Mendoza E., Schmid-Schonbein G.W. A model for mechanics of primary lymphatic valves. J Biomech Eng. 2003;125:407.
16 Szuba A., Shin W.S., Strauss H.W., et al. The third circulation: radionuclide lymphoscintigraphy in the evaluation of lymphedema. J Nucl Med. 2003;44:43.
17 Rockson S.G. Primary lymphedema. Ernst C.B., Stanley J.C. Current therapy in vascular surgery, ed 4, Philadelphia: Mosby, 2000.
18 Rockson S.G. Lymphedema. Am J Med. 2001;110:288.
19 Szuba A., Rockson S.G. Lymphedema: classification, diagnosis and therapy. Vasc Med. 1998;3:145.
20 Kinmonth J.B. The lymphatics, ed 2. London: Arnold; 1982.
21 Rockson S.G. Syndromic lymphedema: keys to the kingdom of lymphatic structure and function? Lymphat Res Biol. 2003;1:181.
22 Milroy W.F. An undescribed variety of hereditary edema. N Y Med J. 1892;56:505.
23 Milroy W.F. Chronic hereditary edema: Milroy’s disease. JAMA. 1928;91:1172.
24 Smeltzer D.M., Stickler G.B., Schirger A. Primary lymphedema in children and adolescents: a followup study and review. Pediatrics. 1985;76:206.
25 Bull L.N., Roche E., Song E.J., et al. Mapping of the locus for cholestasis-lymphedema syndrome (Aagenaes syndrome) to a 6.6-cM interval on chromosome 15q. Am J Hum Genet. 2000;67:994.
26 Karkkainen M.J., Ferrell R.E., Lawrence E.C., et al. Missense mutations interfere with VEGFR-3 signalling in primary lymphoedema. Nat Genet. 2000;25:153.
27 Irrthum A., Karkkainen M.J., Devriendt K., et al. Congenital hereditary lymphedema caused by a mutation that inactivates VEGFR3 tyrosine kinase. Am J Hum Genet. 2000;67:295.
28 Fang J., Dagenais S.L., Erickson R.P., et al. Mutations in FOXC2 (MFH-1), a forkhead family transcription factor, are responsible for the hereditary lymphedema distichiasis syndrome. Am J Hum Genet. 2000;67:1382.
29 Finegold D.N., Kimak M.A., Lawrence E.C., et al. Truncating mutations in FOXC2 cause multiple lymphedema syndromes. Hum Mol Genet. 2001;10:1185.
30 Irrthum A., Devriendt K., Chitayat D., et al. Mutations in the transcription factor gene SOX18 underlie recessive and dominant forms of hypotrichosis-lymphedema-telangiectasia. Am J Hum Genet. 2003;72:1470.
31 Alders M., Hogan B.M., Gjini E., et al. Mutations in CCBE1 cause generalized lymph vessel dysplasia in humans. Nat Genet. 2009;41:1272.
32 Connell F., Kalidas K., Ostergaard P., et al. Linkage and sequence analysis indicate that CCBE1 is mutated in recessively inherited generalised lymphatic dysplasia. Hum Genet. 2010;127:231.
33 Finegold D.N., Schacht V., Kimak M.A., et al. HGF and MET mutations in primary and secondary lymphedema. Lymphat Res Biol. 2008;6:65.
34 Ferrell R.E., Baty C.J., Kimak M.A., et al. GJC2 missense mutations cause human lymphedema. Am J Hum Genet. 2010;86:943.
35 Wheeler E.S., Chan V., Wassman R., et al. Familial lymphedema praecox: Meige’s disease. Plast Reconstr Surg. 1981;67:362.
36 Schirger A. Lymphedema. Cardiovasc Clin. 1983;13:293.
37 Bockarie M., Tisch D., Kastens W., et al. Mass treatment to eliminate filariasis in Papua, New Guinea. N Engl J Med. 2002;347:1841.
38 Kumaraswami V., Ottesen A., Vijayasekaran V., et al. Ivermectin for the treatment of Wuchereria bancrofti filariasis: efficacy and adverse reactions. JAMA. 1988;259:3150.
39 Szuba A., Rockson S. Lymphedema: a review of diagnostic techniques and therapeutic options. Vasc Med. 1998;3:145.
40 Rockson S.G. Precipitating factors in lymphedema: myths and realities. Cancer. 1998;83:2814.
41 Hojris I., Andersen J., Overgaard M., et al. Late treatment-related morbidity in breast cancer patients randomized to postmastectomy radiotherapy and systemic treatment versus systemic treatment alone. Acta Oncol. 2000;39:355.
42 Tengrup I., Tennval-Nittby L., Christiansson I., et al. Arm morbidity after breast-conserving therapy for breast cancer. Acta Oncol. 2000;39:393.
43 Petrek J.A., Heelan M.C. Incidence of breast carcinoma-related lymphedema. Cancer. 1998;83(Suppl 12):2776.
44 Fiorica J.V., Roberts W.S., Greenberg H., et al. Morbidity and survival patterns in patients after radical hysterectomy and postoperative adjuvant pelvic radiotherapy. Gynecol Oncol. 1990;36:343.
45 Werngren-Elgstrom M., Lidman D. Lymphoedema of the lower extremities after surgery and radiotherapy for cancer of the cervix. Scand J Plast Reconstr Surg Hand Surg. 1994;28:289.
46 Soisson A.P., Soper J.T., Clarke-Pearson D.L., et al. Adjuvant radiotherapy following radical hysterectomy for patients with stage IB and IIA cervical cancer. Gynecol Oncol. 1990;37:390.
47 Lynde C.W., Mitchell I.C. Unusual complication of allergic contact dermatitis of the hands-recurrent lymphangitis and persistent lymphoedema. Contact Dermatitis. 1982;8:279.
48 Rockson S.G., Rivera K.K. Estimating the population burden of lymphedema. Ann N Y Acad Sci. 2008;1131:147.
49 Nagai Y., Aoyama K., Endo Y., et al. Lymphedema of the extremities developed as the initial manifestation of rheumatoid arthritis. Eur J Dermatol. 2007;17:175.
50 Majeski I. Lymphedema tarda. Cutis. 1985;38:105.
51 Stemmer R. Ein klinisches Zeichen zur fruh-und differential-Diagnose des Lymphodems. Vasa. 1976;5:261. [A clinical symptom for the early and differential diagnosis of lymphedema]
52 Brorson H., Ohlin K., Olsson G., et al. Breast cancer-related chronic arm lymphedema is associated with excess adipose and muscle tissue. Lymphat Res Biol. 2009;7:3.
53 Rosen E. The molecular control of adipogenesis, with special reference to lymphatic pathology. Ann N Y Acad Sci. 2002;979:143.
54 Schirger A., Harrison E.G., Janes J.M. Idiopathic lymphedema: Review of 131 cases. JAMA. 1962;182:124.
55 Mallon E., Powell S., Mortimer P., et al. Evidence for altered cell-mediated immunity in postmastectomy lymphoedema. Br J Dermatol. 1997;137:928.
56 Beilhack A., Rockson S.G. Immune traffic: a functional overview. Lymphat Res Biol. 2003;1:219.
57 Muller R., Hajdu S.I., Brennan M.F. Lymphangiosarcoma associated with chronic filarial lymphedema. Cancer. 1987;59:179.
58 Benda J.A., Al Jurf A.S., Benson A.B.III. Angiosarcoma of the breast following segmental mastectomy complicated by lymphedema. Am J Clin Pathol. 1987;87:651.
59 Servelle M. Surgical treatment of lymphedema: a report on 652 cases. Surgery. 1987;101:485.
60 Kinmonth J.B., Taylor G.W., Harper R.K. Lymphangiography: a technique for its clinical use in the lower limb. Br Med J. 1955;1:940.
61 O’Brien B.M., et al. Effect of lymphangiography on lymphedema. Plast Reconstr Surg. 1981;68:922.
62 Vaqueiro M., et al. Lymphoscintigraphy in lymphedema: an aid to microsurgery. J Nucl Med. 1986;27:1125.
63 Szuba A., Shin W.S., Strauss H.W., et al. Lymphoscintigraphy in the evaluation of lymphedema. J Nucl Med. 2003;44:43.
64 Hadjis N.S., Carr D.H., Banks L., et al. The role of CT in the diagnosis of primary lymphedema of the lower limb. AJR Am J Roentgenol. 1985;144:361.
65 Gamba J.L., Silverman P.M., Ling D., et al. Primary lower extremity lymphedema: CT diagnosis. Radiology. 1983;149:218.
66 Vaughan B.F. CT of swollen legs. Clin Radiol. 1990;41:24.
67 Liu N.F., Wang C.G. The role of magnetic resonance imaging in the diagnosis of peripheral lymphatic disorders. Lymphology. 1998;31:319.
68 Matsushima S., Ichiba N., Hayashi D., et al. Nonenhanced magnetic resonance lymphoductography: visualization of lymphatic system of the trunk on 3-dimensional heavily T2-weighted image with 2-dimensional prospective acquisition and correction. J Comput Assist Tomogr. 2007;31:299.
69 Gniadecka M. Localization of dermal edema in lipodermatosclerosis, lymphedema, and cardiac insufficiency: high-frequency ultrasound examination of intradermal echogenicity. J Am Acad Dermatol. 1996;35:37.
70 Rockson S.G., Miller L.T., Senie R., et al. American Cancer Society Lymphedema Workshop. Workgroup III: diagnosis and management of lymphedema. Cancer. 1998;83:2882.
71 Kubik S. The role of the lateral upper arm bundle and the lymphatic watersheds in the formation of collateral pathways in lymphedema. Acta Biol Acad Sci Hung. 1980;31:191.
72 Ko D.S., Lerner R., Klose G., et al. Effective treatment of lymphedema of the extremities. Arch Surg. 1998;133:452.
73 Szuba A., Cooke J.P., Yousuf S., et al. Decongestive lymphatic therapy for patients with cancer-related or primary lymphedema. Am J Med. 2000;109:296.
74 Badger C.M., Peacock J.L., Mortimer P.S. A randomized, controlled, parallel-group clinical trial comparing multilayer bandaging followed by hosiery versus hosiery alone in the treatment of patients with lymphedema of the limb. Cancer. 2000;88:2832.
75 Leduc O., Leduc A., Bourgeois P., et al. The physical treatment of upper limb edema. Cancer. 1998;83:2835.
76 Szuba A., Achalu R., Rockson S.G. Decongestive lymphatic therapy for patients with breast carcinoma-associated lymphedema: a randomized, prospective study of a role for adjunctive intermittent pneumatic compression. Cancer. 2002;95:2260.
77 Mayrovitz H.N. The standard of care for lymphedema: current concepts and physiological considerations. Lymphat Res Biol. 2009;7:101.
78 Piller N.B., Thelander A. Treatment of chronic postmastectomy lymphedema with low level laser therapy: a 2.5 year follow-up. Lymphology. 1998;31:74.
79 Ogawa Y., Yoshizumi M., Kitagawa T., et al. Investigation of the mechanism of lymphocyte injection therapy in treatment of lymphedema with special emphasis on cell adhesion molecule (L-selectin). Lymphology. 1999;32:151.
80 Casley-Smith J.R., Morgan R.G., Piller N.B. Treatment of lymphedema of the arms and legs with 5,6-benzo-(alpha)-pyrone. N Engl J Med. 1993;329:1158.
81 Casley-Smith J.R., Wang C.T., Zi-hai C. Treatment of filarial lymphoedema and elephantiasis with 5,6-benzo-alpha-pyrone (coumarin). BMJ. 1993;307:1037.
82 Mortimer P.S., Badger C., Clarke I., et al. A double-blind, randomized parallel-group, placebo-controlled trial of o-(b-hydroxyethyl)-rutosides in chronic arm oedema resulting from breast cancer treatment. Phlebologie. 1995;10:51.
83 Loprinzi C.L., Kugler J.W., Sloan J.A., et al. Lack of effect of coumarin in women with lymphedema after treatment for breast cancer. N Engl J Med. 1999;340:346.
84 Damstra R.J., Voesten H.G., van Schelven W.D., et al. Lymphatic venous anastomosis (LVA) for treatment of secondary arm lymphedema. A prospective study of 11 LVA procedures in 10 patients with breast cancer related lymphedema and a critical review of the literature. Breast Cancer Res Treat. 2009;113:199–206.
85 Campisi C., Boccardo F. Microsurgical techniques for lymphedema treatment: derivative lymphatic-venous microsurgery. World J Surg. 2004;28:609.
86 Olofsson B., Jeltsch M., Eriksson U., et al. Current biology of VEGF-B and VEGF-C. Curr Opin Biotechnol. 1999;10:528.
87 Veikkola T., Karkkainen M., Claesson-Welsh L., et al. Regulation of angiogenesis via vascular endothelial growth factor receptors. Cancer Res. 2000;60:203.
88 Joukov V., Pajusola K., Kaipainen A., et al. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 1996;15:1751.
89 Kaipainen A., Korhonen J., Mustonen T., et al. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci U S A. 1995;92:3566.
90 Jeltsch M., Kaipainen A., Joukov V., et al. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science. 1997;276:1423.
91 Oh S.J., Jeltsch M.M., Birkenhager R., et al. VEGF and VEGF-C: specific induction of angiogenesis and lymphangiogenesis in the differentiated avian chorioallantoic membrane. Dev Biol. 1997;188:96.
92 Enholm B., Karpanen T., Jeltsch M., et al. Adenoviral expression of vascular endothelial growth factor-C induces lymphangiogenesis in the skin. Circ Res. 2001;88:623.
93 Karkkainen M.J., Petrova T.V. Vascular endothelial growth factor receptors in the regulation of angiogenesis and lymphangiogenesis. Oncogene. 2000;19:5598.
94 Karkkainen M.J., Saaristo A., Jussila L., et al. A model for gene therapy of human hereditary lymphedema. Proc Natl Acad Sci U S A. 2001;98:12677.
95 Szuba A., Skobe M., Karkkainen M.J., et al. Therapeutic lymphangiogenesis with human recombinant VEGF-C. FASEB J. 2002;16:1985.
96 Tabibiazar R., Cheung L., Han J., et al. Inflammatory manifestations of experimental lymphatic insufficiency. PLoS Med. 2006;3:e254.
97 Jin D., An A., Liu J., et al. Therapeutic responses to exogenous VEGF-C administration in experimental lymphedema: immunohistochemical and molecular characterization. Lymphat Res Biol. 2009;7:47.
98 Ramani P., Shah A. Lymphangiomatosis: histologic and immunohistochemical analysis of four cases. Am J Surg Pathol. 1993;17:329.
99 Makhija S., von der Weid P.Y., Meddings J., et al. Octreotide in intestinal lymphangiectasia: lack of a clinical response and failure to alter lymphatic function in a guinea pig model. Can J Gastroenterol. 2004;18:681.
100 Lee H.L., Han D.S., Kim J.B., et al. Successful treatment of protein-losing enteropathy induced by intestinal lymphangiectasia in a liver cirrhosis patient with octreotide: a case report. J Korean Med Sci. 2004;19:466.
101 Tibballs J., Soto R., Bharucha T. Management of newborn lymphangiectasia and chylothorax after cardiac surgery with octreotide infusion. Ann Thorac Surg. 2004;77:2213.
102 Bliss C.M., Schroy I.P. Primary intestinal lymphangiectasia. Curr Treat Options Gastroenterol. 2004;7:3.