Chapter 6 Diseases of the aorta
Introduction
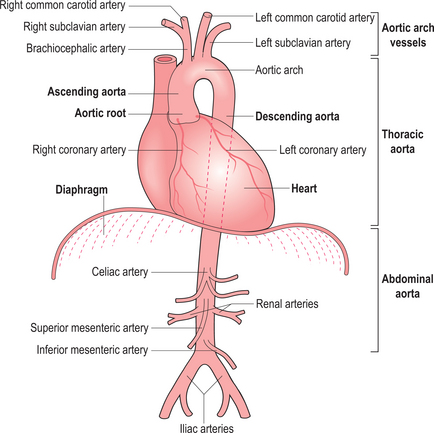
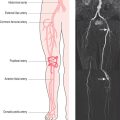
The aorta can be evaluated by a variety of techniques (Figure 6.1). X-ray contrast angiography was the gold standard method for many years but it is invasive and uses ionizing radiation and nephrotoxic contrast agents. Noninvasive options are TOE, CT and CMR (Figure 6.2). TOE is portable but relatively invasive and offers incomplete coverage of the aorta with restricted visualization at the aortic arch. CT is fast and widely available but uses ionizing radiation and nephrotoxic contrast agents. CMR avoids these limitations and offers multiplanar imaging of the aorta with a wide field of view and concurrent cardiac functional assessment. However, it is less available and evaluation of critically ill patients can be hampered by MRI-incompatible life support and monitoring equipment.
CMR reports of scans pertaining to the aorta follow the general pattern of:
Thoracic aortic aneurysm
An aortic aneurysm is a focal dilatation of the aorta. According to the shape the aneurysm is described as fusiform, with symmetrical dilatation of the circumference of the aorta, or saccular where only a part of the aortic wall is dilated. Aortic aneurysms can also be classified into true and false aneurysms. A true aneurysm consists of dilatation of all the layers of the aortic wall and characteristically has a wide neck. By contrast, in a false aneurysm perforation of the intima and media is contained by the surrounding adventitia and peri-aortic tissue, and the neck is usually narrow.
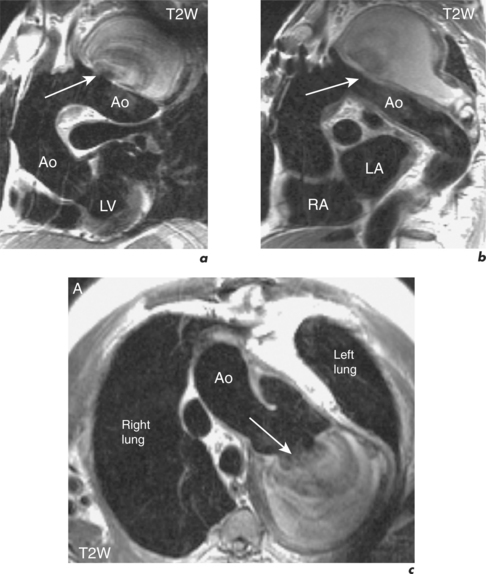
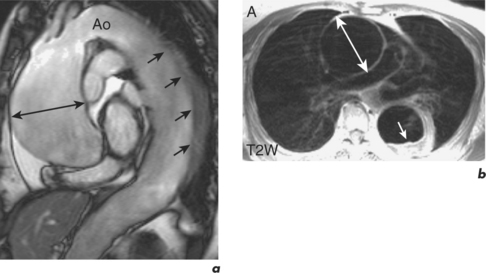
Aortic aneurysms are usually the consequence of atherosclerotic disease and most commonly occur at the descending aorta (Figure 6.3). Other causes are trauma, connective tissue disorders such as Marfan and Ehlers–Danlos syndromes, congenital abnormalities, and infections such as syphilis. Post-stenotic aneurysms are found distal to AV stenosis and aortic coarctation or recoarctation.
CMR can readily detect aortic aneurysms and scan protocols evaluate the following:
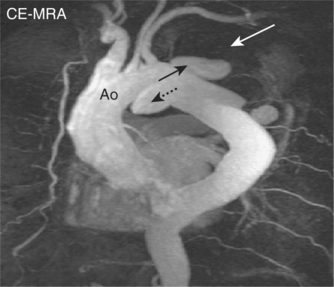
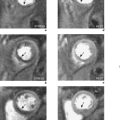
SE images acquired in the transverse and long-axis planes are useful for measuring the diameter of the aorta at different levels and relationship of the aneurysm to major vessels. Coronal and oblique sagittal views clearly depict the aortic root and tortuous segments. Slow or turbulent blood flow can produce images which mimic mural thrombus on SE imaging. SSFP cines and velocity flow mapping help to differentiate slow flow from intraluminal thrombus. Where intraluminal thrombus is present, its thickness and extent should be determined. CMR allows characterization of intraluminal thrombus based on signal changes caused by the paramagnetic properties of deoxyhaemoglobin and methaemoglobin. Methaemoglobin forms from red blood cell lysis and shortens T1 but increases T2, causing hyperintensity on both T1W and T2W SE sequences. Thrombus with homogeneous low signal intensity on both T1W and T2W images corresponds to macroscopic organized thrombus. Some organized thrombi may have an internal rim of hyperintensity which represents recent clot apposition on the luminal surface of the thrombus. Thrombi with homogeneous high signal intensity on T1W and T2W imaging represent unorganized thrombi, composed mainly of fresh clot. Some thrombi may appear partially organized, with areas of high and low signal intensity (Figure 6.5). Inflammatory aortic aneurysms may show an area of peri-aortic inflammation that enhances following gadolinium contrast. This can be better visualized using a T1W CMR sequence with an added fat saturation prepulse. MRA is useful for assessing aortic flow and involvement and patency of aortic branch vessels. Images are acquired in the aortic long-axis plane and can then be reformatted into the transaxial plane. Post-processing techniques, such as maximum intensity projections (MIPs) and shaded surface displays, are used for representation (Figure 6.6). CMR is useful for follow-up evaluation of aneurysm size and serial measurements must be made at the same level.
Aortic dissection
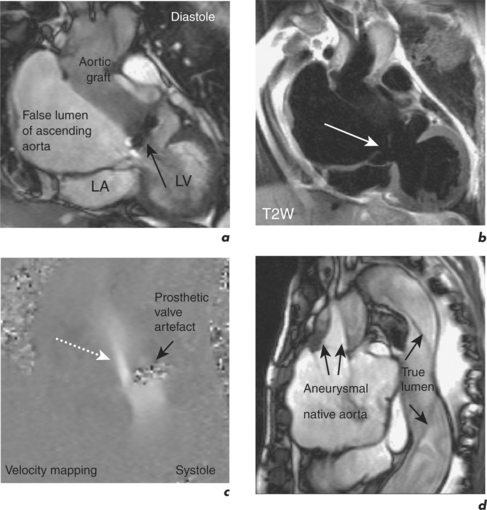
Aortic dissection consists of an intimal flap separating the true and false lumen, and intimal tears are sites of communication between the true and false lumen. A dissection can spread antegradely or retrogradely and may involve the whole length of the aorta. It can lead to occlusion or obstruction of branch vessels, aortic regurgitation and pericardial effusion. Aetiology includes hypertension (the most common), atherosclerosis, congenital lesions, iatrogenic causes and trauma.
Aortic dissections are classified by the De Bakey or Stanford classification system. The De Bakey classification is based on the location of the intimal tear and the extent of the dissection:
The Stanford classification is based on prognosis and has implications for management.
Intramural haematomas and penetrating aortic ulcers
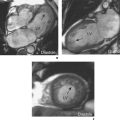
Intramural haematomas are characterized by the presence of intramural blood and/or increased arterial wall thickness which can be asymmetrical or circumferential (Figure 6.9). T1W and T2W SE imaging allows some determination of the age of the haemorrhage. Acutely (first 5–7 days) intramural haematoma appears as increased wall thickness of intermediate signal intensity which is isodense with the aortic wall. Subacutely (more than 8 days) there is high signal intensity similar to fat due to the presence of methaemoglobin. Transaxial planes should be acquired in preference to longitudinal views due to improved differentiation from mediastinal fat. Addition of a fat saturation prepulse sequence further improves distinction between haematoma and fat. MRA will usually not detect intramural haematomas since there is no luminal component.
Aortitis
Takayasu arteritis is a granulomatous vasculitis of unknown aetiology that commonly affects the thoracic and abdominal aorta. Inflammation leads to thickening of the aortic wall and the origin of the main branches, mainly in the aortic arch, with sequelae including aortic branch thrombosis, stenosis or occlusion. The pulmonary arteries can also be affected. SE imaging demonstrates diffuse wall thickening and MRA provides good visualization of aneurysm formation and branch vessel stenosis and/or occlusion.
Marfan syndrome
Aortic dissection and rupture account for most deaths in this condition and affected individuals undergo regular CMR surveillance of the aorta to help plan elective aortic root replacement. Aortic root dimension and the rate of increase are the best predictors of those at greatest risk of aortic dissection, and prophylactic root replacement is recommended before diameters reach 55–60 mm. In patients with family history of aortic dissection, replacement is performed when the aortic root measures 50 mm. Progressive thoracoabdominal vasculopathy postoperatively necessitates continued follow-up after repair of the ascending aorta. Patients with an aortic dissection or peripheral artery aneurysms require more frequent CMR assessment.
Coarctation of the aorta
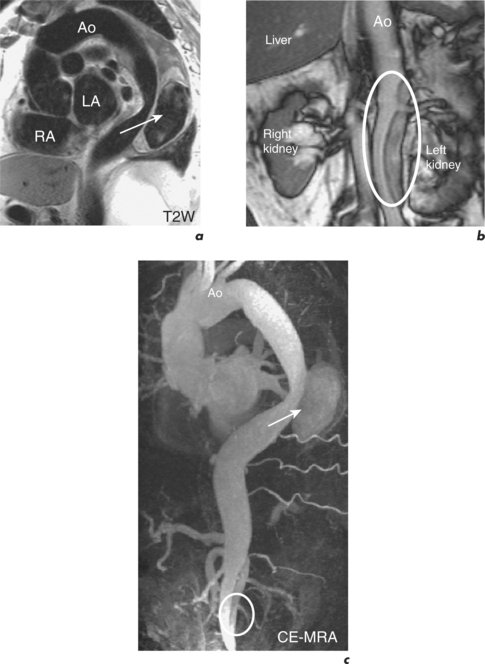
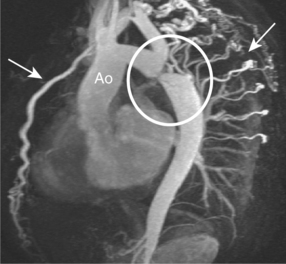
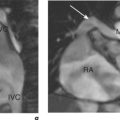
Coarctation of the aorta occurs in approximately 1 in 10 000 people and is usually diagnosed in children or adults under 40 years old. It commonly consists of a stenosis of the aorta just distal to the origin of the left subclavian artery. The area of coarctation can be a localized narrowing or a long hypoplastic segment involving the aortic arch and descending thoracic aorta. Haemodynamically significant lesions are associated with the development of collaterals from the intercostal, internal mammary and anterior spinal arteries which then can supply blood to the descending aorta (Figure 6.10). Bicuspid AV is frequently seen with aortic coarctation and this cohort can progress to AS or AR. Dilatation of the ascending aorta may precede aortic aneurysm formation and type A aortic dissection in these patients.
CMR is the imaging modality of choice in adult aortic coarctation. The study evaluates:
The oblique sagittal plane is imaged using SE and SSFP cines for assessment of anatomy, but several other planes may be required with increased tortuosity. Blood flow across the stenosis is recognized with cines and these are then used to position the planes required for velocity mapping CMR. Flow velocity mapping techniques across the coarctation measure blood flow velocity and a pressure gradient is then estimated with the modified Bernoulli formula. Resting peak velocity of greater than 3 m/s is significant, particularly in the presence of diastolic prolongation of forward flow—a diastolic tail. Flow velocities may normalize in significant coarctation with extensive collateralization. The percentage of collateral blood flow is calculated from measurements of flow just proximal to the coarctation site and within the descending thoracic aorta at the level of the diaphragm. Collateral blood flow is significantly increased in severe coarctation. MRA readily demonstrates tortuosity, involvement of aortic arch vessels and presence of collaterals.
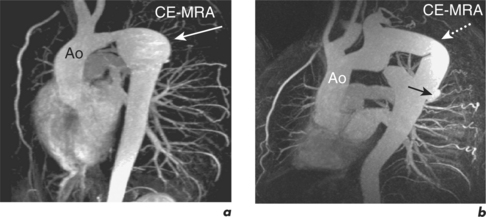
Long-term outcomes following elective intervention in coarctation are optimal when performed at 2–5 years of age. Late complications include hypertension, re-stenosis, residual stenosis and aneurysm formation. CMR is performed at baseline post-intervention and then at intervals thereafter, depending on clinical and imaging findings (Figures 6.11 and 6.12). Haemoptysis in a patient with coarctation may indicate leakage of blood through a false aneurysm. Serial contiguous SE sequences should be used in this situation to identify bright para-aortic haematoma.
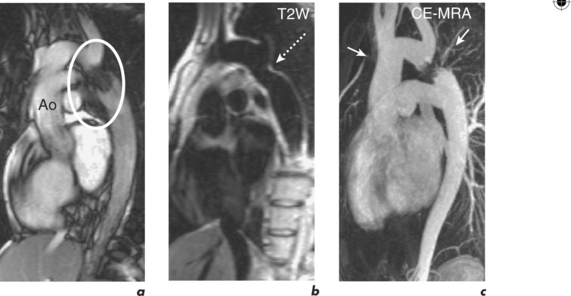
Figure 6.11 One-year post-catheter intervention CMR study of the patient in Figure 6.10. Oblique sagittal views show mild residual in-stent stenosis.
Rare congenital anomalies of the aorta
A right-sided aortic arch consists of a single aortic arch located to the right of the trachea. This congenital anomaly is associated with TOF and truncus arteriosus. A double aortic arch refers to persistence of both the right and left aortic arch leading to the formation of a vascular ring around the trachea and oesophagus. This ring may compress these structures, causing dyspnoea and dysphagia. An aberrant origin of the right subclavian artery from the descending aorta may also cause airways compromise due to direct compression as it crosses obliquely towards the right shoulder behind the trachea.