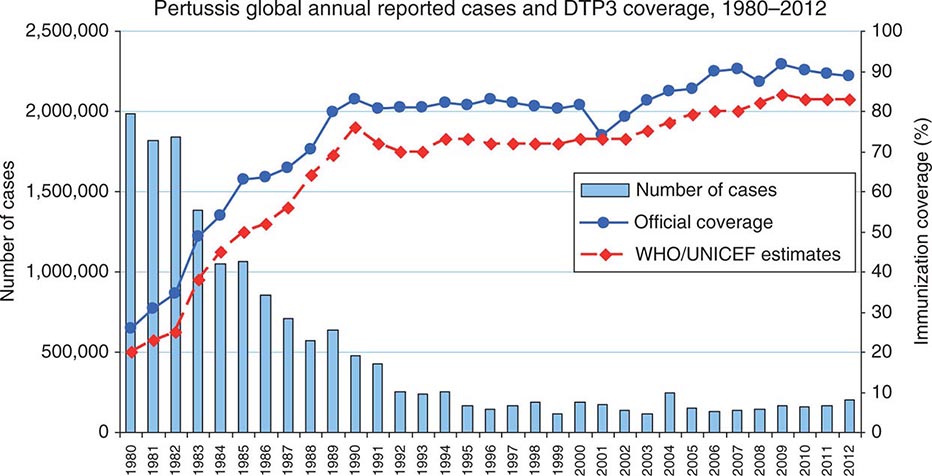
The first widespread use of serogroup C meningococcal conjugate vaccine (MenC) came in 1999 in the United Kingdom after a rise in serogroup C disease. A mass vaccination campaign involving all individuals <19 years of age was undertaken, and the number of laboratory-confirmed serogroup C cases fell from 955 in 1998–1999 to just 29 in 2011–2012. The effectiveness of the immunization program was attributed both to direct protection of immunized persons and to reduced transmission of the organism in the population as a result of decreased rates of colonization among the immunized (herd immunity). Data on immunogenicity and effectiveness have shown that the duration of protection is short when the vaccine is administered in early childhood; thus booster doses are needed to maintain population immunity. In contrast, immunity after a dose of vaccine given in adolescence appears to be prolonged.
The first quadrivalent conjugate meningococcal vaccine containing A, C, Y, and W polysaccharides conjugated to diphtheria toxoid was initially recommended for all children >11 years of age in the United States in 2005. In 2007 the license was extended to high-risk children 2–10 years of age. In the same year, the vaccine was licensed in Canada for persons 2–55 years of age. Uptake was slow, but current U.S. data suggest an efficacy rate of 82% in the first year after vaccination, with waning to 59% at 3–6 years after vaccination. Limited data from the U.S. Vaccine Adverse Events Reporting System indicated that there might be a short-term increase in the risk of Guillain-Barré syndrome after immunization with the diphtheria conjugate vaccine; however, further investigation has not confirmed this finding. Quadrivalent conjugate vaccines with tetanus or CRM197 as carrier protein are now available in many countries.
A monovalent serogroup A vaccine, manufactured in India, was licensed in 2010 and rolled out to countries in the sub-Saharan African meningitis belt. There is strong evidence that this vaccine has been highly effective in controlling epidemic meningococcal disease in the region, with some evidence of a >90% reduction in disease in vaccinated populations. However, disease caused by serogroup × and W persists.
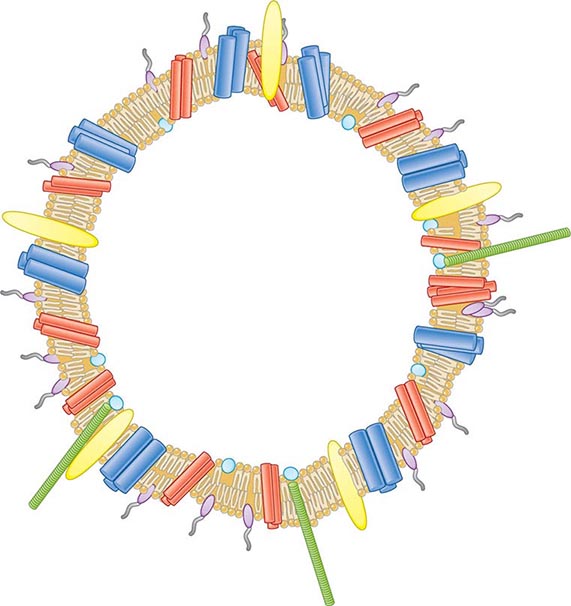
![]() Vaccines Based on Subcapsular Antigens The lack of immunogenicity of the serogroup B capsule has led to the development of vaccines based on subcapsular antigens. Various surface components have been studied in early-phase clinical trials. Outer-membrane vesicles (OMVs) containing outer-membrane proteins, phospholipid, and LPS can be extracted from cultures of N. meningitidis by detergent treatment (Fig. 180-7). OMVs prepared in this way were used in efficacy trials with a Norwegian outbreak strain and reduced the incidence of group B disease among 14- to 16-year-old schoolchildren by 53%. Similarly, OMV vaccines constructed from local outbreak strains in Cuba and New Zealand have had reported efficacy rates of >70%. These OMV vaccines appear to produce strain-specific immune responses, with only limited cross-protection, and are therefore best suited to clonal outbreaks (e.g., those in Cuba and New Zealand as well as others in Norway and the province of Normandy in France).
Vaccines Based on Subcapsular Antigens The lack of immunogenicity of the serogroup B capsule has led to the development of vaccines based on subcapsular antigens. Various surface components have been studied in early-phase clinical trials. Outer-membrane vesicles (OMVs) containing outer-membrane proteins, phospholipid, and LPS can be extracted from cultures of N. meningitidis by detergent treatment (Fig. 180-7). OMVs prepared in this way were used in efficacy trials with a Norwegian outbreak strain and reduced the incidence of group B disease among 14- to 16-year-old schoolchildren by 53%. Similarly, OMV vaccines constructed from local outbreak strains in Cuba and New Zealand have had reported efficacy rates of >70%. These OMV vaccines appear to produce strain-specific immune responses, with only limited cross-protection, and are therefore best suited to clonal outbreaks (e.g., those in Cuba and New Zealand as well as others in Norway and the province of Normandy in France).
FIGURE 180-7 Illustration of meningococcal outer-membrane vesicle containing outer-membrane structures.
Several purified surface proteins have been evaluated in phase 1 clinical trials but have not yet been developed further because of antigenic variability or poor immunogenicity (e.g., transferrin-binding proteins, neisserial surface protein A). Other vaccine candidates have been identified since sequencing of the meningococcal genome. A combination vaccine that includes the New Zealand OMV vaccine and three recombinant proteins (neisserial adhesin A, factor H–binding protein, and neisserial heparin-binding antigen) is immunogenic in infancy and has been licensed for use in Europe and Australia. Recommendations for its use are pending. Finally, a highly immunogenic vaccine based on two variants of the lipoprotein factor H–binding protein is undergoing clinical evaluation
MANAGEMENT OF CONTACTS
Close (household and kissing) contacts of individuals with meningococcal disease are at increased risk (up to 1000 times the rate for the general population) of developing secondary disease; a secondary case follows as many as 3% of sporadic cases. About one-fifth of secondary cases are actually co-primary cases—i.e., cases that occur soon after the primary case and in which transmission is presumed to have originated from the same third party. The rate of secondary cases is highest during the week after presentation of the index case. The risk falls rapidly but remains above baseline for up to 1 year after the index case; 30% of secondary cases occur in the first week, 20% in the second week, and most of the remainder over the next 6 weeks. In outbreaks of meningococcal disease, mass prophylaxis has been used; however, limited data support population intervention, and significant concerns have arisen about adverse events and the development of resistance. For these reasons, prophylaxis is usually restricted to (1) persons at greatest risk who are intimate and/or household contacts of the index case and (2) health care workers who have been directly exposed to respiratory secretions. In most cases, members of wider communities (e.g., at schools or colleges) are not offered prophylaxis.
![]() The aim of prophylaxis is to eradicate colonization of close contacts with the strain that has caused invasive disease in the index case. Prophylaxis should be given to all contacts at the same time to avoid recolonization by meningococci transmitted from untreated contacts and should also be used as soon as possible to treat early disease in secondary cases. If the index patient is treated with an antibiotic that does not reliably clear colonization (e.g., penicillin), he or she should be given a prophylactic agent at the end of treatment to prevent relapse or onward transmission. Although rifampin has been most widely used and studied, it is not the optimal agent because it fails to eradicate carriage in 15–20% of cases, rates of adverse events have been high, compliance is affected by the need for four doses, and emerging resistance has been reported. Ceftriaxone as a single IM or IV injection is highly (97%) effective in carriage eradication and can be used at all ages and in pregnancy. Reduced susceptibility of isolates to ceftriaxone has occasionally been reported. Ciprofloxacin or ofloxacin is preferred in some countries; these agents are highly effective and can be administered by mouth but are not recommended in pregnancy. Resistance to fluoroquinolones has been reported in some meningococci in North America, Europe, and Asia.
The aim of prophylaxis is to eradicate colonization of close contacts with the strain that has caused invasive disease in the index case. Prophylaxis should be given to all contacts at the same time to avoid recolonization by meningococci transmitted from untreated contacts and should also be used as soon as possible to treat early disease in secondary cases. If the index patient is treated with an antibiotic that does not reliably clear colonization (e.g., penicillin), he or she should be given a prophylactic agent at the end of treatment to prevent relapse or onward transmission. Although rifampin has been most widely used and studied, it is not the optimal agent because it fails to eradicate carriage in 15–20% of cases, rates of adverse events have been high, compliance is affected by the need for four doses, and emerging resistance has been reported. Ceftriaxone as a single IM or IV injection is highly (97%) effective in carriage eradication and can be used at all ages and in pregnancy. Reduced susceptibility of isolates to ceftriaxone has occasionally been reported. Ciprofloxacin or ofloxacin is preferred in some countries; these agents are highly effective and can be administered by mouth but are not recommended in pregnancy. Resistance to fluoroquinolones has been reported in some meningococci in North America, Europe, and Asia.
In documented serogroup A, C, Y, or W disease, contacts may be offered immunization (preferably with a conjugate vaccine) in addition to chemoprophylaxis to provide protection beyond the duration of antibiotic therapy. Mass vaccination has been used successfully to control disease during outbreaks in closed communities (educational and military establishments) as well as during epidemics in open communities.
181 |
Gonococcal Infections |
DEFINITION
Gonorrhea is a sexually transmitted infection (STI) of epithelium and commonly manifests as cervicitis, urethritis, proctitis, and conjunctivitis. If untreated, infections at these sites can lead to local complications such as endometritis, salpingitis, tuboovarian abscess, bartholinitis, peritonitis, and perihepatitis in female patients; periurethritis and epididymitis in male patients; and ophthalmia neonatorum in newborns. Disseminated gonococcemia is an uncommon event whose manifestations include skin lesions, tenosynovitis, arthritis, and (in rare cases) endocarditis or meningitis.
MICROBIOLOGY
Neisseria gonorrhoeae is a gram-negative, nonmotile, non-spore-forming organism that grows singly and in pairs (i.e., as monococci and diplococci, respectively). Exclusively a human pathogen, the gonococcus contains, on average, three genome copies per coccal unit; this polyploidy permits a high level of antigenic variation and the survival of the organism in its host. Gonococci, like all other Neisseria species, are oxidase positive. They are distinguished from other neisseriae by their ability to grow on selective media and to use glucose but not maltose, sucrose, or lactose.
EPIDEMIOLOGY
![]() The incidence of gonorrhea has declined significantly in the United States, but there were still ~311,000 newly reported cases in 2012. Gonorrhea remains a major public health problem worldwide, is a significant cause of morbidity in developing countries, and may play a role in enhancing transmission of HIV.
The incidence of gonorrhea has declined significantly in the United States, but there were still ~311,000 newly reported cases in 2012. Gonorrhea remains a major public health problem worldwide, is a significant cause of morbidity in developing countries, and may play a role in enhancing transmission of HIV.
Gonorrhea predominantly affects young, nonwhite, unmarried, less educated members of urban populations. The number of reported cases probably represents half of the true number of cases—a discrepancy resulting from underreporting, self-treatment, and nonspecific treatment without a laboratory-proven diagnosis. The number of reported new cases of gonorrhea in the United States rose from ~250,000 in the early 1960s to a high of 1.01 million in 1978. The recorded incidence of gonorrhea in modern times peaked in 1975, with 468 reported new cases per 100,000 population in the United States. This peak was attributable to the interaction of several variables, including improved accuracy of diagnosis, changes in patterns of contraceptive use, and changes in sexual behavior. The incidence of the disease has since declined gradually and is currently estimated at 120 cases per 100,000, a figure that is still the highest among industrialized countries. A further decline in the overall incidence of gonorrhea in the United States over the past quarter-century may reflect increased condom use resulting from public health efforts to curtail HIV transmission. At present, the attack rate in the United States is highest among 15- to 19-year-old women and 20- to 24-year-old men; 60% of all reported cases occur in the preceding two groups together. From the standpoint of ethnicity, rates are highest among African Americans and lowest among persons of Asian or Pacific Island descent.
The incidence of gonorrhea is higher in developing countries than in industrialized nations. The exact incidence of any STI is difficult to ascertain in developing countries because of limited surveillance and variable diagnostic criteria. Studies in Africa have clearly demonstrated that nonulcerative STIs such as gonorrhea (in addition to ulcerative STIs) are an independent risk factor for the transmission of HIV (Chap. 226).
Gonorrhea is transmitted from males to females more efficiently than in the opposite direction. The rate of transmission to a woman during a single unprotected sexual encounter with an infected man is ~50–70%. Oropharyngeal gonorrhea occurs in ~20% of women who practice fellatio with infected partners. Transmission in either direction by cunnilingus is rare.
In any population, there exists a small minority of individuals who have high rates of new-partner acquisition. These “core-group members” or “high-frequency transmitters” are vital in sustaining STI transmission at the population level. Another instrumental factor in sustaining gonorrhea in the population is the large number of infected individuals who are asymptomatic or have minor symptoms that are ignored. These persons, unlike symptomatic individuals, may not cease sexual activity and therefore continue to transmit the infection. This situation underscores the importance of contact tracing and empirical treatment of the sex partners of index cases.
PATHOGENESIS, IMMUNOLOGY, AND ANTIMICROBIAL RESISTANCE
Outer-Membrane Proteins • PILI Fresh clinical isolates of N. gonorrhoeae initially form piliated (fimbriated) colonies distinguishable on translucent agar. Pilus expression is rapidly switched off with unselected subculture because of rearrangements in pilus genes. This change is a basis for antigenic variation of gonococci. Piliated strains adhere better to cells derived from human mucosal surfaces and are more virulent in organ culture models and human inoculation experiments than nonpiliated variants. In a fallopian tube explant model, pili mediate gonococcal attachment to nonciliated columnar epithelial cells. This event initiates gonococcal phagocytosis and transport through these cells to intercellular spaces near the basement membrane or directly into the subepithelial tissue. Pili are also essential for genetic competence and transformation of N. gonorrhoeae, which permit horizontal transfer of genetic material between different gonococcal lineages in vivo.
OPACITY-ASSOCIATED PROTEIN Another gonococcal surface protein that is important in adherence to epithelial cells is opacity-associated protein (Opa, formerly called protein II). Opa contributes to intergonococcal adhesion, which is responsible for the opaque nature of gonococcal colonies on translucent agar and the organism’s adherence to a variety of eukaryotic cells, including polymorphonuclear leukocytes (PMNs). Certain Opa variants promote invasion of epithelial cells, and this effect has been linked with the ability of Opa to bind vitronectin, glycosaminoglycans, and several members of the carcinoembryonic antigen–related cell adhesion molecule (CEACAM) receptor family. N. gonorrhoeae Opa proteins that bind CEACAM1, which is expressed by primary CD4+ T lymphocytes, suppress the activation and proliferation of these lymphocytes. This phenomenon may serve to explain the transient decrease in CD4+ T lymphocyte counts associated with gonococcal infection. Select Opa proteins can engage CEACAM3, which is expressed on neutrophils, with consequent nonopsonic phagocytosis (i.e., phagocytosis independent of antibody and complement) and killing of bacteria.
PORIN Porin (previously designated protein I) is the most abundant gonococcal surface protein, accounting for >50% of the organism’s total outer-membrane protein. Porin molecules exist as trimers that provide anion-transporting aqueous channels through the otherwise hydrophobic outer membrane. Porin exhibits stable interstrain antigenic variation and forms the basis for gonococcal serotyping. Two main serotypes have been identified: PorB.1A strains are often associated with disseminated gonococcal infection (DGI), whereas PorB.1B strains usually cause local genital infections only. DGI strains are generally resistant to the killing action of normal human serum and do not incite a significant local inflammatory response; therefore, they may not cause symptoms at genital sites. These characteristics may be related to the ability of PorB.1A strains to bind to complement-inhibitory molecules, resulting in a diminished inflammatory response. Porin can translocate to the cytoplasmic membrane of host cells—a process that could initiate gonococcal endocytosis and invasion.
OTHER OUTER-MEMBRANE PROTEINS Other notable outer-membrane proteins include H.8, a lipoprotein that is present in high concentration on the surface of all gonococcal strains and is an excellent target for antibody-based diagnostic testing. Transferrin-binding proteins (Tbp1 and Tbp2) and lactoferrin-binding protein are required for scavenging iron from transferrin and lactoferrin in vivo. Transferrin and iron have been shown to enhance the attachment of iron-deprived N. gonorrhoeae to human endometrial cells. IgA1 protease is produced by N. gonorrhoeae and may protect the organism from the action of mucosal IgA.
Lipooligosaccharide Gonococcal lipooligosaccharide (LOS) consists of a lipid A and a core oligosaccharide that lacks the repeating O-carbohydrate antigenic side chain seen in other gram-negative bacteria (Chap. 145e). Gonococcal LOS possesses marked endotoxic activity and contributes to the local cytotoxic effect in a fallopian tube model. LOS core sugars undergo a high degree of phase variation under different conditions of growth; this variation reflects genetic regulation and expression of glycotransferase genes that dictate the carbohydrate structure of LOS. These phenotypic changes may affect interactions of N. gonorrhoeae with elements of the humoral immune system (antibodies and complement) and may also influence direct binding of organisms to both professional phagocytes and nonprofessional phagocytes (epithelial cells). For example, gonococci that are sialylated at their LOS sites bind complement factor H and inhibit the alternative pathway of complement. LOS sialylation may also decrease nonopsonic Opa-mediated association with neutrophils and inhibit the oxidative burst in PMNs. The binding of the unsialylated terminal lactosamine residue of LOS to an asialoglycoprotein receptor on male epithelial cells facilitates adherence and subsequent gonococcal invasion of these cells. Moreover, oligosaccharide structures in LOS can modulate host immune responses. For example, the terminal monosaccharide expressed by LOS determines the C-type lectin receptor on dendritic cells that is targeted by the bacteria. In turn, the specific C-type lectin receptor engaged influences whether a TH1- or TH2-type response is elicited; the latter response may be less favorable for clearance of gonococcal infection.
Host Factors In addition to gonococcal structures that interact with epithelial cells, host factors seem to be important in mediating entry of gonococci into nonphagocytic cells. Activation of phosphatidylcholine-specific phospholipase C and acidic sphingomyelinase by N. gonorrhoeae, which results in the release of diacylglycerol and ceramide, is a requirement for the entry of N. gonorrhoeae into epithelial cells. Ceramide accumulation within cells leads to apoptosis, which may disrupt epithelial integrity and facilitate entry of gonococci into subepithelial tissue. Release of chemotactic factors as a result of complement activation contributes to inflammation, as does the toxic effect of LOS in provoking the release of inflammatory cytokines.
The importance of humoral immunity in host defenses against neisserial infections is best illustrated by the predisposition of persons deficient in terminal complement components (C5 through C9) to recurrent bacteremic gonococcal infections and to recurrent meningococcal meningitis or meningococcemia. Gonococcal porin induces T cell–proliferative responses in persons with urogenital gonococcal disease. A significant increase in porin-specific interleukin (IL) 4–producing CD4+ as well as CD8+ T lymphocytes is seen in individuals with mucosal gonococcal disease. A portion of these lymphocytes that show a porin-specific TH2-type response could traffic to mucosal surfaces and play a role in immune protection against the disease. Few data clearly indicate that protective immunity is acquired from a previous gonococcal infection, although bactericidal and opsonophagocytic antibodies to porin and LOS may offer partial protection. On the other hand, women who are infected and acquire high levels of antibody to another outer-membrane protein, Rmp (reduction modifiable protein, formerly called protein III), may be especially likely to become reinfected with N. gonorrhoeae because Rmp antibodies block the effect of bactericidal antibodies to porin and LOS. Rmp shows little, if any, interstrain antigenic variation; therefore, Rmp antibodies potentially may block antibody-mediated killing of all gonococci. The mechanism of blocking has not been fully characterized, but Rmp antibodies may noncompetitively inhibit binding of porin and LOS antibodies because of the proximity of these structures in the gonococcal outer membrane. In male volunteers who have no history of gonorrhea, the net effect of these events may influence the outcome of experimental challenge with N. gonorrhoeae. Because Rmp bears extensive homology to enterobacterial OmpA and meningococcal class 4 proteins, it is possible that these blocking antibodies result from prior exposure to cross-reacting proteins from these species and also play a role in first-time infection with N. gonorrhoeae.
Gonococcal Resistance to Antimicrobial Agents It is no surprise that N. gonorrhoeae, with its remarkable capacity to alter its antigenic structure and adapt to changes in the microenvironment, has become resistant to numerous antibiotics. The first effective agents against gonorrhea were the sulfonamides, which were introduced in the 1930s and became ineffective within a decade. Penicillin was then used as the drug of choice for the treatment of gonorrhea. By 1965, 42% of gonococcal isolates had developed low-level resistance to penicillin G. Resistance due to the production of penicillinase arose later.
![]() Gonococci become fully resistant to antibiotics either by chromosomal mutations or by acquisition of R factors (plasmids). Two types of chromosomal mutations have been described. The first type, which is drug specific, is a single-step mutation leading to high-level resistance. The second type involves mutations at several chromosomal loci that combine to determine the level as well as the pattern of resistance. Strains with mutations in chromosomal genes were first observed in the late 1950s. As recently as 2007, chromosomal mutations accounted for resistance to penicillin, tetracycline, or both in ~16% of strains surveyed in the United States.
Gonococci become fully resistant to antibiotics either by chromosomal mutations or by acquisition of R factors (plasmids). Two types of chromosomal mutations have been described. The first type, which is drug specific, is a single-step mutation leading to high-level resistance. The second type involves mutations at several chromosomal loci that combine to determine the level as well as the pattern of resistance. Strains with mutations in chromosomal genes were first observed in the late 1950s. As recently as 2007, chromosomal mutations accounted for resistance to penicillin, tetracycline, or both in ~16% of strains surveyed in the United States.
β-Lactamase (penicillinase)–producing strains of N. gonorrhoeae (PPNG) carrying plasmids with the Pcr determinant had rapidly spread worldwide by the early 1980s. N. gonorrhoeae strains with plasmid-borne tetracycline resistance (TRNG) can mobilize some β-lactamase plasmids, and PPNG and TRNG occur together, sometimes along with strains exhibiting chromosomally mediated resistance (CMRNG). Penicillin, ampicillin, and tetracycline are no longer reliable for the treatment of gonorrhea and should not be used.
![]() Quinolone-containing regimens were also recommended for treatment of gonococcal infections; the fluoroquinolones offered the advantage of antichlamydial activity when administered for 7 days. However, quinolone-resistant N. gonorrhoeae (QRNG) appeared soon after these agents were first used to treat gonorrhea. QRNG is particularly common in the Pacific Islands (including Hawaii) and Asia, where, in certain areas, all gonococcal strains are now resistant to quinolones. At present, QRNG is also common in parts of Europe and the Middle East. In the United States, QRNG has been identified in midwestern and eastern areas as well as in states on the Pacific coast, where resistant strains were first seen. Alterations in DNA gyrase and topoisomerase IV have been implicated as mechanisms of fluoroquinolone resistance.
Quinolone-containing regimens were also recommended for treatment of gonococcal infections; the fluoroquinolones offered the advantage of antichlamydial activity when administered for 7 days. However, quinolone-resistant N. gonorrhoeae (QRNG) appeared soon after these agents were first used to treat gonorrhea. QRNG is particularly common in the Pacific Islands (including Hawaii) and Asia, where, in certain areas, all gonococcal strains are now resistant to quinolones. At present, QRNG is also common in parts of Europe and the Middle East. In the United States, QRNG has been identified in midwestern and eastern areas as well as in states on the Pacific coast, where resistant strains were first seen. Alterations in DNA gyrase and topoisomerase IV have been implicated as mechanisms of fluoroquinolone resistance.
Resistance to spectinomycin, which has been used in the past as an alternative agent, has been reported. Because this agent usually is not associated with resistance to other antibiotics, spectinomycin can be reserved for use against multidrug-resistant strains of N. gonorrhoeae. Nevertheless, outbreaks caused by strains resistant to spectinomycin have been documented in Korea and England when the drug has been used for primary treatment of gonorrhea.
Third-generation cephalosporins have remained highly effective as single-dose therapy for gonorrhea, but the recent isolation of strains highly resistant to ceftriaxone (minimal inhibitory concentrations [MICs], 2 μg/mL) in Japan and some European countries is cause for concern. Even though the MICs of ceftriaxone against certain strains may reach 0.015–0.125 μg/mL (higher than the MICs of 0.0001–0.008 μg/mL for fully susceptible strains), these levels are greatly exceeded in the blood, the urethra, and the cervix when the routinely recommended parenteral dose of ceftriaxone is administered. The rising MICs of oral cefixime (the previously recommended alternative oral third-generation cephalosporin) against N. gonorrhoeae, combined with this drug’s limited capacity to reach levels sufficiently higher than MICs in the blood, the urethra, the cervix, and especially the pharynx, have resulted in the removal of cefixime from the list of first-line agents for treatment of uncomplicated gonorrhea. All N. gonorrhoeae strains with reduced susceptibility to ceftriaxone and cefixime (i.e., cephalosporin-intermediate/resistant strains) contain (1) a mosaic penA allele, which is the principal resistance determinant and encodes a penicillin-binding protein (PBP2) whose sequence differs in 60 amino acids from that of wild-type PBP2, and (2) additional genetic resistance determinants that are also required for high-level penicillin resistance.
CLINICAL MANIFESTATIONS
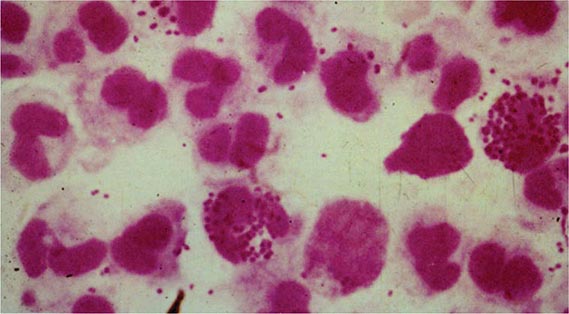
Gonococcal Infections in Men Acute urethritis is the most common clinical manifestation of gonorrhea in male patients. The usual incubation period after exposure is 2–7 days, although the interval can be longer and some men remain asymptomatic. Strains of the PorB.1A serotype tend to cause a greater proportion of cases of mild and asymptomatic urethritis than do PorB.1B strains. Urethral discharge and dysuria, usually without urinary frequency or urgency, are the major symptoms. The discharge initially is scant and mucoid but becomes profuse and purulent within a day or two. Gram’s staining of the urethral discharge may reveal PMNs and gram-negative intracellular monococci and diplococci (Fig. 181-1). The clinical manifestations of gonococcal urethritis are usually more severe and overt than those of nongonococcal urethritis, including urethritis caused by Chlamydia trachomatis (Chap. 213); however, exceptions are common, and it is often impossible to differentiate the causes of urethritis on clinical grounds alone. The majority of cases of urethritis seen in the United States today are not caused by N. gonorrhoeae and/or C. trachomatis. Although a number of other organisms may be responsible, many cases do not have a specific etiologic agent identified.
FIGURE 181-1 Gram’s stain of urethral discharge from a male patient with gonorrhea shows gram-negative intracellular monococci and diplococci. (From the Public Health Agency of Canada.)
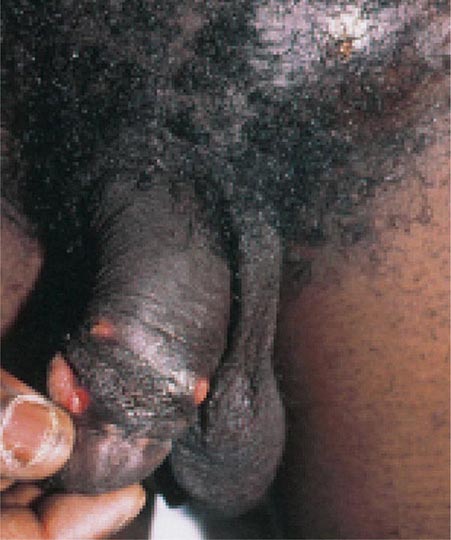
Most symptomatic men with gonorrhea seek treatment and cease to be infectious. The remaining men, who are largely asymptomatic, accumulate in number over time and constitute about two-thirds of all infected men at any point in time; together with men incubating the organism (who shed the organism but are asymptomatic), they serve as the source of spread of infection. Before the antibiotic era, symptoms of urethritis persisted for ~8 weeks. Epididymitis is now an uncommon complication, and gonococcal prostatitis occurs rarely, if at all. Other unusual local complications of gonococcal urethritis include edema of the penis due to dorsal lymphangitis or thrombophlebitis, submucous inflammatory “soft” infiltration of the urethral wall, periurethral abscess or fistula, inflammation or abscess of Cowper’s gland, and seminal vesiculitis. Balanitis may develop in uncircumcised men.
Gonococcal Infections in Women • GONOCOCCAL CERVICITIS Mucopurulent cervicitis is a common STI diagnosis in American women and may be caused by N. gonorrhoeae, C. trachomatis, and other organisms, including Mycoplasma genitalium (Chap. 212). Cervicitis may coexist with candidal or trichomonal vaginitis. N. gonorrhoeae primarily infects the columnar epithelium of the cervical os. Bartholin’s glands occasionally become infected.
Women infected with N. gonorrhoeae usually develop symptoms. However, the women who either remain asymptomatic or have only minor symptoms may delay in seeking medical attention. These minor symptoms may include scant vaginal discharge issuing from the inflamed cervix (without vaginitis or vaginosis per se) and dysuria (often without urgency or frequency) that may be associated with gonococcal urethritis. Although the incubation period of gonorrhea is less well defined in women than in men, symptoms usually develop within 10 days of infection and are more acute and intense than those of chlamydial cervicitis.
The physical examination may reveal a mucopurulent discharge (mucopus) issuing from the cervical os. Because Gram’s stain is not sensitive for the diagnosis of gonorrhea in women, specimens should be submitted for culture or a nonculture assay (see “Laboratory Diagnosis,” below). Edematous and friable cervical ectopy and endocervical bleeding induced by gentle swabbing are more often seen in chlamydial infection. Gonococcal infection may extend deep enough to produce dyspareunia and lower abdominal or back pain. In such cases, it is imperative to consider a diagnosis of pelvic inflammatory disease (PID) and to administer treatment for that disease (Chaps. 163 and 213).
N. gonorrhoeae may also be recovered from the urethra and rectum of women with cervicitis, but these are rarely the only infected sites. Urethritis in women may produce symptoms of internal dysuria, which is often attributed to “cystitis.” Pyuria in the absence of bacteriuria seen on Gram’s stain of unspun urine, accompanied by urine cultures that fail to yield >102 colonies of bacteria usually associated with urinary tract infection, signifies the possibility of urethritis due to C. trachomatis. Urethral infection with N. gonorrhoeae may also occur in this context, but in this instance urethral cultures are usually positive.
GONOCOCCAL VAGINITIS The vaginal mucosa of healthy women is lined by stratified squamous epithelium and is rarely infected by N. gonorrhoeae. However, gonococcal vaginitis can occur in anestrogenic women (e.g., prepubertal girls and postmenopausal women), in whom the vaginal stratified squamous epithelium is often thinned down to the basilar layer, which can be infected by N. gonorrhoeae. The intense inflammation of the vagina makes the physical (speculum and bimanual) examination extremely painful. The vaginal mucosa is red and edematous, and an abundant purulent discharge is often present. Infection in the urethra and in Skene’s and Bartholin’s glands often accompanies gonococcal vaginitis. Inflamed cervical erosion or abscesses in nabothian cysts may also occur. Coexisting cervicitis may result in pus in the cervical os.
Anorectal Gonorrhea Because the female anatomy permits the spread of cervical exudate to the rectum, N. gonorrhoeae is sometimes recovered from the rectum of women with uncomplicated gonococcal cervicitis. The rectum is the sole site of infection in only 5% of women with gonorrhea. Such women are usually asymptomatic but occasionally have acute proctitis manifested by anorectal pain or pruritus, tenesmus, purulent rectal discharge, and rectal bleeding. Among men who have sex with men (MSM), the frequency of gonococcal infection, including rectal infection, fell by ≥90% throughout the United States in the early 1980s, but a resurgence of gonorrhea among MSM has been documented in several cities since the 1990s. Gonococcal isolates from the rectum of MSM tend to be more resistant to antimicrobial agents than are gonococcal isolates from other sites. Gonococcal isolates with a mutation in mtrR (multiple transferable resistance repressor) or in the promoter region of the gene that encodes for this transcriptional repressor develop increased resistance to antimicrobial hydrophobic agents such as bile acids and fatty acids in feces and thus are found with increased frequency in MSM. This situation may have been responsible for higher rates of failure of treatment for rectal gonorrhea with older regimens consisting of penicillin or tetracyclines.
Pharyngeal Gonorrhea Pharyngeal gonorrhea is usually mild or asymptomatic, although symptomatic pharyngitis does occasionally occur with cervical lymphadenitis. The mode of acquisition is oral-genital sexual exposure, with fellatio being a more efficient means of transmission than cunnilingus. In certain female adolescent populations in the United States, pharyngeal gonorrhea has become as common as genital gonorrhea. Most cases resolve spontaneously, and transmission from the pharynx to sexual contacts is rare. Pharyngeal infection almost always coexists with genital infection. Swabs from the pharynx should be plated directly onto gonococcal selective media. Pharyngeal colonization with Neisseria meningitidis needs to be differentiated from that with other Neisseria species.
Ocular Gonorrhea in Adults Ocular gonorrhea in an adult usually results from autoinoculation of N. gonorrhoeae from an infected genital site. As in genital infection, the manifestations range from severe to occasionally mild or asymptomatic disease. The variability in clinical manifestations may be attributable to differences in the ability of the infecting strain to elicit an inflammatory response. Infection may result in a markedly swollen eyelid, severe hyperemia and chemosis, and a profuse purulent discharge. The massively inflamed conjunctiva may be draped over the cornea and limbus. Lytic enzymes from the infiltrating PMNs occasionally cause corneal ulceration and rarely cause perforation.
Prompt recognition and treatment of this condition are of paramount importance. Gram’s stain and culture of the purulent discharge establish the diagnosis. Genital cultures should also be performed.
Gonorrhea in Pregnant Women, Neonates, and Children Gonorrhea in pregnancy can have serious consequences for both the mother and the infant. Recognition of gonorrhea early in pregnancy also identifies a population at risk for other STIs, particularly chlamydial infection, syphilis, and trichomoniasis. The risks of salpingitis and PID—conditions associated with a high rate of fetal loss—are highest during the first trimester. Pharyngeal infection, most often asymptomatic, may be more common during pregnancy because of altered sexual practices. Prolonged rupture of the membranes, premature delivery, chorioamnionitis, funisitis (infection of the umbilical cord stump), and sepsis in the infant (with N. gonorrhoeae detected in the newborn’s gastric aspirate during delivery) are common complications of maternal gonococcal infection at term. Other conditions and microorganisms, including Mycoplasma hominis, Ureaplasma urealyticum, C. trachomatis, and bacterial vaginosis (often accompanied by infection with Trichomonas vaginalis), have been associated with similar complications.
The most common form of gonorrhea in neonates is ophthalmia neonatorum, which results from exposure to infected cervical secretions during parturition. Ocular neonatal instillation of a prophylactic agent (e.g., 1% silver nitrate eye drops or ophthalmic preparations containing erythromycin or tetracycline) prevents ophthalmia neonatorum but is not effective for its treatment, which requires systemic antibiotics. The clinical manifestations are acute and usually begin 2–5 days after birth. An initial nonspecific conjunctivitis with a serosanguineous discharge is followed by tense edema of the eyelids, chemosis, and a profuse, thick, purulent discharge. Corneal ulcerations that result in nebulae or perforation may lead to anterior synechiae, anterior staphyloma, panophthalmitis, and blindness. Infections described at other mucosal sites in infants, including vaginitis, rhinitis, and anorectal infection, are likely to be asymptomatic. Pharyngeal colonization has been demonstrated in 35% of infants with gonococcal ophthalmia, and coughing is the most prominent symptom in these cases. Septic arthritis (see below) is the most common manifestation of systemic infection or DGI in the newborn. The onset usually comes at 3–21 days of age, and polyarticular involvement is common. Sepsis, meningitis, and pneumonia are seen in rare instances.
Any STI in children beyond the neonatal period raises the possibility of sexual abuse. Gonococcal vulvovaginitis is the most common manifestation of gonococcal infection in children beyond infancy. Anorectal and pharyngeal infections are common in these children and are frequently asymptomatic. The urethra, Bartholin’s and Skene’s glands, and the upper genital tract are rarely involved. All children with gonococcal infection should also be evaluated for chlamydial infection, syphilis, and possibly HIV infection.
Gonococcal Arthritis (DGI) DGI (gonococcal arthritis) results from gonococcal bacteremia. In the 1970s, DGI occurred in ~0.5–3% of persons with untreated gonococcal mucosal infection. The lower incidence of DGI at present is probably attributable to a decline in the prevalence of particular strains that are likely to disseminate. DGI strains resist the bactericidal action of human serum and generally do not incite inflammation at genital sites, probably because of limited generation of chemotactic factors. Strains recovered from DGI cases in the 1970s were often of the PorB.1A serotype, were highly susceptible to penicillin, and had special growth requirements—including arginine, hypoxanthine, and uracil—that made the organism more fastidious and more difficult to isolate.
Menstruation is a risk factor for dissemination, and approximately two-thirds of cases of DGI are in women. In about half of affected women, symptoms of DGI begin within 7 days of onset of menses. Complement deficiencies, especially of the components involved in the assembly of the membrane attack complex (C5 through C9), predispose to neisserial bacteremia, and persons with more than one episode of DGI should be screened with an assay for total hemolytic complement activity.
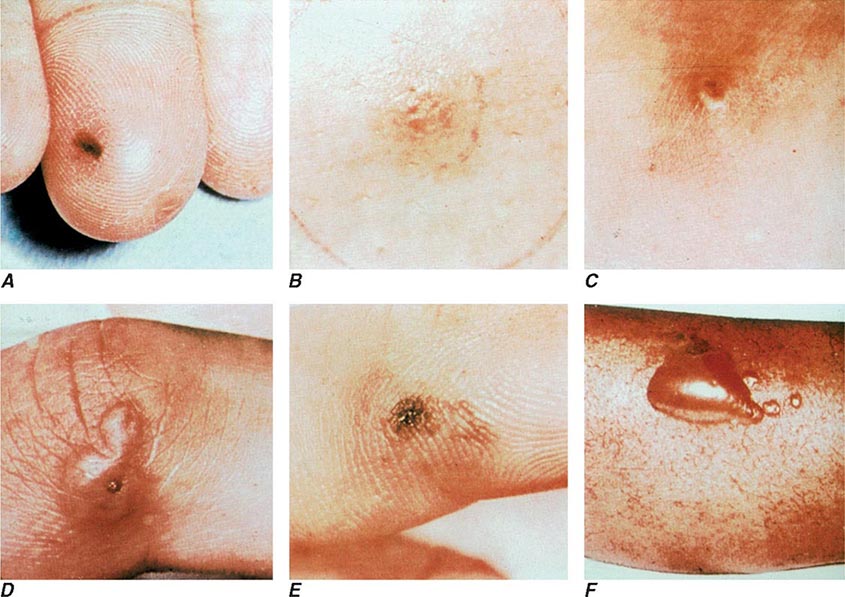
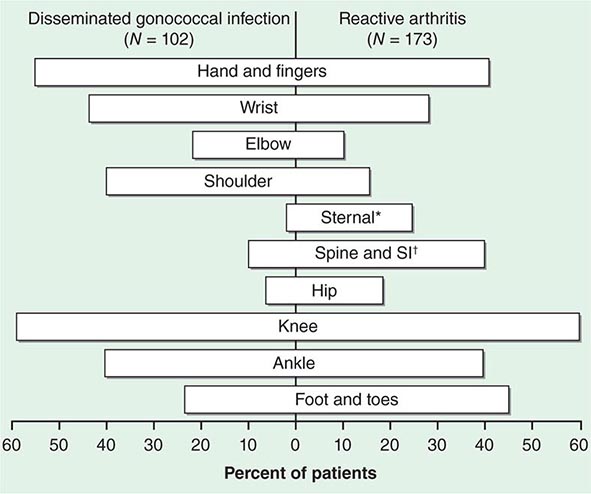
The clinical manifestations of DGI have sometimes been classified into two stages: a bacteremic stage, which is less common today, and a joint-localized stage with suppurative arthritis. A clear-cut progression usually is not evident. Patients in the bacteremic stage have higher temperatures, and chills more frequently accompany their fever. Painful joints are common and often occur together with tenosynovitis and skin lesions. Polyarthralgias usually include the knees, elbows, and more distal joints; the axial skeleton is generally spared. Skin lesions are seen in ~75% of patients and include papules and pustules, often with a hemorrhagic component (Fig. 181-2; see also Fig. 25e-44). Other manifestations of noninfectious dermatitis, such as nodular lesions, urticaria, and erythema multiforme, have been described. These lesions are usually on the extremities and number between 5 and 40. The differential diagnosis of the bacteremic stage of DGI includes reactive arthritis, acute rheumatoid arthritis, sarcoidosis, erythema nodosum, drug-induced arthritis, and viral infections (e.g., hepatitis B and acute HIV infection). The distribution of joint symptoms in reactive arthritis differs from that in DGI (Fig. 181-3), as do the skin and genital manifestations (Chap. 384).
FIGURE 181-2 Characteristic skin lesions in patients with proven gonococcal bacteremia. The lesions are in various stages of evolution. A. Very early petechia on finger. B. Early papular lesion, 7 mm in diameter, on lower leg. C. Pustule with central eschar resulting from early petechial lesion. D. Pustular lesion on finger. E. Mature lesion with central necrosis (black) on hemorrhagic base. F. Bullae on anterior tibial surface. (Reprinted with permission from KK Holmes et al: Disseminated gonococcal infection. Ann Intern Med 74:979, 1971.)
FIGURE 181-3 Distribution of joints with arthritis in 102 patients with disseminated gonococcal infection and 173 patients with reactive arthritis. *Includes the sternoclavicular joints. †SI, sacroiliac joint.
Suppurative arthritis involves one or two joints, most often the knees, wrists, ankles, and elbows (in decreasing order of frequency); other joints occasionally are involved. Most patients who develop gonococcal septic arthritis do so without prior polyarthralgias or skin lesions; in the absence of symptomatic genital infection, this disease cannot be distinguished from septic arthritis caused by other pathogens. The differential diagnosis of acute arthritis in young adults is discussed in Chap. 157. Rarely, osteomyelitis complicates septic arthritis involving small joints of the hand.
Gonococcal endocarditis, although rare today, was a relatively common complication of DGI in the preantibiotic era, accounting for about one-quarter of reported cases of endocarditis. Another unusual complication of DGI is meningitis.
Gonococcal Infections in HIV-Infected Persons The association between gonorrhea and the acquisition of HIV has been demonstrated in several well-controlled studies, mainly in Kenya and Zaire. The nonulcerative STIs enhance the transmission of HIV by three- to fivefold; transmission of HIV-infected immune cells and increased viral shedding by persons with urethritis or cervicitis may contribute (Chap. 226). HIV has been detected by polymerase chain reaction (PCR) more commonly in ejaculates from HIV-positive men with gonococcal urethritis than in those from HIV-positive men with nongonococcal urethritis. PCR positivity diminishes by twofold after appropriate therapy for urethritis. Not only does gonorrhea enhance the transmission of HIV, but it may also increase the individual’s risk for acquisition of HIV. A proposed mechanism is the significantly greater number of CD4+ T lymphocytes and dendritic cells that can be infected by HIV in endocervical secretions from women with nonulcerative STIs than in those from women with ulcerative STIs.
LABORATORY DIAGNOSIS
A rapid diagnosis of gonococcal infection in men may be obtained by Gram’s staining of urethral exudates (Fig. 181-1). The detection of gram-negative intracellular monococci and diplococci is usually highly specific and sensitive in diagnosing gonococcal urethritis in symptomatic males but is only ~50% sensitive in diagnosing gonococcal cervicitis. Samples should be collected with Dacron or rayon swabs. Part of the sample should be inoculated onto a plate of modified Thayer-Martin or other gonococcal selective medium for culture. It is important to process all samples immediately because gonococci do not tolerate drying. If plates cannot be incubated immediately, they can be held safely for several hours at room temperature in candle extinction jars prior to incubation. If processing is to occur within 6 h, transport of specimens may be facilitated by the use of nonnutritive swab transport systems such as Stuart or Amies medium. For longer holding periods (e.g., when specimens for culture are to be mailed), culture media with self-contained CO2-generating systems (such as the JEMBEC or Gono-Pak systems) may be used. Specimens should also be obtained for the diagnosis of chlamydial infection (Chap. 213).
PMNs are often seen in the endocervix on a Gram’s stain, and an abnormally increased number (≥30 PMNs per field in five 1000× oil-immersion microscopic fields) establishes the presence of an inflammatory discharge. Unfortunately, the presence or absence of gram-negative intracellular monococci or diplococci in cervical smears does not accurately predict which patients have gonorrhea, and the diagnosis in this setting should be made by culture or another suitable nonculture diagnostic method. The sensitivity of a single endocervical culture is ~80–90%. If a history of rectal sex is elicited, a rectal wall swab (uncontaminated with feces) should be cultured. A presumptive diagnosis of gonorrhea cannot be made on the basis of gram-negative diplococci in smears from the pharynx, where other Neisseria species are components of the normal flora.
Increasingly, nucleic acid probe tests are being substituted for culture for the direct detection of N. gonorrhoeae in urogenital specimens. A common assay uses a nonisotopic chemiluminescent DNA probe that hybridizes specifically with gonococcal 16S ribosomal RNA; this assay is as sensitive as conventional culture techniques. A disadvantage of non-culture-based assays is that N. gonorrhoeae cannot be grown from the transport systems. Thus a culture-confirmatory test and formal antimicrobial susceptibility testing, if needed, cannot be performed. Nucleic acid amplification tests (NAATs), including the Roche Cobas® Amplicor, Gen-Probe APTIMA COMBO 2®, and BD ProbeTec™ ET, also detect C. trachomatis and are more sensitive than culture identification of either N. gonorrhoeae or C. trachomatis. The Gen-Probe and BD tests offer the advantage that urine samples can be tested with a sensitivity similar to or greater than that obtained when urethral or cervical swab samples are assessed by other non-NAATs or culture, respectively. Several amplification tests are now available on semiautomated or fully automated platforms.
Because of the legal implications, the preferred method for the diagnosis of gonococcal infection in children is a standardized culture. Two positive NAATs, each targeting a different nucleic acid sequence, may be substituted for culture of the cervix or the urethra as legal evidence of infection in children. Although nonculture tests for gonococcal infection have not been approved by the U.S. Food and Drug Administration for use with specimens obtained from the pharynx and rectum of infected children, NAATs from these sites are preferred for diagnostic evaluation in adult victims of suspected sexual abuse, especially if the NAATs have been evaluated by the local laboratory and found to be superior. Cultures should be obtained from the pharynx and anus of both girls and boys, the urethra of boys, and the vagina of girls; cervical specimens are not recommended for prepubertal girls. For boys with a urethral discharge, a meatal specimen of the discharge is adequate for culture. Presumptive colonies of N. gonorrhoeae should be identified definitively by at least two independent methods.
Blood should be cultured in suspected cases of DGI. The use of Isolator blood culture tubes may enhance the yield. The probability of positive blood cultures decreases after 48 h of illness. Synovial fluid should be inoculated into blood culture broth medium and plated onto chocolate agar rather than selective medium because this fluid is not likely to be contaminated with commensal bacteria. Gonococci are infrequently recovered from early joint effusions containing <20,000 leukocytes/μL but may be recovered from effusions containing >80,000 leukocytes/μL. The organisms are seldom recovered from blood and synovial fluid of the same patient.
|
TREATMENT |
GONOCOCCAL INFECTIONS |
Treatment failure can lead to continued transmission and the emergence of antibiotic resistance. The importance of adequate treatment with a regimen that the patient will adhere to cannot be overemphasized. Thus highly effective single-dose regimens have been developed for uncomplicated gonococcal infections. The modified 2010 treatment guidelines for gonococcal infections from the Centers for Disease Control and Prevention (CDC) are summarized in Table 181-1. Rising MICs of cefixime worldwide have led the CDC to discontinue its recommendation of this agent as first-line treatment for uncomplicated gonorrhea. The recommendations for uncomplicated gonorrhea apply to HIV-infected as well as HIV-uninfected patients.
|
RECOMMENDED TREATMENT FOR GONOCOCCAL INFECTIONS: ADAPTED FROM THE 2010 GUIDELINES OF THE CENTERS FOR DISEASE CONTROL AND PREVENTION |
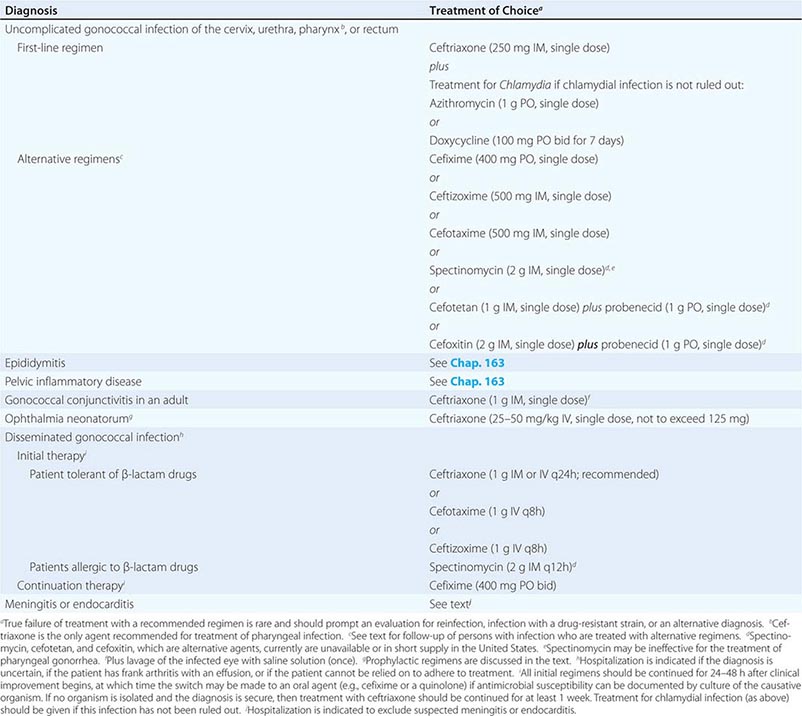
Currently, a single IM dose of the third-generation cephalosporin ceftriaxone is the mainstay of therapy for uncomplicated gonococcal infection of the urethra, cervix, rectum, or pharynx and almost always results in an effective cure. Quinolone-containing regimens are no longer recommended in the United States as first-line treatment because of widespread resistance. A recent multicenter trial of treatment for uncomplicated gonorrhea in the United States showed ≥99.5% efficacy of two combination regimens: (1) gemifloxacin (320 mg, single oral dose) plus azithromycin (2 g, single oral dose) or (2) azithromycin (2 g, single oral dose) plus gentamicin (a single IM dose of 240 mg or, in individuals who weigh ≤45 kg, 5 mg/kg).
Because co-infection with C. trachomatis occurs frequently, initial treatment regimens must also incorporate an agent (e.g., azithromycin or doxycycline) that is effective against chlamydial infection. Pregnant women with gonorrhea, who should not take doxycycline, should receive concurrent treatment with a macrolide antibiotic for possible chlamydial infection. A single 1-g dose of azithromycin, which is effective therapy for uncomplicated chlamydial infections, results in an unacceptably low cure rate (93%) for gonococcal infections and should not be used alone. A single 2-g dose of azithromycin, particularly in the extended-release microsphere formulation, delivers azithromycin to the lower gastrointestinal tract, thereby improving tolerability. Azithromycin is effective against sensitive strains, but this drug is expensive, causes gastrointestinal distress, and is not recommended for routine or first-line treatment of gonorrhea. Spectinomycin has been used as an alternative agent for the treatment of uncomplicated gonococcal infections in penicillin-allergic persons outside the United States but is not currently available in this country. Of note, the limited effectiveness of spectinomycin for the treatment of pharyngeal infection reduces its utility in populations among whom such infection is common, such as MSM.
Persons with uncomplicated infections who receive ceftriaxone do not need a test of cure; however, cultures for N. gonorrhoeae should be performed if symptoms persist after therapy with an established regimen, and any gonococci isolated should be tested for antimicrobial susceptibility. Persons given an alternative regimen should return for a test of cure targeting the infected anatomic site. This test ideally should be a culture. If culture is not readily available and a NAAT is positive, every effort should be made to perform a confirmatory culture. All positive cultures for test of cure should undergo antimicrobial susceptibility testing. Because of high rates of reinfection with N. gonorrhoeae and C. trachomatis within 6 months, repeat testing is recommended 3 months after treatment.
Symptomatic gonococcal pharyngitis is more difficult to eradicate than genital infection. Persons who cannot tolerate ceftriaxone and those in whom quinolones are contraindicated may be treated with spectinomycin if it is available, but this agent results in a cure rate of ≤52%. Persons given spectinomycin should have a pharyngeal sample cultured 3–5 days after treatment as a test of cure. A single 2-g dose of azithromycin may be used in areas where rates of resistance to azithromycin are low.
Treatments for gonococcal epididymitis and PID are discussed in Chap. 163. Ocular gonococcal infections in older children and adults should be managed with a single dose of ceftriaxone combined with saline irrigation of the conjunctivae (both undertaken expeditiously), and patients should undergo a careful ophthalmologic evaluation that includes a slit-lamp examination.
DGI may require higher dosages and longer durations of therapy (Table 181-1). Hospitalization is indicated if the diagnosis is uncertain, if the patient has localized joint disease that requires aspiration, or if the patient cannot be relied on to comply with treatment. Open drainage is necessary only occasionally—e.g., for management of hip infections that may be difficult to drain percutaneously. Nonsteroidal anti-inflammatory agents may be indicated to alleviate pain and hasten clinical improvement of affected joints.
Gonococcal meningitis and endocarditis should be treated in the hospital with high-dose IV ceftriaxone (1–2 g every 12 h); therapy should continue for 10–14 days for meningitis and for at least 4 weeks for endocarditis. All persons who experience more than one episode of DGI should be evaluated for complement deficiency.
PREVENTION AND CONTROL
Condoms, if properly used, provide effective protection against the transmission and acquisition of gonorrhea as well as other infections that are transmitted to and from genital mucosal surfaces. Spermicidal preparations used with a diaphragm or cervical sponges impregnated with nonoxynol 9 offer some protection against gonorrhea and chlamydial infection. However, the frequent use of preparations that contain nonoxynol 9 is associated with mucosal disruption that paradoxically may enhance the risk of HIV infection in the event of exposure. All patients should be instructed to refer sex partners for evaluation and treatment. All sex partners of persons with gonorrhea should be evaluated and treated for N. gonorrhoeae and C. trachomatis infections if their last contact with the patient took place within 60 days before the onset of symptoms or the diagnosis of infection in the patient. If the patient’s last sexual encounter was >60 days before onset of symptoms or diagnosis, the patient’s most recent sex partner should be treated. Partner-delivered medications or prescriptions for medications to treat gonorrhea and chlamydial infection diminish the likelihood of reinfection (or relapse) in the infected patient. In states where it is legal, this approach is an option for partner management. Patients should be instructed to abstain from sexual intercourse until therapy is completed and until they and their sex partners no longer have symptoms. Greater emphasis must be placed on prevention by public health education, individual patient counseling, and behavior modification. Sexually active persons, especially adolescents, should be offered screening for STIs. For male patients, a NAAT on urine or a urethral swab may be used for screening. Preventing the spread of gonorrhea may help reduce the transmission of HIV. No effective vaccine for gonorrhea is yet available, but efforts to test several candidates are under way.
ACKNOWLEDGMENT
The authors acknowledge the contributions of Dr. King K. Holmes and Dr. Stephen A. Morse to the chapter on this subject in earlier editions.
182 |
Haemophilus and Moraxella Infections |
HAEMOPHILUS INFLUENZAE
MICROBIOLOGY
Haemophilus influenzae was first recognized in 1892 by Pfeiffer, who erroneously concluded that the bacterium was the cause of influenza. H. influenzae is a small (1- × 0.3-μm) gram-negative organism of variable shape; thus, it is often described as a pleomorphic coccobacillus. In clinical specimens such as cerebrospinal fluid (CSF) and sputum, H. influenzae frequently stains only faintly with safranin and therefore can easily be overlooked.
H. influenzae grows both aerobically and anaerobically. Its aerobic growth requires two factors: hemin (X factor) and nicotinamide adenine dinucleotide (V factor). These requirements are used in the clinical laboratory to identify the bacterium. Caution must be used to distinguish H. influenzae from H. haemolyticus, a respiratory tract commensal that has identical growth requirements. H. haemolyticus has classically been distinguished from H. influenzae by the hemolysis of the former species on horse blood agar. However, a significant proportion of isolates of H. haemolyticus have now been recognized as nonhemolytic. Analysis of various genotypic and phenotypic markers, including16S ribosomal sequences, superoxide dismutase, outer-membrane protein P6, protein D, and fuculose kinase, can be used to distinguish these two species.
Six major serotypes of H. influenzae have been identified; designated a through f, they are based on antigenically distinct polysaccharide capsules. In addition, some strains lack a polysaccharide capsule and are referred to as nontypable strains. Type b and nontypable strains are the most relevant strains clinically (Table 182-1), although encapsulated strains other than type b can cause disease. H. influenzae was the first free-living organism to have its entire genome sequenced.
|
CHARACTERISTICS OF TYPE b AND NONTYPABLE STRAINS OF HAEMOPHILUS INFLUENZAE |
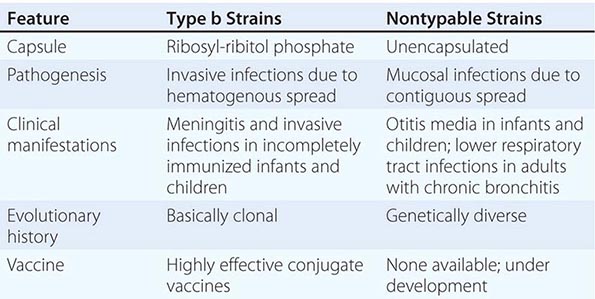
The antigenically distinct type b capsule is a linear polymer composed of ribosyl-ribitol phosphate. Strains of H. influenzae type b (Hib) cause disease primarily in infants and children <6 years of age. Nontypable strains are primarily mucosal pathogens but occasionally cause invasive disease.
EPIDEMIOLOGY AND TRANSMISSION
H. influenzae, an exclusively human pathogen, is spread by airborne droplets or by direct contact with secretions or fomites. Colonization with nontypable H. influenzae is a dynamic process; new strains are acquired and other strains are replaced periodically.
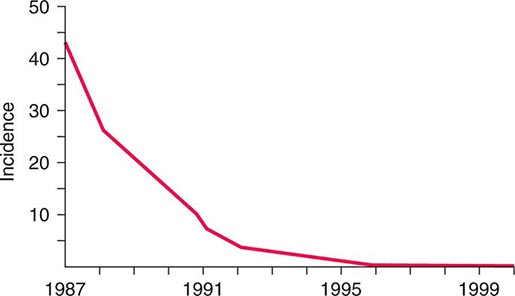
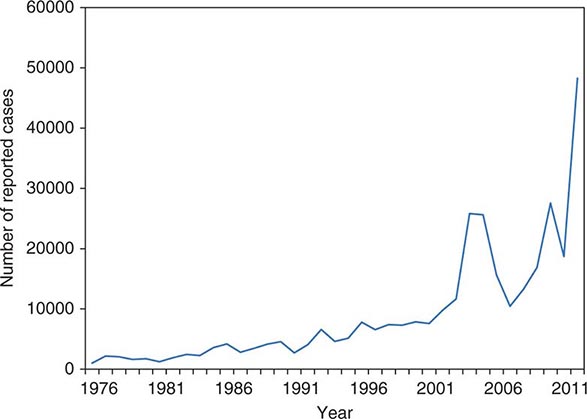
![]() The widespread use of Hib conjugate vaccines in many industrialized countries has resulted in striking decreases in the rate of nasopharyngeal colonization by Hib and in the incidence of Hib infection (Fig. 182-1). However, the majority of the world’s children remain unimmunized. Worldwide, invasive Hib disease occurs predominantly in unimmunized children and in those who have not completed the primary immunization series. Certain groups have a higher incidence of invasive Hib disease than the general population, including African-American children and Native American groups. Although this increased incidence has not yet been accounted for, several factors may be relevant, including age at exposure to the bacterium, socioeconomic conditions, and genetic differences.
The widespread use of Hib conjugate vaccines in many industrialized countries has resulted in striking decreases in the rate of nasopharyngeal colonization by Hib and in the incidence of Hib infection (Fig. 182-1). However, the majority of the world’s children remain unimmunized. Worldwide, invasive Hib disease occurs predominantly in unimmunized children and in those who have not completed the primary immunization series. Certain groups have a higher incidence of invasive Hib disease than the general population, including African-American children and Native American groups. Although this increased incidence has not yet been accounted for, several factors may be relevant, including age at exposure to the bacterium, socioeconomic conditions, and genetic differences.
FIGURE 182-1 Estimated incidence (rate per 100,000) of invasive disease due to Haemophilus influenzae type b among children <5 years of age: 1987–2000. Fewer than 40 cases per year have been reported since 2000. (Data from the Centers for Disease Control and Prevention.)
PATHOGENESIS
Hib strains cause systemic disease by invasion and hematogenous spread from the respiratory tract to distant sites such as the meninges, bones, and joints. The type b polysaccharide capsule is an important virulence factor affecting the bacterium’s ability to avoid opsonization and cause systemic disease.
Nontypable strains cause disease by local invasion of mucosal surfaces. Otitis media results when bacteria reach the middle ear by way of the eustachian tube. Adults with chronic bronchitis experience recurrent lower respiratory tract infection due to nontypable strains. In addition, persistent nontypable H. influenzae colonization of the lower airways of adults with chronic obstructive pulmonary disease (COPD) contributes to the airway inflammation that is a hallmark of the disease. Nontypable strains that cause infection in adults with COPD differ in pathogenic potential and genome content from strains that cause otitis media. In the middle ear, nontypable strains form biofilms. More resistant to host clearance mechanisms and to antibiotics than are planktonic bacteria, biofilms are associated with chronic and recurrent otitis media. The incidence of invasive disease caused by nontypable strains is low. Strains that cause invasive disease are genetically and phenotypically diverse.
IMMUNE RESPONSE
Antibody to the capsule is important in protection from infection by Hib strains. The level of (maternally acquired) serum antibody to the capsular polysaccharide, which is a polymer of polyribitol ribose phosphate (PRP), declines from birth to 6 months of age and, in the absence of vaccination, remains low until ~2 or 3 years of age. The age at the antibody nadir correlates with that of the peak incidence of type b disease. Antibody to PRP then appears partly as a result of exposure to Hib or cross-reacting antigens. Systemic Hib disease is unusual after the age of 6 years because of the presence of protective antibody. Vaccines in which PRP is conjugated to protein carrier molecules have been developed and are now used widely. These vaccines generate an antibody response to PRP in infants and effectively prevent invasive infections in infants and children.
Since nontypable strains lack a capsule, the immune response to infection is directed at noncapsular antigens. These antigens have generated considerable interest as immune targets and potential vaccine components. The human immune response to nontypable strains appears to be strain-specific, a characteristic that accounts in part for the propensity of these strains to cause recurrent otitis media and recurrent exacerbations of chronic bronchitis in immunocompetent hosts.
CLINICAL MANIFESTATIONS
Hib The most serious manifestation of infection with Hib is meningitis (Chap. 164), which primarily affects children <2 years of age. The clinical manifestations of Hib meningitis are similar to those of meningitis caused by other bacterial pathogens. Fever and altered central nervous system function are the most common features at presentation. Nuchal rigidity may or may not be evident. Subdural effusion, the most common complication, is suspected when, despite 2 or 3 days of appropriate antibiotic therapy, the infant has seizures, hemiparesis, or continued obtundation. The overall mortality rate from Hib meningitis is ~5%, and the morbidity rate is high. Of survivors, 6% have permanent sensorineural hearing loss, and about one-fourth have a significant handicap of some type. If more subtle handicaps are sought, up to half of survivors are found to have some neurologic sequelae, such as partial hearing loss and delayed language development.
Epiglottitis (Chap. 44) is a life-threatening Hib infection involving cellulitis of the epiglottis and supraglottic tissues. It can lead to acute upper airway obstruction. Its unique epidemiologic features are its occurrence in an older age group (2–7 years old) than other Hib infections and its absence among Navajo Indians and Alaskan Eskimos. Sore throat and fever rapidly progress to dysphagia, drooling, and airway obstruction. Epiglottitis also occurs in adults.
Cellulitis (Chap. 156) due to Hib occurs in young children. The most common location is on the head or neck, and the involved area sometimes takes on a characteristic bluish-red color. Most patients have bacteremia, and 10% have an additional focus of infection.
Hib causes pneumonia in infants. The infection is clinically indistinguishable from other types of bacterial pneumonia (e.g., pneumococcal pneumonia) except that Hib is more likely to involve the pleura. Several less common invasive conditions can be important clinical manifestations of Hib infection in children. These include osteomyelitis, septic arthritis, pericarditis, orbital cellulitis, endophthalmitis, urinary tract infection, abscesses, and bacteremia without an identifiable focus.
Non–type b encapsulated strains of H. influenzae (types a, c, d, e, and f) are unusual causes of invasive infection manifested predominantly by bacteremia and pneumonia. Most such infections occur in the setting of underlying conditions.
Nontypable H. influenzae Nontypable H. influenzae is the most common bacterial cause of exacerbations of COPD; these exacerbations are characterized by increased cough, sputum production, and shortness of breath. Fever is low-grade, and no infiltrates are evident on chest x-ray. Nontypable strains also cause community-acquired bacterial pneumonia in adults, especially among patients with COPD or AIDS. The clinical features of H. influenzae pneumonia are similar to those of other types of bacterial pneumonia, including pneumococcal pneumonia.
Nontypable H. influenzae is one of the three most common causes of childhood otitis media (the other two being Streptococcus pneumoniae and Moraxella catarrhalis) (Chap. 44). Infants are febrile and irritable, while older children report ear pain. Symptoms of viral upper respiratory infection often precede otitis media. The diagnosis is made by pneumatic otoscopy. An etiologic diagnosis, although not routinely sought, can be established by tympanocentesis and culture of middle-ear fluid. Clinical features associated with H. influenzae otitis media include a history of recurrent episodes, treatment failure, concomitant conjunctivitis, bilateral otitis media, and recent antimicrobial therapy. The increasing use of pneumococcal polysaccharide conjugate vaccines in infants is resulting in a relative increase in the proportion of otitis media cases that are caused by H. influenzae.
Nontypable H. influenzae also causes puerperal sepsis and is an important cause of neonatal bacteremia. These nontypable strains, which are closely related to H. haemolyticus, tend to be of biotype IV and cause invasive disease after colonizing the female genital tract.
Nontypable H. influenzae causes sinusitis (Chap. 44) in adults and children. In addition, the bacterium is a less common cause of various invasive infections. These infections include empyema, adult epiglottitis, pericarditis, cellulitis, septic arthritis, osteomyelitis, endocarditis, cholecystitis, intraabdominal infections, urinary tract infections, mastoiditis, aortic graft infection, and bacteremia without a detectable focus. While most H. influenzae invasive infections in countries where Hib vaccines are used widely are caused by nontypable strains, there is no convincing evidence of an increased incidence of infection by nontypable H. influenzae as a result of use of Hib vaccines. Continued monitoring will be important. Many patients with H. influenzae bacteremia have an underlying condition, such as HIV infection, cardiopulmonary disease, alcoholism, or cancer.
DIAGNOSIS
The most reliable method for establishing a diagnosis of Hib infection is recovery of the organism in culture. The presence of gram-negative coccobacilli in Gram-stained CSF is strong evidence for Hib meningitis. Recovery of the organism from CSF confirms the diagnosis. Cultures of other normally sterile body fluids, such as blood, joint fluid, pleural fluid, pericardial fluid, and subdural effusion, are confirmatory in other infections.
Detection of PRP is an important adjunct to culture in rapid diagnosis of Hib meningitis. Immunoelectrophoresis, latex agglutination, coagglutination, and enzyme-linked immunosorbent assay are effective in detecting PRP. These assays are particularly helpful when patients have received prior antimicrobial therapy and thus are especially likely to have negative cultures.
Because nontypable H. influenzae is primarily a mucosal pathogen, it is a component of a mixed flora; thus etiologic diagnosis is challenging. Nontypable H. influenzae infection is strongly suggested by the predominance of gram-negative coccobacilli among abundant polymorphonuclear leukocytes in a Gram-stained sputum specimen from a patient in whom pneumonia is suspected. Although bacteremia is detectable in a small proportion of patients with pneumonia due to nontypable H. influenzae, most such patients have negative blood cultures.
A diagnosis of otitis media is based on the detection by pneumatic otoscopy of fluid in the middle ear. An etiologic diagnosis requires tympanocentesis but is not routinely sought. An invasive procedure is also required to determine the etiology of sinusitis; thus, treatment is often empirical once the diagnosis is suspected in light of clinical symptoms and sinus radiographs.
|
TREATMENT |
HAEMOPHILUS INFLUENZAE |
Initial therapy for meningitis due to Hib should consist of a cephalosporin such as ceftriaxone or cefotaxime. For children, the dosage of ceftriaxone is 75–100 mg/kg daily given in two doses 12 h apart. The pediatric dosage of cefotaxime is 200 mg/kg daily given in four doses 6 h apart. Adult dosages are 2 g every 12 h for ceftriaxone and 2 g every 4–6 h for cefotaxime. An alternative regimen for initial therapy is ampicillin (200–300 mg/kg daily in four divided doses) plus chloramphenicol (75–100 mg/kg daily in four divided doses). Therapy should continue for a total of 1–2 weeks.
Administration of glucocorticoids to patients with Hib meningitis reduces the incidence of neurologic sequelae. The presumed mechanism is reduction of the inflammation induced by bacterial cell-wall mediators of inflammation when cells are killed by antimicrobial agents. Dexamethasone (0.6 mg/kg per day intravenously in four divided doses for 2 days) is recommended for the treatment of Hib meningitis in children >2 months of age.
Invasive infections other than meningitis are treated with the same antimicrobial agents. For epiglottitis, the dosage of ceftriaxone is 50 mg/kg daily, and the dosage of cefotaxime is 150 mg/kg daily, given in three divided doses 8 h apart. Epiglottitis constitutes a medical emergency, and maintenance of an airway is critical. The duration of therapy is determined by the clinical response. A course of 1–2 weeks is usually appropriate.
Many infections caused by nontypable strains of H. influenzae, such as otitis media, sinusitis, and exacerbations of COPD, can be treated with oral antimicrobial agents. Approximately 20–35% of nontypable strains produce β-lactamase (with the exact proportion depending on geographic location), and these strains are resistant to ampicillin. Several agents have excellent activity against nontypable H. influenzae, including amoxicillin/clavulanic acid, various extended-spectrum cephalosporins, and the macrolides azithromycin and clarithromycin. Fluoroquinolones are highly active against H. influenzae and are useful in adults with exacerbations of COPD. However, fluoroquinolones are not currently recommended for the treatment of children or pregnant women because of possible effects on articular cartilage.
![]() In addition to β-lactamase production, alteration of penicillin-binding proteins—a second mechanism of ampicillin resistance—has been detected in isolates of H. influenzae. Although rare in the United States, these β-lactamase-negative ampicillin-resistant strains are common in Japan and are increasing in prevalence in Europe. Continued monitoring of the evolving antimicrobial susceptibility patterns of H. influenzae will be important.
In addition to β-lactamase production, alteration of penicillin-binding proteins—a second mechanism of ampicillin resistance—has been detected in isolates of H. influenzae. Although rare in the United States, these β-lactamase-negative ampicillin-resistant strains are common in Japan and are increasing in prevalence in Europe. Continued monitoring of the evolving antimicrobial susceptibility patterns of H. influenzae will be important.
PREVENTION
![]() Vaccination (See also Chap. 148) Two conjugate vaccines that prevent invasive infections with Hib in infants and children are licensed in the United States. In addition to eliciting protective antibody, these vaccines prevent disease by reducing rates of pharyngeal colonization with Hib. The widespread use of conjugate vaccines has dramatically reduced the incidence of Hib disease in developed countries. Even though the manufacture of Hib vaccines is costly, vaccination is cost-effective. The Global Alliance for Vaccines and Immunizations has recognized the underuse of Hib conjugate vaccines.
Vaccination (See also Chap. 148) Two conjugate vaccines that prevent invasive infections with Hib in infants and children are licensed in the United States. In addition to eliciting protective antibody, these vaccines prevent disease by reducing rates of pharyngeal colonization with Hib. The widespread use of conjugate vaccines has dramatically reduced the incidence of Hib disease in developed countries. Even though the manufacture of Hib vaccines is costly, vaccination is cost-effective. The Global Alliance for Vaccines and Immunizations has recognized the underuse of Hib conjugate vaccines.
The disease burden has been reduced in developing countries that have implemented routine vaccination (e.g., The Gambia, Chile). An important obstacle to more widespread vaccination is the lack of data on the epidemiology and burden of Hib disease in many developing countries.
All children should be immunized with an Hib conjugate vaccine, receiving the first dose at ~2 months of age, the rest of the primary series at 2–6 months of age, and a booster dose at 12–15 months of age. Specific recommendations vary for the different conjugate vaccines. The reader is referred to the recommendations of the American Academy of Pediatrics (Chap. 148 and www.cispimmunize.org).
Currently, no vaccines are available specifically for the prevention of disease caused by nontypable H. influenzae. However, a vaccine that contains protein D—a surface protein of H. influenzae—conjugated to pneumococcal polysaccharides is licensed in other countries and is used widely in Europe. The vaccine has shown partial efficacy in preventing H. influenzae otitis media in clinical trials. Additional progress in the development of vaccines against nontypable H. influenzae is anticipated.
Chemoprophylaxis The risk of secondary disease is greater than normal among household contacts of patients with Hib disease. Therefore, all children and adults (except pregnant women) in households with an index case and at least one incompletely immunized contact <4 years of age should receive prophylaxis with oral rifampin. When two or more cases of invasive Hib disease have occurred within 60 days at a child-care facility attended by incompletely vaccinated children, administration of rifampin to all attendees and personnel is indicated, as is recommended for household contacts. Chemoprophylaxis is not indicated in nursery and child-care contacts of a single index case. The reader is referred to the recommendations of the American Academy of Pediatrics.
HAEMOPHILUS DUCREYI
Haemophilus ducreyi is the etiologic agent of chancroid (Chap. 163), a sexually transmitted disease characterized by genital ulceration and inguinal adenitis. In addition to being a cause of morbidity in itself, chancroid is associated with HIV infection because of the role played by genital ulceration in HIV transmission. Chancroid increases the efficiency of transmission of and the degree of susceptibility to HIV infection.
MICROBIOLOGY
H. ducreyi is a highly fastidious coccobacillary gram-negative bacterium whose growth requires × factor (hemin). Although, in light of this requirement, the bacterium has been classified in the genus Haemophilus, DNA homology and chemotaxonomic studies have established substantial differences between H. ducreyi and other Haemophilus species. Taxonomic reclassification of the organism is likely in the future but awaits further study. Ulcers contain predominantly T cells. The fact that patients who have had chancroid may have repeated infections indicates that infection does not confer protection.
EPIDEMIOLOGY AND PREVALENCE
![]() The prevalence of chancroid has declined in the United States and worldwide. However, prevalence data must be interpreted with caution because of the difficulty of establishing a diagnosis. The infection appears to be more common in developing countries. Transmission is predominantly heterosexual, and cases in males have outnumbered those in females by ratios of 3:1 to 25:1 during outbreaks. Contact with commercial sex workers and illicit drug use are strongly associated with chancroid.
The prevalence of chancroid has declined in the United States and worldwide. However, prevalence data must be interpreted with caution because of the difficulty of establishing a diagnosis. The infection appears to be more common in developing countries. Transmission is predominantly heterosexual, and cases in males have outnumbered those in females by ratios of 3:1 to 25:1 during outbreaks. Contact with commercial sex workers and illicit drug use are strongly associated with chancroid.
CLINICAL MANIFESTATIONS AND DIFFERENTIAL DIAGNOSIS
Infection is acquired as the result of a break in the epithelium during sexual contact with an infected individual. After an incubation period of 4–7 days, the initial lesion—a papule with surrounding erythema—appears. In 2 or 3 days, the papule evolves into a pustule, which spontaneously ruptures and forms a sharply circumscribed ulcer that generally is not indurated (Fig. 182-2). The ulcers are painful and bleed easily; little or no inflammation of the surrounding skin is evident. Approximately half of patients develop enlarged, tender inguinal lymph nodes, which frequently become fluctuant and spontaneously rupture. Patients usually seek medical care after 1–3 weeks of painful symptoms.
FIGURE 182-2 Chancroid with characteristic penile ulcers and associated left inguinal adenitis (bubo).
The presentation of chancroid does not usually include all of the typical clinical features and is sometimes atypical. Multiple ulcers can coalesce to form giant ulcers. Ulcers can appear and then resolve, with inguinal adenitis (Fig. 182-2) and suppuration following 1–3 weeks later; this clinical picture can be confused with that of lymphogranuloma venereum (Chap. 213). Multiple small ulcers can resemble folliculitis. Other differential diagnostic considerations include the various infections causing genital ulceration, such as primary syphilis, secondary syphilis (condyloma latum), genital herpes, and donovanosis. In rare cases, chancroid lesions become secondarily infected with bacteria; the result is extensive inflammation.
DIAGNOSIS
Clinical diagnosis of chancroid is often inaccurate, and laboratory confirmation should be attempted in suspected cases. An accurate diagnosis of chancroid relies on culture of H. ducreyi from the lesion or from an aspirate of suppurative lymph nodes. Since the organism can be difficult to grow, the use of selective and supplemented media is necessary. No polymerase chain reaction assay for H. ducreyi is commercially available; such tests can be performed by Clinical Laboratory Improvement Amendment (CLIA)–certified clinical laboratories that have developed their own assays.
A probable diagnosis of chancroid can be made when the following criteria are met: (1) one or more painful genital ulcers; (2) no evidence of Treponema pallidum infection by dark-field examination of ulcer exudate or by a negative serologic test for syphilis performed at least 7 days after ulcer onset; (3) a typical clinical presentation for chancroid; and (4) a negative test for herpes simplex virus in the ulcer exudate.
|
TREATMENT |
HAEMOPHILUS DUCREYI |
Treatment regimens recommended by the Centers for Disease Control and Prevention include (1) a single 1-g oral dose of azithromycin; (2) ceftriaxone (250 mg intramuscularly in a single dose); (3) ciprofloxacin (500 mg by mouth twice a day for 3 days); and (4) erythromycin base (500 mg by mouth three times a day for 7 days). Isolates from patients who do not respond promptly to treatment should be tested for antimicrobial resistance. In patients with HIV infection, healing may be slow and longer courses of treatment may be necessary. Clinical treatment failure in HIV-seropositive patients may reflect co-infection, especially with herpes simplex virus. Contacts of patients with chancroid should be identified and treated, whether or not symptoms are present, if they have had sexual contact with the patient during the 10 days preceding the patient’s onset of symptoms.
MORAXELLA CATARRHALIS
MICROBIOLOGY
M. catarrhalis is an unencapsulated gram-negative diplococcus whose ecologic niche is the human respiratory tract. The organism was initially designated Micrococcus catarrhalis. Its name was changed to Neisseria catarrhalis in 1970 because of phenotypic similarities to commensal Neisseria species. On the basis of more rigorous analysis of genetic relatedness, Moraxella catarrhalis is now the widely accepted name for this species.
EPIDEMIOLOGY
![]() Nasopharyngeal colonization by M. catarrhalis is common in infancy, with colonization rates ranging between 33% and 100% and depending on geographic location. Several factors probably account for this geographic variation, including living conditions, day-care attendance, hygiene, household smoking, and population genetics. The prevalence of colonization decreases steadily with age.
Nasopharyngeal colonization by M. catarrhalis is common in infancy, with colonization rates ranging between 33% and 100% and depending on geographic location. Several factors probably account for this geographic variation, including living conditions, day-care attendance, hygiene, household smoking, and population genetics. The prevalence of colonization decreases steadily with age.
The widespread use of pneumococcal conjugate vaccines in some countries has resulted in alterations in patterns of nasopharyngeal colonization in resident populations. A relative increase in colonization by nonvaccine pneumococcal serotypes, nontypable H. influenzae, and M. catarrhalis has occurred. These changes in colonization patterns may be altering the distribution of pathogens of both otitis media and sinusitis in children.
PATHOGENESIS
M. catarrhalis causes mucosal infections of the respiratory tract by contiguous spread from its colonizing site in the upper airway. A preceding viral upper respiratory tract infection is a common inciting event for otitis media. In exacerbations of COPD, the acquisition of new strains is critical for pathogenesis. Strains exhibit substantial genetic diversity and differences in virulence properties.
The expression of several adhesin molecules with differing specificities for various host cell receptors reflects the importance of adherence to the respiratory epithelial surface in the pathogenesis of infection. M. catarrhalis invades multiple cell types. Its intracellular residence in lymphoid tissue provides a potential reservoir for persistence in the human respiratory tract. Like many gram-negative bacteria, M. catarrhalis sheds vesicles into the surrounding environment. The vesicles are internalized by host cells and mediate several virulence mechanisms, including induction of inflammation and delivery of β-lactamase, that can promote the survival of co-pathogens.
CLINICAL MANIFESTATIONS
In children, M. catarrhalis causes predominantly mucosal infections when the bacterium migrates from the nasopharynx to the middle ear or the sinuses (Chap. 44). The inciting event for both otitis media and sinusitis is often a preceding viral infection. Overall, cultures of middle-ear fluid obtained by tympanocentesis indicate that M. catarrhalis causes 15–20% of cases of acute otitis media. Acute otitis media caused by M. catarrhalis or nontypable H. influenzae is clinically milder than otitis media caused by S. pneumoniae, with less fever and a lower prevalence of a red bulging tympanic membrane. However, substantial overlap makes it impossible to predict etiology in an individual child on the basis of clinical features.
A small proportion of viral upper respiratory tract infections are complicated by bacterial sinusitis. Cultures of sinus puncture aspirates show that M. catarrhalis accounts for ~20% of cases of acute bacterial sinusitis in children and for a smaller proportion in adults.
M. catarrhalis is a common cause of exacerbations in adults with COPD. The bacterium has been overlooked in this clinical setting because it has long been considered to be a commensal and because it is easily mistaken for commensal Neisseria species in cultures of respiratory secretions (see “Diagnosis,” below). Several independent lines of evidence have established M. catarrhalis as a pathogen in COPD. These include (1) the demonstration of M. catarrhalis in the lower airways during exacerbations, (2) the association of exacerbation with acquisition of new strains, (3) elevations of inflammatory markers in association with M. catarrhalis, and (4) the development of specific immune responses following infection. M. catarrhalis is the second most common bacterial cause of COPD exacerbations (after H. influenzae), as shown in a 10-year prospective study; the distribution of exacerbations associated with new-strain acquisitions is shown in Fig. 182-3. Not included are culture-negative cases or cases from which a pathogen had been previously isolated. With the application of rigorous clinical criteria for defining the etiology of exacerbations (both culture-positive and culture-negative), ~10% of all exacerbations in the same study were caused by M. catarrhalis. The clinical features of an exacerbation due to M. catarrhalis are similar to those of exacerbations due to other bacterial pathogens, including H. influenzae and S. pneumoniae. The cardinal symptoms are cough with increased sputum production, sputum purulence, and dyspnea in comparison with baseline symptoms.
FIGURE 182-3 Cumulative results of a prospective study (1994–2004) of bacterial infection in chronic obstructive pulmonary disease (COPD) showing etiology of exacerbations. The numbers of exacerbations shown indicate the acquisition of a new strain simultaneous with clinical symptoms of an exacerbation. NTHI, nontypable H. influenzae; M.cat, M. catarrhalis; S.pn, Streptococcus pneumoniae; PA, Pseudomonas aeruginosa. (Adapted from TF Murphy, GI Parameswaran: Clin Infect Dis 49:124, 2009, with permission. © 2009 Infectious Diseases Society of America.)
Pneumonia due to M. catarrhalis occurs in the elderly, particularly in the setting of underlying cardiopulmonary disease, but is infrequent. Invasive infections, such as bacteremia, endocarditis, neonatal meningitis, and septic arthritis, are rare.
DIAGNOSIS
Tympanocentesis is required for etiologic diagnosis of otitis media, but this procedure is not performed routinely. Therefore, treatment of otitis media is generally empirical. Similarly, an etiologic diagnosis of sinusitis requires an invasive procedure and thus is usually not available to the clinician. Isolation of M. catarrhalis from an expectorated sputum sample from an adult experiencing clinical symptoms of an exacerbation is suggestive, but not diagnostic, of M. catarrhalis as the cause.
Upon culture, colonies of M. catarrhalis resemble commensal neisseriae that are part of the normal upper airway flora. As mentioned above, the difficulty in distinguishing colonies of M. catarrhalis from neisserial colonies in cultures of respiratory secretions explains in part why M. catarrhalis has been overlooked as a pathogen. In contrast to these Neisseria species, M. catarrhalis colonies can be slid across the agar surface without disruption (the “hockey puck sign”). In addition, after 48 h of growth, M. catarrhalis colonies take on a pink color and tend to be larger than neisserial colonies. A variety of biochemical tests can distinguish M. catarrhalis from neisseriae. Kits that rely on these biochemical reactions are commercially available.
|
TREATMENT |
MORAXELLA CATARRHALIS |
M. catarrhalis rapidly acquired β-lactamases during the 1970s and 1980s; antimicrobial susceptibility patterns have remained relatively stable since that time, with >90% of strains now producing β-lactamase and thus resistant to amoxicillin. Otitis media in children and exacerbations of COPD in adults are generally managed empirically with antimicrobial agents that are active against S. pneumoniae, H. influenzae, and M. catarrhalis. Most strains of M. catarrhalis are susceptible to amoxicillin/clavulanic acid, extended-spectrum cephalosporins, newer macrolides (azithromycin, clarithromycin), trimethoprim-sulfamethoxazole, and fluoroquinolones.
183e |
Infections Due to the HACEK Group and Miscellaneous Gram-Negative Bacteria |
THE HACEK GROUP
HACEK organisms are a group of fastidious, slow-growing, gram-negative bacteria whose growth requires an atmosphere of carbon dioxide. Species belonging to this group include several Haemophilus species, Aggregatibacter (formerly Actinobacillus) species, Cardio-bacterium hominis, Eikenella corrodens, and Kingella kingae. HACEK bacteria normally reside in the oral cavity and have been associated with local infections in the mouth. They are also known to cause severe systemic infections—most often bacterial endocarditis, which can develop on either native or prosthetic valves (Chap. 155).
In large series, 0.8–6% of cases of infective endocarditis are attributable to HACEK organisms, most often Aggregatibacter species, Haemophilus species, and C. hominis. Invasive infection typically occurs in patients with a history of cardiac valvular disease, often in the setting of a recent dental procedure or nasopharyngeal infection. The aortic and mitral valves are most commonly affected. Compared with non-HACEK endocarditis, HACEK endocarditis occurs in younger patients and is more frequently associated with embolic, vascular, and immunologic manifestations but less commonly associated with congestive heart failure. The clinical course of HACEK endocarditis tends to be subacute, particularly with Aggregatibacter or Cardiobacterium. However, K. kingae endocarditis may have a more aggressive presentation. Systemic embolization is common. The overall prevalence of major emboli associated with HACEK endocarditis ranges from 28% to 71% in different series. On echocardiography, valvular vegetations are seen in up to 85% of patients. Aggregatibacter and Haemophilus species cause mitral valve vegetations most often; Cardiobacterium is associated with aortic valve vegetations. The microbiology laboratory should be alerted when a HACEK organism is being considered; however, most cultures that ultimately yield a HACEK organism become positive within the first week, especially with improved culture systems such as BACTEC. In addition, polymerase chain reaction (PCR) techniques (e.g., of cardiac valves) and other tools, such as matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry performed directly on agar colonies, are facilitating the diagnosis of HACEK infections. Because of the organisms’ slow growth, antimicrobial testing may be difficult, and β-lactamase production may not be detected. Resistance is most commonly noted in Haemophilus and Aggregatibacter species. E-test methodology may increase the accuracy of susceptibility testing. The overall prognosis in HACEK endocarditis is excellent and significantly better than that in endocarditis caused by non-HACEK pathogens.
Haemophilus Species Haemophilus parainfluenzae is the most common species isolated from cases of HACEK endocarditis. Of patients with HACEK endocarditis due to Haemophilus species, 60% have been ill for <2 months before presentation, and 19–50% develop congestive heart failure. Mortality rates as high as 30–50% were reported in older series; however, more recent studies have documented mortality rates of <5%. H. parainfluenzae has been isolated from other infections, such as meningitis; brain, dental, pelvic, and liver abscess; pneumonia; urinary tract infection; and septicemia.
Aggregatibacter Species The species of Aggregatibacter that most frequently cause infective endocarditis are A. actinomycetemcomitans, A. (formerly Haemophilus) aphrophilus, and A. paraphrophilus. Aggregatibacter is associated with prosthetic valve endocarditis more often than are Haemophilus species. A. actinomycetemcomitans can be isolated from soft tissue infections and abscesses in association with Actinomyces israelii. Typically, patients who develop endocarditis with Aggregatibacter have periodontal disease or have recently undergone dental procedures in the setting of underlying cardiac valvular damage. The disease is insidious; patients may be sick for several months before diagnosis. Frequent complications include embolic phenomena, congestive heart failure, and renal failure. A. actinomycetemcomitans has been isolated from patients with brain abscess, meningitis, endoph-thalmitis, parotitis, osteomyelitis, urinary tract infection, pneumonia, and empyema, among other infections, while A. aphrophilus is often associated with bone and joint infection.
Cardiobacterium hominis C. hominis primarily causes endocarditis in patients with underlying valvular heart disease or with prosthetic valves. This organism most frequently affects the aortic valve. Many patients have signs and symptoms of long-standing infection before diagnosis, with evidence of arterial embolization, vasculitis, cerebrovascular accidents, immune complex glomerulonephritis, or arthritis at presentation. Embolization, mycotic aneurysms, and congestive heart failure are common complications. A second species, C. valvarum, has now been described in association with endocarditis.
Eikenella corrodens E. corrodens is most frequently recovered from sites of infection in conjunction with other bacterial species. Clinical sources of E. corrodens include sites of human bite wounds (clenched-fist injuries), endocarditis, soft tissue infections, osteomyelitis, head and neck infections, respiratory infections, chorioamnionitis, gynecologic infections associated with intrauterine devices, meningitis, brain abscesses, and visceral abscesses. This organism is the least common cause of HACEK endocarditis.
Kingella kingae Because of improved microbiologic methodology and molecular methods such as real-time PCR, the isolation of K. kingae is increasingly common. Inoculation of clinical specimens (e.g., synovial fluid) into aerobic blood culture bottles enhances recovery of this organism. More than half of cases of K. kingae infection are bone and joint infections; the majority of the remaining infections are infective endocarditis and bacteremia. PCR studies of joint fluid can identify K. kingae in culture-negative cases. Some studies now demonstrate that K. kingae has surpassed Staphylococcus aureus as the leading cause of septic arthritis in children. Invasive K. kingae infections with bacteremia are associated with upper respiratory tract infections and stomatitis in 80% of cases. Rates of oropharyngeal colonization with K. kingae are highest in the first 3 years of life (detected in ~10% of children), coinciding with an increased incidence of skeletal infections due to this organism. K. kingae bacteremia can present with a petechial rash similar to that seen in Neisseria meningitidis sepsis.
Infective endocarditis, unlike other infections with K. kingae, occurs in older children and adults. The majority of patients have preexisting valvular disease. There is a high incidence of complications, including arterial emboli, cerebrovascular accidents, tricuspid insufficiency, and congestive heart failure with cardiovascular collapse.
|
TREATMENT |
HACEK ENDOCARDITIS |
(Table 183e-1) Ceftriaxone (2 g/d) is first-line therapy for HACEK endocarditis. Data on the use of levofloxacin (500–750 mg/d) for HACEK endocarditis remain limited, but this drug can be considered an alternative for treatment of patients intolerant of β-lactam therapy. Of note, Eikenella is resistant to clindamycin, metronidazole, and aminoglycosides.
|
TREATMENT OF INFECTIONS CAUSED BY HACEK GROUP ORGANISMS |
Native-valve endocarditis should be treated for 4 weeks with antibiotics, whereas prosthetic-valve endocarditis requires 6 weeks of therapy. The cure rates for HACEK prosthetic-valve endocarditis appear to be high. Unlike prosthetic-valve endocarditis caused by other gram-negative organisms, HACEK endocarditis is often cured with antibiotic treatment alone—i.e., without surgical intervention.
OTHER GRAM-NEGATIVE BACTERIA
Achromobacter xylosoxidans Achromobacter (previously Alcaligenes) xylosoxidans is probably part of the endogenous intestinal flora and has been isolated from a variety of water sources, including well water, IV fluids, and humidifiers. Immunocompromised hosts, including patients with cancer and postchemotherapy neutropenia, cirrhosis, chronic renal failure, and cystic fibrosis, are at increased risk. Nosocomial outbreaks and pseudo-outbreaks of A. xylosoxidans infection have been attributed to contaminated fluids, and clinical illness has been associated with isolates from many sites, including blood (often in the setting of intravascular devices). Community-acquired A. xylosoxidans bacteremia usually occurs in the setting of pneumonia. Metastatic skin lesions are present in one-fifth of cases. The reported mortality rate is as high as 67%—a figure similar to rates for other bacteremic gram-negative pneumonias.
|
TREATMENT |
ACHROMOBACTER XYLOSOXIDANS INFECTIONS |
(Table 183e-2) Treatment is based on in vitro susceptibility testing of all clinically relevant isolates; multidrug resistance is common. Meropenem, tigecycline, and colistin are typically the most active agents.
|
TREATMENT OPTIONS FOR OTHER SELECTED GRAM-NEGATIVE BACTERIA |
Aeromonas Species More than 85% of Aeromonas infections are caused by A. hydrophila, A. caviae, and A. veronii biovar sobria. Aeromonas proliferates in potable water, freshwater, and soil. It remains controversial whether Aeromonas is a cause of bacterial gastroenteritis; asymptomatic colonization of the intestinal tract with Aeromonas occurs frequently. However, rare cases of hemolytic-uremic syndrome following bloody diarrhea have been shown to be secondary to the presence of Aeromonas.
Aeromonas causes sepsis and bacteremia in infants with multiple medical problems and in immunocompromised hosts, particularly those with cancer or hepatobiliary disease. A. caviae is associated with health care–related bacteremia. Aeromonas infection and sepsis can occur in patients with trauma (including severe trauma with myonecrosis) and in burn patients exposed to Aeromonas by environmental (freshwater or soil) contamination of their wounds. Reported mortality rates range from 25% among immunocompromised adults with sepsis to >90% among patients with myonecrosis. Aeromonas can produce ecthyma gangrenosum (hemorrhagic vesicles surrounded by a rim of erythema with central necrosis and ulceration; see Fig. 25e-35) resembling the lesions seen in Pseudomonas aeruginosa infection. This organism causes nosocomial infections related to catheters, surgical incisions, or use of leeches. Other manifestations include necrotizing fasciitis, meningitis, peritonitis, pneumonia, and ocular infections.
|
TREATMENT |
AEROMONAS INFECTIONS |
(Table 183e-2) Aeromonas species are generally susceptible to fluoroquinolones (e.g., ciprofloxacin at a dosage of 500 mg every 12 h PO or 400 mg every 12 h IV), third- and fourth-generation cephalosporins, carbapenems, and aminoglycosides, but resistance has been described to all those agents. Because Aeromonas can produce various β-lactamases, including carbapenemases, susceptibility testing must be used to guide therapy. Antibiotic prophylaxis (e.g., with ciprofloxacin) is indicated when medicinal leeches are used.
Capnocytophaga Species This genus of fastidious, fusiform, gram-negative coccobacilli is facultatively anaerobic and requires an atmosphere enriched in carbon dioxide for optimal growth. C. ochracea, C. gingivalis, C. haemolytica, and C. sputigena have been associated with sepsis in immunocompromised hosts, particularly neutropenic patients with oral ulcerations. These species have been isolated from many other sites as well, usually as part of a polymicrobial infection. Most Capnocytophaga infections are contiguous with the oropharynx (e.g., periodontal disease, respiratory tract infections, cervical abscesses, and endophthalmitis).
C. canimorsus and C. cynodegmi are endogenous to the canine mouth (Chap. 167e). Patients infected with these species frequently have a history of dog bites or of canine exposure without scratches or bites. Asplenia, glucocorticoid therapy, and alcohol abuse are predisposing conditions that can be associated with severe sepsis with shock and disseminated intravascular coagulation. Patients typically have a petechial rash that can progress from purpuric lesions to gangrene. Meningitis, endocarditis, cellulitis, osteomyelitis, and septic arthritis also have been associated with these organisms.
|
TREATMENT |
CAPNOCYTOPHAGA INFECTIONS |
(Table 183e-2) Because of increasing β-lactamase production, a penicillin derivative plus a β-lactamase inhibitor—such as ampicillin/sulbactam (1.5–3.0 g of ampicillin every 6 h)—is currently recommended for empirical treatment of infections caused by Capnocytophaga species. If the isolate is known to be susceptible, infections with C. canimorsus should be treated with penicillin (12–18 million units every 4 h). Capnocytophaga is also susceptible to clindamycin (600–900 mg every 6–8 h). This regimen or ampicillin/sulbactam should be given prophylactically to asplenic patients who have sustained dog-bite injuries.
Elizabethkingia/Chryseobacterium Species Elizabethkingia meningoseptica (formerly Chryseobacterium meningosepticum) is an important cause of nosocomial infections, including outbreaks due to contaminated fluids (e.g., disinfectants and aerosolized antibiotics) and sporadic infections due to indwelling devices, feeding tubes, and other fluid-associated apparatuses. Nosocomial E. meningoseptica infection usually involves neonates or patients with underlying immunosuppression (e.g., related to malignancy or diabetes). E. meningoseptica has been reported to cause meningitis (primarily in neonates), pneumonia, sepsis, endocarditis, bacteremia, and soft tissue infections. Most published reports have originated from Taiwan. Chryseobacterium indologenes has caused bacteremia, sepsis, and pneumonia, typically in immunocompromised patients with indwelling devices.
|
TREATMENT |
ELIZABETHKINGIA/CHRYSEOBACTERIUM INFECTIONS |
(Table 183e-2) These organisms are often susceptible to fluoroquinolones and trimethoprim-sulfamethoxazole. They may be susceptible to β-lactam/β-lactamase inhibitor combinations such as piperacillin/tazobactam but can possess extended-spectrum β-lactamases and metallo-β-lactamases. Susceptibility testing should be performed.
Pasteurella multocida P. multocida is a bipolar-staining, gram-negative coccobacillus that colonizes the respiratory and gastrointestinal tracts of domestic animals; oropharyngeal colonization rates are 70–90% in cats and 50–65% in dogs. P. multocida can be transmitted to humans through bites or scratches, via the respiratory tract from contact with contaminated dust or infectious droplets, or via deposition of the organism on injured skin or mucosal surfaces during licking. Most human infections affect skin and soft tissue; almost two-thirds of these infections are caused by cats. Patients at the extremes of age or with serious underlying disorders (e.g., cirrhosis, diabetes) are at increased risk for systemic manifestations, including meningitis, peritonitis, osteomyelitis and septic arthritis, endocarditis, and septic shock, but cases have also occurred in healthy individuals of all ages. If inhaled, P. multocida can cause acute respiratory tract infection, particularly in patients with underlying sinus and pulmonary disease.
|
TREATMENT |
PASTEURELLA MULTOCIDA INFECTIONS |
P. multocida is susceptible to penicillin, ampicillin, ampicillin/sulbactam, second- and third-generation cephalosporins, tetracyclines, and fluoroquinolones. β-lactamase-producing strains have been reported.
MISCELLANEOUS ORGANISMS
Rhizobium (formerly Agrobacterium) radiobacter has usually been associated with infection in the presence of medical devices, including intravascular catheter–related infections, prosthetic-joint and prosthetic-valve infections, and peritonitis caused by dialysis catheters. Cases of endophthalmitis after cataract surgery also have been described. Most R. radiobacter infections occur in immunocompromised hosts, especially individuals with malignancy or HIV infection. Strains are usually susceptible to fluoroquinolones, third- and fourth-generation cephalosporins, and carbapenems (Table 183e-2).
Shewanella putrefaciens and S. algae are ubiquitous organisms found primarily in seawater. Devitalized tissues can become colonized with Shewanella and serve as a nidus for systemic infection. Shewanella species cause skin and soft tissue infections, chronic ulcers of the lower extremities, ear infections, biliary tract infections, pneumonia, necrotizing fasciitis, bacteremia, and sepsis. A fulminant course is associated with cirrhosis, diabetes mellitus, malignancy, or other severe underlying conditions. Organisms are often susceptible to fluoroquinolones, third- and fourth-generation cephalosporins, β-lactam/β-lactamase inhibitors, carbapenems, and aminoglycosides (Table 183e-2).
Chromobacterium violaceum has been responsible for life-threatening infections with severe sepsis and metastatic abscesses, particularly in children with defective neutrophil function (e.g., those with chronic granulomatous disease). Ochrobactrum anthropi causes infections related to central venous catheters in compromised hosts; other invasive infections have been described. Other organisms implicated in human infections include Weeksella species; various CDC groups, such as Ve-1 and Ve-2; Flavimonas species; Sphingobacterium species; and Oligella urethralis. The reader is advised to consult subspecialty texts and references for further guidance on these organisms.
184 |
Legionella Infections |
Legionellosis refers to the two clinical syndromes caused by bacteria of the genus Legionella. Pontiac fever is an acute, febrile, self-limited illness that has been serologically linked to Legionella species, whereas Legionnaires’ disease is the designation for pneumonia caused by these species. Legionnaires’ disease was first recognized in 1976, when an outbreak of pneumonia took place at a Philadelphia hotel during an American Legion convention.
MICROBIOLOGY
The family Legionellaceae comprises more than 50 species with more than 70 serogroups. The species L. pneumophila causes 80–90% of human infections and includes at least 16 serogroups; serogroups 1, 4, and 6 are most commonly implicated in human infections. To date, 18 species other than L. pneumophila have been associated with human infections, among which L. micdadei (Pittsburgh pneumonia agent), L. bozemanii, L. dumoffii, and L. longbeachae are the most common. Members of the Legionellaceae are aerobic gram-negative bacilli that do not grow on routine microbiologic media. Buffered charcoal yeast extract (BCYE) agar is the medium used to grow Legionella.
ECOLOGY AND TRANSMISSION
![]() The natural habitats for L. pneumophila are aquatic bodies, including lakes and streams. L. longbeachae has been isolated from natural soil. Commercial potting soil has been suggested as the reservoir for L. longbeachae infections in Australia and New Zealand. Legionella can survive under a wide range of environmental conditions; for example, the organisms can live for years in refrigerated water samples. Natural bodies of water contain only small numbers of legionellae. However, once the organisms enter human-constructed aquatic reservoirs (such as drinking-water systems), they can grow and proliferate. Factors known to enhance colonization by and amplification of legionellae include warm temperatures (25°–42°C) and the presence of scale and sediment. L. pneumophila can form microcolonies within biofilms; its eradication from drinking-water systems requires disinfectants that can penetrate the biofilm. The presence of symbiotic microorganisms, including algae, amebas, ciliated protozoa, and other water-dwelling bacteria, promotes the growth of Legionella. The organisms can invade and multiply within free-living protozoa.
The natural habitats for L. pneumophila are aquatic bodies, including lakes and streams. L. longbeachae has been isolated from natural soil. Commercial potting soil has been suggested as the reservoir for L. longbeachae infections in Australia and New Zealand. Legionella can survive under a wide range of environmental conditions; for example, the organisms can live for years in refrigerated water samples. Natural bodies of water contain only small numbers of legionellae. However, once the organisms enter human-constructed aquatic reservoirs (such as drinking-water systems), they can grow and proliferate. Factors known to enhance colonization by and amplification of legionellae include warm temperatures (25°–42°C) and the presence of scale and sediment. L. pneumophila can form microcolonies within biofilms; its eradication from drinking-water systems requires disinfectants that can penetrate the biofilm. The presence of symbiotic microorganisms, including algae, amebas, ciliated protozoa, and other water-dwelling bacteria, promotes the growth of Legionella. The organisms can invade and multiply within free-living protozoa.
Heavy rainfall and flooding can result in the entry of high numbers of legionellae into water-distribution systems, leading to an upsurge of cases. Large buildings over three stories high are commonly colonized with Legionella. Sporadic community-acquired Legionnaires’ disease has been linked to colonization of hotels, office buildings, factories, and even private homes. Drinking-water systems in hospitals and extended-care facilities have been the source for health care–associated Legionnaires’ disease.
In contrast, cooling towers and evaporative condensers have been overestimated as sources of Legionella causing human illness. Early investigations that implicated cooling towers antedated the discovery that the organism could also exist in drinking water. In many outbreaks attributed to cooling towers, cases of Legionnaires’ disease continued to occur despite disinfection of the towers; drinking water was found to be the actual source. Koch’s postulates have never been fulfilled for Legionella links to cooling tower–associated outbreaks as they have been for hospital-acquired Legionnaires’ disease. Nevertheless, cooling towers have, in rare instances, been implicated in community-acquired outbreaks, including an outbreak in Murcia, Spain. As mentioned above, L. longbeachae infections have been linked to potting soil, but the mode of transmission remains to be clarified.
Multiple modes of transmission of Legionella to humans exist, including aerosolization, aspiration, and direct instillation into the lungs during respiratory tract manipulations. Aspiration is now known to be the predominant mode of transmission, but it is unclear whether Legionella enters the lungs via oropharyngeal colonization or directly via the drinking of contaminated water. Oropharyngeal colonization with Legionella has been demonstrated in patients undergoing transplantation. Nasogastric tubes have been linked to hospital-acquired Legionnaires’ disease; microaspiration of contaminated water was the hypothesized mode of transmission. Surgery with general anesthesia is a known risk factor that is consistent with aspiration. Especially compelling is the reported 30% incidence of postoperative Legionnaires’ disease among patients undergoing head and neck surgery at a hospital with a contaminated water supply; aspiration is a recognized postoperative complication in such cases. One observational study showed that patients with hospital-acquired Legionnaires’ disease underwent endotracheal intubation significantly more often and for a significantly longer duration than patients with hospital-acquired pneumonias of other etiologies.
Aerosolization of Legionella by devices filled with tap water, including whirlpools, nebulizers, and humidifiers, has been linked to cases in patients. An ultrasonic mist machine in the produce section of a grocery store has been the source in community outbreaks. Pontiac fever has been linked to Legionella-containing aerosols from water-using machinery, a cooling tower, air conditioners, and whirlpools.
EPIDEMIOLOGY
![]() Community-Acquired Pneumonia The incidence of Legionnaires’ disease depends on the degree of contamination of the aquatic reservoir, the immune status of the persons exposed to water from that reservoir, the intensity of exposure, and the availability of specialized laboratory tests on which the correct diagnosis can be based. Numerous prospective studies have ranked Legionella among the top four microbial causes of community-acquired pneumonia, finding that it accounts for 2–13% of cases. (Streptococcus pneumoniae, Haemophilus influenzae, and Chlamydia pneumoniae are usually ranked first, second, and third, respectively.) On the basis of a multihospital study of community-acquired pneumonia in Ohio, the Centers for Disease Control and Prevention (CDC) estimated that only 3% of community-acquired cases of Legionnaires’ disease are diagnosed as such. Observational studies of community-acquired pneumonia showed that Legionnaires’ disease was largely unrecognized unless Legionella diagnostic testing was routinely applied to all patients with pneumonia; such studies in Spain and Germany resulted in detection of increased numbers of cases throughout Europe. It is likely that observational studies in Taiwan and Australia will have a similar result, with more cases identified throughout Asia as the index of suspicion rises.
Community-Acquired Pneumonia The incidence of Legionnaires’ disease depends on the degree of contamination of the aquatic reservoir, the immune status of the persons exposed to water from that reservoir, the intensity of exposure, and the availability of specialized laboratory tests on which the correct diagnosis can be based. Numerous prospective studies have ranked Legionella among the top four microbial causes of community-acquired pneumonia, finding that it accounts for 2–13% of cases. (Streptococcus pneumoniae, Haemophilus influenzae, and Chlamydia pneumoniae are usually ranked first, second, and third, respectively.) On the basis of a multihospital study of community-acquired pneumonia in Ohio, the Centers for Disease Control and Prevention (CDC) estimated that only 3% of community-acquired cases of Legionnaires’ disease are diagnosed as such. Observational studies of community-acquired pneumonia showed that Legionnaires’ disease was largely unrecognized unless Legionella diagnostic testing was routinely applied to all patients with pneumonia; such studies in Spain and Germany resulted in detection of increased numbers of cases throughout Europe. It is likely that observational studies in Taiwan and Australia will have a similar result, with more cases identified throughout Asia as the index of suspicion rises.
Hospital-Acquired Pneumonia Legionella is responsible for 10–50% of cases of nosocomial pneumonia when a hospital’s water system is colonized with the organism. The incidence of hospital-acquired Legionnaires’ disease depends on the degree of contamination of drinking water, as defined by the rate of positivity of distal water sites; in contrast, the use of quantitative criteria of the number of colony-forming units per milliliter has proven useless.
![]() Proactive culture of the hospital water supply has increased the detection of hospital-acquired Legionnaires’ disease and simultaneously allowed expeditious diagnosis resulting in early administration of antibiotic therapy. In the early years after its recognition, Legionnaires’ disease was documented primarily in the United States. As diagnostic modalities (especially the urinary antigen test) became more widely used, cases were documented in European hospitals. Likewise, following the enactment of public health guidelines in Taiwan, cases attributable to hospital tap water were found in Taiwanese hospitals. Risk factors for Legionnaires’ disease include cigarette smoking, chronic lung disease, advanced age, prior hospitalization with discharge within 10 days before onset of pneumonia symptoms, and immunosuppression. Immunosuppressive conditions that predispose to Legionnaires’ disease include transplantation, HIV infection, and treatment with glucocorticoids or tumor necrosis factor α antagonists. However, in a large prospective study of community-acquired pneumonia, 28% of patients with Legionnaires’ disease did not have these classic risk factors. Hospital-acquired cases are now being recognized among neonates and immunosuppressed children.
Proactive culture of the hospital water supply has increased the detection of hospital-acquired Legionnaires’ disease and simultaneously allowed expeditious diagnosis resulting in early administration of antibiotic therapy. In the early years after its recognition, Legionnaires’ disease was documented primarily in the United States. As diagnostic modalities (especially the urinary antigen test) became more widely used, cases were documented in European hospitals. Likewise, following the enactment of public health guidelines in Taiwan, cases attributable to hospital tap water were found in Taiwanese hospitals. Risk factors for Legionnaires’ disease include cigarette smoking, chronic lung disease, advanced age, prior hospitalization with discharge within 10 days before onset of pneumonia symptoms, and immunosuppression. Immunosuppressive conditions that predispose to Legionnaires’ disease include transplantation, HIV infection, and treatment with glucocorticoids or tumor necrosis factor α antagonists. However, in a large prospective study of community-acquired pneumonia, 28% of patients with Legionnaires’ disease did not have these classic risk factors. Hospital-acquired cases are now being recognized among neonates and immunosuppressed children.
Pneumonia in Transplant Recipients Transplant recipients appear to be at unusually high risk of Legionella pneumonia. This elevated risk may be due to diagnostic bias, given the extensive workup for opportunistic pathogens with pneumonic symptoms as well as the long-standing immunosuppression in this population of patients. Legionnaires’ disease usually occurs in the 3 months after transplantation. Cavitation is seen on chest radiograph more frequently in transplant recipients, and mortality rates are higher.
Pontiac Fever Pontiac fever occurs in epidemics. The high attack rate (>90%) reflects airborne transmission.
PATHOGENESIS AND IMMUNITY
Legionella enters the lungs through aspiration or direct inhalation. Attachment to host cells is mediated by bacterial type IV pili, heat-shock proteins, a major outer-membrane protein, and complement. Because the organism possesses pili that mediate adherence to respiratory tract epithelial cells, conditions that impair mucociliary clearance, including cigarette smoking, lung disease, or alcoholism, predispose to Legionnaires’ disease.
Both innate and adaptive immune responses play a role in host defense. Toll-like receptors mediate recognition of L. pneumophila in alveolar macrophages and enhance early neutrophil recruitment to the site of infection. Alveolar macrophages phagocytose legionellae by a conventional or a coiling mechanism. After phagocytosis, L. pneumophila evades intracellular killing by inhibiting phagosome–lysosome fusion. Although many legionellae are killed, some proliferate intracellularly until the cells rupture; the bacteria are then phagocytosed again by newly recruited phagocytes, and the cycle begins anew. The role of neutrophils in immunity appears to be minimal: neutropenic patients are not predisposed to Legionnaires’ disease. Although L. pneumophila is susceptible to oxygen-dependent microbiologic systems in vitro, it resists killing by neutrophils. The humoral immune system is active against Legionella. Type-specific IgM and IgG antibodies are measurable within weeks of infection. In vitro, antibodies promote killing of Legionella by phagocytes (neutrophils, monocytes, and alveolar macrophages). Immunized animals develop a specific antibody response, with subsequent resistance to Legionella challenge. However, antibodies neither enhance lysis by complement nor inhibit intracellular multiplication within phagocytes.
![]() The genome of L. pneumophila has been sequenced. A broad range of membrane transporters within the genome are thought to optimize the use of nutrients in water and soil.
The genome of L. pneumophila has been sequenced. A broad range of membrane transporters within the genome are thought to optimize the use of nutrients in water and soil.
Some L. pneumophila strains are clearly more virulent than others, although the precise factors mediating virulence remain uncertain. For example, although multiple strains may colonize water-distribution systems, only a few cause disease in patients exposed to water from these systems. At least one surface epitope of L. pneumophila serogroup 1 is associated with virulence. Monoclonal antibody subtype mAb2 has been linked to virulence. L. pneumophila serogroup 6 is more commonly involved in hospital-acquired Legionnaires’ disease and is especially likely to be associated with a poor outcome.
CLINICAL AND LABORATORY FEATURES
Pontiac Fever Pontiac fever is an acute, self-limiting, flu-like illness with an incubation period of 24–48 h. Pneumonia does not develop. Malaise, fatigue, and myalgias are the most common symptoms, occurring in 97% of cases. Fever (usually with chills) develops in 80–90% of cases and headache in 80%. Other symptoms (seen in fewer than 50% of cases) include arthralgias, nausea, cough, abdominal pain, and diarrhea. Modest leukocytosis with a neutrophilic predominance is sometimes detected. Complete recovery occurs within a few days; antibiotic therapy is unnecessary. A few patients may experience lassitude for some weeks after recovery. The diagnosis is established by antibody seroconversion. Pontiac fever due to L. longbeachae has been reported in individuals exposed to potting soil.
Legionnaires’ Disease (Pneumonia) Legionnaires’ disease is often included in the differential diagnosis of “atypical pneumonia,” along with pneumonia due to C. pneumoniae, Chlamydia psittaci, Mycoplasma pneumoniae, Coxiella burnetii, and some viruses. The clinical similarities among “atypical” pneumonias include a nonproductive cough with a low frequency of grossly purulent sputum. The clinical manifestations of Legionnaires’ disease are usually more severe than those of most “atypical” pneumonias. The course and prognosis of Legionella pneumonia more closely resemble those of bacteremic pneumococcal pneumonia than those of pneumonia due to other “atypical” pathogens. Patients with community-acquired Legionnaires’ disease are significantly more likely than patients with pneumonia of other etiologies to be admitted to an intensive care unit (ICU) on presentation.
The incubation period for Legionnaires’ disease is usually 2–10 days, although slightly longer incubation periods have been documented. Fever is almost universal. In one observational study, 20% of patients had temperatures in excess of 40°C (104°F). The symptoms and signs may range from a mild cough and a slight fever to stupor with widespread pulmonary infiltrates and multisystem failure. The mild cough of Legionnaires’ disease is only slightly productive. Sometimes the sputum is streaked with blood. Chest pain—either pleuritic or nonpleuritic—can be a prominent feature and, when coupled with hemoptysis, can lead to an incorrect diagnosis of pulmonary embolism. Shortness of breath is reported by one-third to one-half of patients. Gastrointestinal difficulties are often pronounced; abdominal pain, nausea, and vomiting affect 10–20% of patients. Diarrhea (watery rather than bloody) is reported in 25–50% of cases. The most common neurologic abnormalities are confusion or changes in mental status; however, the multitudinous neurologic symptoms reported range from headache and lethargy to encephalopathy. Nonspecific symptoms—malaise, fatigue, anorexia, and headache—are reported early in the illness. Myalgias and arthralgias are uncommon but are prominent in a few patients. Upper respiratory symptoms, including coryza, are rare.
Relative bradycardia has been overemphasized as a useful diagnostic finding; it occurs primarily in older patients with severe pneumonia. Rales are detected by chest examination early in the course, and evidence of consolidation is found as the disease progresses. Abdominal examination may reveal generalized or local tenderness.
Although the clinical manifestations often considered classic for Legionnaires’ disease may suggest the diagnosis (Table 184-1), prospective comparative studies have shown that clinical manifestations are generally nonspecific and that Legionnaires’ disease is not readily distinguishable from pneumonia of other etiologies. In a review of 13 studies of community-acquired pneumonia, clinical manifestations that occurred significantly more often in Legionnaires’ disease included diarrhea, neurologic findings (including confusion), and a temperature of >39°C. Hyponatremia, elevated values in liver function tests, and hematuria also occurred more frequently in Legionnaires’ disease. Other laboratory abnormalities include creatine phosphokinase elevation, hypophosphatemia, serum creatinine elevation, and proteinuria.
|
CLINICAL CLUES SUGGESTIVE OF LEGIONNAIRES’ DISEASE |
Sporadic cases of Legionnaires’ disease appear to be more severe than outbreak-associated and hospital-acquired cases, presumably because their diagnosis is delayed. Results of the German CAPNETZ Study showed that, among cases of community-acquired Legionella pneumonia, ambulatory patients were as common as hospitalized patients.
Extrapulmonary Legionellosis Because the portal of entry for Legionella is the lung in virtually all cases, extrapulmonary manifestations usually result from bloodborne dissemination from the lung. Legionella has been identified in lymph nodes, spleen, liver, or kidneys in autopsied cases. Sinusitis, peritonitis, pyelonephritis, skin and soft tissue infection, septic arthritis, and pancreatitis have developed predominantly in immunosuppressed patients. The most severe sequela, neurologic dysfunction, is rare but can be debilitating. The most common neurologic deficits in the long term—ataxia and speech difficulties—result from cerebellar dysfunction.
We speculate that cardiac abnormalities in patients without pneumonia are caused by Legionella-contaminated water entering through an intravenous site, chest tube, or surgical wound, with subsequent seeding of a prosthetic valve, the myocardium, or the pericardium. This scenario is supported by cases occurring at Stanford University Hospital in which sternal wound infections and prosthetic valve endocarditis due to L. pneumophila were observed. The source was a sink in the postoperative surgical recovery ward.
Chest Radiography Virtually all patients with Legionnaires’ disease have abnormal chest radiographs showing pulmonary infiltrates at the time of clinical presentation. In a few cases of hospital-acquired disease, fever and respiratory tract symptoms have preceded the radiographic appearance of the infiltrate. Radiologic findings are nonspecific. Pleural effusion is evident in 28–63% of patients on hospital admission. In immunosuppressed patients, especially those receiving glucocorticoids, distinctive rounded nodular opacities may be seen; these lesions may expand and cavitate (Fig. 184-1). Likewise, abscesses can occur in immunosuppressed hosts. The progression of infiltrates and pleural effusion on chest radiography despite appropriate antibiotic therapy within the first week is common, and radiographic improvement lags behind clinical improvement by several days. Complete clearing of infiltrates requires 1–4 months. Computed tomography (CT) is more sensitive than chest radiography, may show more extensive disease, and should be performed if fever persists during treatment with presumably effective antibiotics (Fig. 184-2).
FIGURE 184-1 Chest radiographic findings in a 52-year-old man who presented with pneumonia subsequently diagnosed as Legionnaires’ disease. The patient was a cigarette smoker with chronic obstructive pulmonary disease and alcoholic cardiomyopathy; he had received glucocorticoids. Legionella pneumophila was identified by direct fluorescent antibody staining and culture of sputum. Left: Baseline chest radiograph showing long-standing cardiomegaly. Center: Admission chest radiograph showing new rounded opacities. Right: Chest radiograph taken 3 days after admission, during treatment with erythromycin.
FIGURE 184-2 Computed tomography (CT) scans of a 49-year-old woman with no underlying conditions who presented with community-acquired pneumonia. CT revealed multilobar infiltrates, some of which were not as prominent on chest x-ray. Cultures of both the patient’s sputum and her home water supply yielded Legionella pneumophila serogroup 1. (Images courtesy of Dr. Wen-Chien Ko, National Cheng Kung University Hospital, Tainan, Taiwan.)
DIAGNOSIS
Given the nonspecific clinical manifestations of Legionnaires’ disease and the high mortality rates for untreated Legionnaires’ disease, Legionella testing—especially the Legionella urinary antigen test—is recommended for all patients with pneumonia, including patients with ambulatory pneumonia and hospitalized children. Legionella cultures should be made more widely available because the urinary antigen test can diagnose only L. pneumophila serogroup 1. Hospitals in which the drinking water is known to be colonized with Legionella species should have Legionella cultures routinely available.
The diagnosis of Legionnaires’ disease requires special microbiologic tests (Table 184-2). The sensitivity of bronchoscopy specimens is similar to that of sputum samples for culture on selective media; if sputum is not available, bronchoscopy specimens may yield the organism. Bronchoalveolar lavage fluid gives higher yields than bronchial wash specimens. Thoracentesis should be performed if pleural effusion is found, and the fluid should be evaluated by direct fluorescent antibody (DFA) staining, culture, and the antigen assay designed for use with urine.
|
UTILITY OF SPECIAL LABORATORY TESTS FOR THE DIAGNOSIS OF LEGIONNAIRES’ DISEASE |
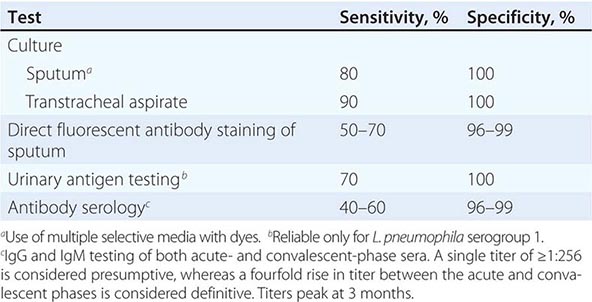
Stains Gram’s staining of material from normally sterile sites, such as pleural fluid or lung tissue, occasionally suggests the diagnosis; efforts to detect Legionella in sputum by Gram’s staining typically reveal numerous leukocytes but no organisms. When they are visualized, the organisms appear as small, pleomorphic, faint, gram-negative bacilli. L. micdadei organisms can be detected as weakly or partially acid-fast bacilli in clinical specimens.
The DFA stain is rapid and highly specific but is less sensitive than culture because large numbers of organisms are required for microscopic visualization. This test is more likely to be positive in advanced than in early disease.
Culture The definitive method for diagnosis of Legionella infection is isolation of the organism from respiratory secretions, although culture for 3–5 days is required. Antibiotics added to the medium suppress the growth of competing flora from nonsterile sites, and dyes color the colonies and assist in identification. The use of multiple selective BCYE media is necessary for maximal sensitivity. When culture plates are overgrown with other microflora, pretreatment of the specimen with acid or heat can markedly improve the yield. L. pneumophila is often isolated from sputum that is not grossly or microscopically purulent; sputum containing more than 25 epithelial cells per high-power field (a finding that classically suggests contamination) may still yield L. pneumophila.
Antibody Detection Antibody testing of both acute- and convalescent-phase sera is necessary. A fourfold rise in titer is diagnostic; 12 weeks are often required for the detection of an antibody response. A single titer of 1:128 in a patient with pneumonia constitutes circumstantial evidence for Legionnaires’ disease. The CDC uses a titer of 1:256 as presumptive evidence for Legionnaires’ disease. Serology is of use primarily in epidemiologic studies. The specificity of serology for Legionella species other than L. pneumophila is uncertain; there is cross-reactivity within Legionella species and with some gram-negative bacilli. Serology is used as the criterion for the diagnosis of Pontiac fever.
Urinary Antigen The assay for Legionella soluble antigen in urine is second only to culture in terms of sensitivity and is highly specific. A rapid immunochromatographic assay is commercially available (BinaxNOW; Alere, Waltham, MA). This assay is relatively inexpensive and easy to perform. Its drawback is that the urinary antigen test is reliable only for L. pneumophila serogroup 1, which causes ~80% of Legionella infections. Cross-reactivity with other L. pneumophila serogroups and other Legionella species has been detected in up to 22% of urine samples from patients with culture-proven cases. Antigen in urine is detectable 3 days after the onset of clinical disease and disappears over 2 months; positivity can be prolonged when patients receive glucocorticoids. The test is not affected by antibiotic administration.
Molecular Methods DFA stains can identify a number of Legionella species. Both polyclonal and monoclonal antibody stains are commercially available. Polymerase chain reaction (PCR) with DNA probes is being applied in-house in selected hospitals but is not yet commercially available. PCR has proven somewhat useful in the identification of Legionella from environmental water specimens. Epidemiologic links cannot easily be made with PCR because the infecting pathogen is not available for molecular subtyping.
Procalcitonin can be used as an indicator of severity of illness in patients in ICUs. Clinical response to antibiotics can be monitored by procalcitonin levels.
|
TREATMENT |
LEGIONELLA INFECTION |
Because Legionella is an intracellular pathogen, antibiotics that can attain high intracellular concentrations are most likely to be effective. The dosages for various drugs used in the treatment of Legionella infection are listed in Table 184-3.
|
ANTIBIOTIC THERAPY FOR LEGIONELLA INFECTION |
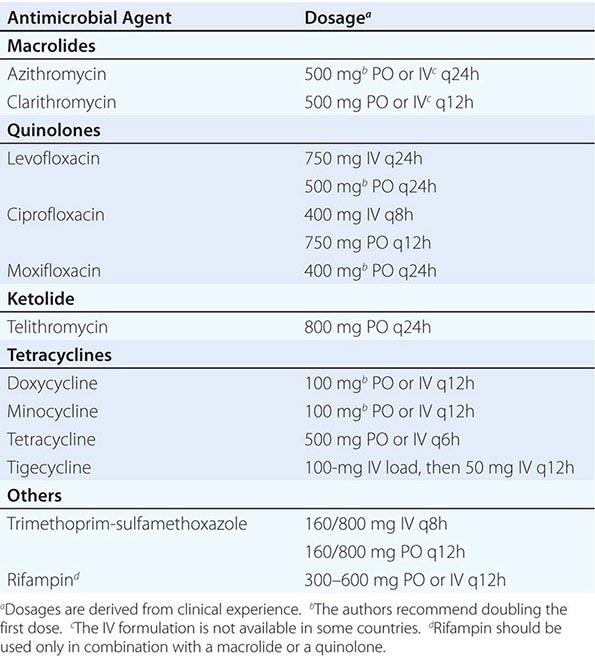
The macrolides (especially azithromycin) and the respiratory quinolones are now the antibiotics of choice and are effective as monotherapy. Compared with erythromycin, the newer macrolides have superior in vitro activity, display greater intracellular activity, reach higher concentrations in respiratory secretions and lung tissue, and have fewer adverse effects. The pharmacokinetics of the newer macrolides and quinolones also allow once- or twice-daily dosing. Quinolones are the preferred antibiotics for transplant recipients because both macrolides and rifampin interact pharmacologically with cyclosporine and tacrolimus. Retrospective uncontrolled studies have shown that complications of pneumonia are fewer and clinical response is more rapid in patients receiving quinolones than in those receiving macrolides. Initial therapy should be given by the IV route. A clinical response usually occurs within 3–5 days, after which oral therapy can be substituted. The total duration of therapy in the immunocompetent host is 10–14 days.
Alternative agents include tetracycline and its analogues doxycycline and minocycline. Tigecycline is active in vitro, but clinical experience with this drug is minimal. Anecdotal reports have described both successes and failures with trimethoprim-sulfamethoxazole, imipenem, and clindamycin.
For critically ill patients, the authors use combination regimens of azithromycin, a quinolone, and/or rifampin. This practice is empirical and is not supported by comparative studies. Rifampin is highly active in vitro and in cell models. Its interaction with other medications and its side effect of reversible hyperbilirubinemia can be minimized by limiting the duration of therapy to 3–5 days. A longer course of therapy (3 weeks) may be appropriate for immunosuppressed patients and those with advanced disease. For azithromycin, with its long half-life, a 5- to 10-day course is sufficient.
Pontiac fever requires only symptom-based treatment, not antimicrobial therapy.
PROGNOSIS
Mortality rates for Legionnaires’ disease vary with the patient’s underlying disease, the patient’s immune status, the severity of pneumonia, and the timing of administration of appropriate antimicrobial therapy. Mortality rates are highest (80%) among immunosuppressed patients who do not receive appropriate antimicrobial therapy early in the course of illness. With timely antibiotic treatment, mortality rates from community-acquired Legionnaires’ disease among immunocompetent patients range from 0 to 11%; without treatment, the figure may be as high as 31%. In a study of survivors of an outbreak of community-acquired Legionnaires’ disease, sequelae of fatigue, neurologic symptoms, and weakness were found in 63–75% of patients 17 months after receipt of antibiotics.
PREVENTION
Routine environmental culture of hospital water supplies for Legionella is recommended as an approach to the prevention of hospital-acquired Legionnaires’ disease. Guidelines mandating this proactive approach have been adopted throughout Europe and in several U.S. states. The presence of Legionella in the water supply mandates the use of specialized laboratory tests (especially culture on selective media and the urinary antigen test) for patients with hospital-acquired pneumonia. A 30% cutoff for the presence of Legionella in water from multiple hospital sites prompts an increased index of suspicion. When the 30% cutoff point is exceeded, diagnostic tests for Legionella need to be applied in all cases of hospital-acquired pneumonia, and measures directed at eliminating the organism from the water supply should be considered. Quantitative criteria at a given water site (colony-forming units [CFU]/mL) have proven unreliable and inconsistent in the prediction of disease.
Studies have shown that neither a high degree of outward cleanliness of the water system nor routine application of maintenance measures decreases the frequency or intensity of Legionella contamination. Thus, engineering guidelines and building codes, although routinely advocated as preventive measures, have little impact on the presence of Legionella.
Environmental cultures for Legionella from cold-water taps, hot-water taps, the hot-water recirculating line, and water-storage tanks will reveal the source of hospital-acquired infections. Disinfection of the hospital drinking-water system is an effective preventive measure for hospital-acquired cases of Legionnaires’ diseases because this system is the reservoir for Legionella. In geographic areas where the climate is semitropical, cold-water lines may be colonized by Legionella.
Copper-silver ionization is a reliable method for eradication of Legionella. Unlike the efficacy of chlorine dioxide decontamination and chlorination, that of ionization is not affected by high water temperature. Ionization systems are easy to install, and the ions are odorless, with minimal adverse effects. The efficacy of copper-silver ionization has been documented in hospitals worldwide. A comprehensive review of 10 published studies concluded that copper-silver ionization is effective for Legionella control as long as ion levels are monitored. If cold-water colonization by Legionella is the source of an outbreak, chlorine dioxide and monochloramine offer advantages. Chlorine dioxide, often the least expensive option, penetrates biofilms better and is less corrosive than chlorine. The major disadvantage of chlorine dioxide is the need to maintain an effective residual throughout the drinking-water system, especially in the hot-water system. Eradication of Legionella by chlorine dioxide may require several months—a drawback in outbreak situations. Monochloramine is a promising approach in disinfection. Hyperchlorination is no longer recommended because of its expense, carcinogenicity, corrosive effects on piping, and unreliable efficacy.
Point-of-use disposable water filters (0.2 μm) may be an economical and effective option in high-risk areas (e.g., ICUs and transplantation units). These filters can be used in an outbreak situation for a limited period.
Ineffective yet expensive methods that are often promulgated include removal of stagnation (“dead legs”) in the water-distribution system and replacement or disinfection/cleaning of distal outlets. Infection control personnel should oversee the selection of disinfection technology and should apply evidence-based criteria when making their choice. Managers of health care facilities should not be given the primary responsibility for selection and subsequent monitoring of measures to eliminate and control Legionella.
185 |
Pertussis and Other Bordetella Infections |
Pertussis is an acute infection of the respiratory tract caused by Bordetella pertussis. The name pertussis means “violent cough,” which aptly describes the most consistent and prominent feature of the illness. The inspiratory sound made at the end of an episode of paroxysmal coughing gives rise to the common name for the illness, “whooping cough.” However, this feature is variable: it is uncommon among infants ≤6 months of age and is frequently absent in older children and adults. The Chinese name for pertussis is “the 100-day cough,” which accurately describes the clinical course of the illness. The identification of B. pertussis was first reported by Bordet and Gengou in 1906, and vaccines were produced over the following two decades.
MICROBIOLOGY
Of the 10 identified species in the genus Bordetella, only four are of major medical significance. B. pertussis infects only humans and is the most important Bordetella species causing human disease. B. parapertussis causes an illness in humans that is similar to pertussis but is typically milder; co-infections with B. parapertussis and B. pertussis have been documented. With improved polymerase chain reaction (PCR) diagnostic methodology, up to 20% of patients with a pertussis-like syndrome have been found to be infected with B. holmesii, formerly thought to be an unusual cause of bacteremia. B. bronchiseptica is an important pathogen of domestic animals that causes kennel cough in dogs, atrophic rhinitis and pneumonia in pigs, and pneumonia in cats. Both respiratory infection and opportunistic infection due to B. bronchiseptica are occasionally reported in humans. B. petrii, B. hinzii, and B. ansorpii have been isolated from patients who are immunocompromised.
![]() Bordetella species are gram-negative pleomorphic aerobic bacilli that share common genotypic characteristics. B. pertussis and B. parapertussis are the most similar of the species, but B. parapertussis does not express the gene coding for pertussis toxin. B. pertussis is a slow-growing fastidious organism that requires selective medium and forms small, glistening, bifurcated colonies. Suspicious colonies are presumptively identified as B. pertussis by direct fluorescent antibody testing or by agglutination with species-specific antiserum. B. pertussis is further differentiated from other Bordetella species by biochemical and motility characteristics.
Bordetella species are gram-negative pleomorphic aerobic bacilli that share common genotypic characteristics. B. pertussis and B. parapertussis are the most similar of the species, but B. parapertussis does not express the gene coding for pertussis toxin. B. pertussis is a slow-growing fastidious organism that requires selective medium and forms small, glistening, bifurcated colonies. Suspicious colonies are presumptively identified as B. pertussis by direct fluorescent antibody testing or by agglutination with species-specific antiserum. B. pertussis is further differentiated from other Bordetella species by biochemical and motility characteristics.
B. pertussis produces a wide array of toxins and biologically active products that are important in its pathogenesis and in immunity. Most of these virulence factors are under the control of a single genetic locus that regulates their production, resulting in antigenic modulation and phase variation. Although these processes occur both in vitro and in vivo, their importance in the pathobiology of the organism is unknown; they may play a role in intracellular persistence and person-to-person spread. The organism’s most important virulence factor is pertussis toxin, which is composed of a B oligomer–binding subunit and an enzymatically active A protomer that ADP-ribosylates a guanine nucleotide-binding regulatory protein (G protein) in target cells, producing a variety of biologic effects. Pertussis toxin has important mitogenic activity, affects the circulation of lymphocytes, and serves as an adhesin for bacterial binding to respiratory ciliated cells. Other important virulence factors and adhesins are filamentous hemagglutinin, a component of the cell wall, and pertactin, an outer-membrane protein. Fimbriae, bacterial appendages that play a role in bacterial attachment, are the major antigens against which agglutinating antibodies are directed. These agglutinating antibodies have historically been the primary means of serotyping B. pertussis strains. Other virulence factors include tracheal cytotoxin, which causes respiratory epithelial damage; adenylate cyclase toxin, which impairs host immune-cell function; dermonecrotic toxin, which may contribute to respiratory mucosal damage; and lipooligosaccharide, which has properties similar to those of other gram-negative bacterial endotoxins.
EPIDEMIOLOGY
![]() Pertussis is a highly communicable disease, with attack rates of 80–100% among unimmunized household contacts and 20% within households in well-immunized populations. The infection has a worldwide distribution, with cyclical outbreaks every 3–5 years (a pattern that has persisted despite widespread immunization). Pertussis occurs in all months; however, in North America, its activity peaks in summer and autumn.
Pertussis is a highly communicable disease, with attack rates of 80–100% among unimmunized household contacts and 20% within households in well-immunized populations. The infection has a worldwide distribution, with cyclical outbreaks every 3–5 years (a pattern that has persisted despite widespread immunization). Pertussis occurs in all months; however, in North America, its activity peaks in summer and autumn.
In developing countries, pertussis remains an important cause of infant morbidity and death. The reported incidence of pertussis worldwide has decreased as a result of improved vaccine coverage (Fig. 185-1). However, coverage rates are still <50% in many developing nations; the World Health Organization (WHO) estimates that 90% of the burden of pertussis is in developing regions. In addition, overreporting of immunization coverage and underreporting of disease result in substantial underestimation of the global burden of pertussis. The WHO estimates that there were 195,000 deaths from pertussis among children in 2008.
FIGURE 185-1 Global annual reported cases of pertussis and rate of coverage with DTP3 (diphtheria toxoid, tetanus toxoid, and pertussis vaccine; 3 doses), 1980–2012. (© World Health Organization, 2013. All rights reserved. From www.who.int/immunization/monitoring_surveillance/burden/vpd/surveillance_type/passive/Pertussis_coverage.JPG. Source: WHO/IVB database, 2013.)
Before the institution of widespread immunization programs in the developed world, pertussis was one of the most common infectious causes of morbidity and death. In the United States before the 1940s, between 115,000 and 270,000 cases of pertussis were reported annually, with an average yearly rate of 150 cases per 100,000 population. With universal childhood immunization, the number of reported cases fell by >95%, and mortality rates decreased even more dramatically. Only 1010 cases of pertussis were reported in 1976 (Fig. 185-2). After that historic low, rates of pertussis slowly increased. In recent years, pertussis epidemics have been reported with increasing frequency worldwide. The United States experienced widespread outbreaks of pertussis in 2005, 2010, and 2012 at levels not seen in 40–50 years (>40,000 reported cases in 2012).
FIGURE 185-2 Reported cases of pertussis by year—United States, 1976–2012. (From the Centers for Disease Control and Prevention, www.cdc.gov/pertussis/surv-reporting/cases-by-year.html. Accessed December 17, 2013.)
Although thought of as a disease of childhood, pertussis can affect people of all ages and is increasingly being identified as a cause of prolonged coughing illness in adolescents and adults. In unimmunized populations, pertussis incidence peaks during the preschool years, and well over half of children have the disease before reaching adulthood. In highly immunized populations such as those in North America, the peak incidence is among infants <1 year of age who have not completed the three-dose primary immunization series. An increase in pertussis incidence among adolescents and adults began in the late 1990s and led to the introduction of an adolescent booster across North America by 2006. While the disease burden among adolescents has started to decrease, children 7–10 years of age have recently emerged as a high-risk group. In major outbreaks in 2010 and 2012, the incidence of pertussis among children 10 years of age, most of whom were fully immunized, was as high as that among infants <6 months of age. Although adults contribute a smaller proportion of reported cases of pertussis than do children and adolescents, this difference may be related to a greater degree of underrecognition and underreporting. A number of studies of prolonged coughing illness suggest that B. pertussis may be the etiologic agent in 12–30% of adults with cough that does not improve within 2 weeks. In one study of the efficacy of an acellular pertussis vaccine in adolescents and adults, the incidence of pertussis in the placebo group was 3.7–4.5 cases per 1000 person-years. Although this prospective cohort study yielded a lower estimate than the studies of cough illness, its results still translate to 600,000–800,000 cases of pertussis annually among adults in the United States.
Severe morbidity and high mortality rates, however, are restricted almost entirely to infants. In Canada, there were 16 deaths from pertussis between 1991 and 2001; all those who died were infants ≤6 months of age. Similarly, in the United States between 1993 and 2004, all pertussis deaths and 86% of hospitalizations for pertussis involved infants ≤3 months of age. Although school-age children are the source of infection for most households, adults are the likely source for cases in high-risk infants and may serve as the reservoir of infection between epidemic years.
PATHOGENESIS
Infection with B. pertussis is initiated by attachment of the organism to the ciliated epithelial cells of the nasopharynx. Attachment is mediated by surface adhesins (e.g., pertactin and filamentous hemagglutinin), which bind to the integrin family of cell-surface proteins, probably in conjunction with pertussis toxin. The role of fimbriae in adhesion and in maintenance of infection has not been fully delineated. At the site of attachment, the organism multiplies, producing a variety of other toxins that cause local mucosal damage (tracheal cytotoxin, dermonecrotic toxin). Impairment of host defense by B. pertussis is mediated by pertussis toxin and adenylate cyclase toxin. There is local cellular invasion, with intracellular bacterial persistence; however, systemic dissemination does not occur. Systemic manifestations (lymphocytosis) result from the effects of the toxins.
The pathogenesis of the clinical manifestations of pertussis is poorly understood. It is not known what causes the hallmark paroxysmal cough. A pivotal role for pertussis toxin has been proposed. Proponents of this position point to the efficacy of preventing clinical symptoms with a vaccine containing only pertussis toxoid. Detractors counter that pertussis toxin is not the critical factor because paroxysmal cough also occurs in patients infected with B. parapertussis, which does not produce pertussis toxin. It is thought that neurologic events in pertussis, such as seizures and encephalopathy, are due to hypoxia from coughing paroxysms or apnea rather than to the effects of specific bacterial products. B. pertussis pneumonia, which occurs in up to 10% of infants with pertussis, is usually a diffuse bilateral primary infection. In older children and adults with pertussis, pneumonia is often due to secondary bacterial infection with streptococci or staphylococci. Deaths from pertussis among young infants are frequently associated with very high levels of leukocytosis and pulmonary hypertension.
IMMUNITY
Both humoral and cell-mediated immunity are thought to be important in pertussis. Antibodies to pertussis toxin, filamentous hemagglutinin, pertactin, and fimbriae are all protective in animal models. Pertussis agglutinins were correlated with protection in early studies of whole-cell pertussis vaccines. Serologic correlates of protection conferred by acellular pertussis vaccines have not been established, although antibody to pertactin, fimbriae, and (to a lesser degree) pertussis toxin correlated best with protection in two efficacy trials. The duration of immunity after whole-cell pertussis vaccination is short-lived, with little protection remaining after 10–12 years. Recent studies have demonstrated early waning of immunity—i.e., within 2–4 years after the fifth dose of acellular pertussis vaccine in children who received acellular pertussis vaccine for their primary series in infancy. These data suggest that boosters may be needed more frequently than every 10 years, as previously thought. Although immunity after natural infection was thought to be lifelong, seroepidemiologic evidence demonstrates that it clearly is not and that subsequent episodes of clinical pertussis are prevented by intermittent subclinical infection.
CLINICAL MANIFESTATIONS
Pertussis is a prolonged coughing illness with clinical manifestations that vary by age (Table 185-1). Although not uncommon among adolescents and adults, classic pertussis is most often seen in preschool and school-age children. After an incubation period averaging 7–10 days, an illness develops that is indistinguishable from the common cold and is characterized by coryza, lacrimation, mild cough, low-grade fever, and malaise. After 1–2 weeks, this catarrhal phase evolves into the paroxysmal phase: the cough becomes more frequent and spasmodic with repetitive bursts of 5–10 coughs, often within a single expiration. Posttussive vomiting is frequent, with a mucous plug occasionally expelled at the end of an episode. The episode may be terminated by an audible whoop, which occurs upon rapid inspiration against a closed glottis at the end of a paroxysm. During a spasm, there may be impressive neck-vein distension, bulging eyes, tongue protrusion, and cyanosis. Paroxysms may be precipitated by noise, eating, or physical contact. Between attacks, the patient’s appearance is normal but increasing fatigue is evident. The frequency of paroxysmal episodes varies widely, from several per hour to 5–10 per day. Episodes are often worse at night and interfere with sleep. Weight loss is not uncommon as a result of the illness’s interference with eating. Most complications occur during the paroxysmal stage. Fever is uncommon and suggests bacterial superinfection.
|
CLINICAL FEATURES OF PERTUSSIS, BY AGE GROUP AND DIAGNOSTIC STATUS |
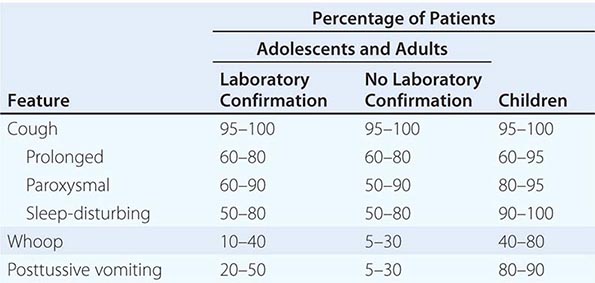
After 2–4 weeks, the coughing episodes become less frequent and less severe—changes heralding the onset of the convalescent phase. This phase can last 1–3 months and is characterized by gradual resolution of coughing episodes. For 6–12 months, intercurrent viral infections may be associated with a recrudescence of paroxysmal cough.
Not all individuals who develop pertussis have classic disease. The clinical manifestations in adolescents and adults are more often atypical. In a German study of pertussis in adults, more than two-thirds had paroxysmal cough and more than one-third had whoop. Adult illness in North America differs from this experience: the cough may be severe and prolonged but is less frequently paroxysmal, and a whoop is uncommon. Vomiting with cough is the best predictor of pertussis as the cause of prolonged cough in adults. Other predictive features are a cough at night, sweating episodes between paroxysms of coughing, and exposure to other individuals with a prolonged coughing illness.
COMPLICATIONS
Complications are frequently associated with pertussis and are more common among infants than among older children or adults. Subconjunctival hemorrhages, abdominal and inguinal hernias, pneumothoraces, and facial and truncal petechiae can result from increased intrathoracic pressure generated by severe fits of coughing. Weight loss can follow decreased caloric intake. In a series of more than 1100 children <2 years of age who were hospitalized with pertussis, 27.1% had apnea, 9.4% had pneumonia, 2.6% had seizures, and 0.4% had encephalopathy; 10 children (0.9%) died. Pneumonia is reported in <5% of adolescents and adults and increases in frequency after 50 years of age. In contrast to the primary B. pertussis pneumonia that develops in infants, pneumonia in adolescents and adults with pertussis is usually caused by a secondary infection with encapsulated organisms such as Streptococcus pneumoniae or Haemophilus influenzae. Pneumothorax, severe weight loss, inguinal hernia, rib fracture, carotid artery aneurysm, and cough syncope have all been reported in adolescents and adults with pertussis.
DIAGNOSIS
If the classic symptoms of pertussis are present, clinical diagnosis is not difficult. However, particularly in older children and adults, it is difficult to differentiate infections caused by B. pertussis and B. parapertussis from other respiratory tract infections on clinical grounds. Therefore, laboratory confirmation should be attempted in all cases. Lymphocytosis (an absolute lymphocyte count of >108–109/L) is common among young children, in whom it is unusual with other infections, but not among adolescents and adults. Culture of nasopharyngeal secretions remains the gold standard of diagnosis, although DNA detection by PCR has replaced culture in many laboratories because of increased sensitivity and quicker results. Appropriate PCR methodology must include primers to differentiate among B. pertussis, B. parapertussis, and B. holmesii. The best specimen is collected by nasopharyngeal aspiration, in which a fine flexible plastic catheter attached to a 10-mL syringe is passed into the nasopharynx and withdrawn while gentle suction is applied. Since B. pertussis is highly sensitive to drying, secretions for culture should be inoculated without delay onto appropriate medium (Bordet-Gengou or Regan-Lowe), or the catheter should be flushed with a phosphate-buffered saline solution for culture and/or PCR. An alternative to the aspirate is a Dacron or rayon nasopharyngeal swab; again, inoculation of culture plates should be immediate or an appropriate transport medium (e.g., Regan-Lowe charcoal medium) should be used. Results of PCR can be available within hours; cultures become positive by day 5 of incubation. B. pertussis and B. parapertussis can be differentiated by agglutination with specific antisera or by direct immunofluorescence.
Nasopharyngeal cultures in untreated pertussis remain positive for a mean of 3 weeks after the onset of illness; these cultures become negative within 5 days of the institution of appropriate antimicrobial therapy. The duration of a positive PCR in untreated pertussis or after therapy is not known but exceeds that of positive cultures. Since much of the period during which the organism can be recovered from the nasopharynx falls into the catarrhal phase, when the etiology of the infection is not suspected, there is only a small window of opportunity for culture-proven diagnosis. Cultures from infants and young children are more frequently positive than those from older children and adults; this difference may reflect earlier presentation of the former age group for medical care. Direct fluorescent antibody tests of nasopharyngeal secretions for direct diagnosis may still be available in some laboratories but should not be used because of poor sensitivity and specificity. Pseudo-outbreaks of pertussis have been reported as a result of false-positive PCR results. Greater standardization of PCR methodology can alleviate this problem.
As a result of the difficulties with laboratory diagnosis of pertussis in adolescents, adults, and patients who have been symptomatic for >4 weeks, increasing attention is being given to serologic diagnosis. Enzyme immunoassays detecting IgA and IgG antibodies to pertussis toxin, filamentous hemagglutinin, pertactin, and fimbriae have been developed and assessed for reproducibility. Two- or fourfold increases in antibody titer are suggestive of pertussis, although cross-reactivity of some antigens (such as filamentous hemagglutinin and pertactin) among Bordetella species makes it difficult to depend diagnostically on seroconversion involving a single type of antibody. Late presentation for medical care and prior immunization also complicate serologic diagnosis because the first sample obtained may in fact be a convalescent-phase specimen. Criteria for serologic diagnosis based on comparison of results for a single serum specimen with established population values are gaining acceptance, and serologic measurement of antibody to pertussis toxin is becoming more widely standardized and available for diagnostic purposes, particularly in outbreak settings and for surveillance.
DIFFERENTIAL DIAGNOSIS
A child presenting with paroxysmal cough, posttussive vomiting, and whoop is likely to have an infection caused by B. pertussis or B. parapertussis; lymphocytosis increases the likelihood of a B. pertussis etiology. Viruses such as respiratory syncytial virus and adenovirus have been isolated from patients with clinical pertussis but probably represent co-infection.
In adolescents and adults, who often do not have paroxysmal cough or whoop, the differential diagnosis of a prolonged coughing illness is more extensive. Pertussis should be suspected when any patient has a cough that does not improve within 14 days, a paroxysmal cough of any duration, a cough followed by vomiting (adolescents and adults), or any respiratory symptoms after contact with a laboratory-confirmed case of pertussis. Other etiologies to consider include infections caused by Mycoplasma pneumoniae, Chlamydia pneumoniae, adenovirus, influenza virus, and other respiratory viruses. Use of angiotensin-converting enzyme (ACE) inhibitors, reactive airway disease, and gastroesophageal reflux disease are well-described noninfectious causes of prolonged cough in adults.
|
TREATMENT |
PERTUSSIS |
ANTIBIOTICS
The purpose of antibiotic therapy for pertussis is to eradicate the infecting bacteria from the nasopharynx; therapy does not substantially alter the clinical course unless given early in the catarrhal phase. Macrolide antibiotics are the drugs of choice for treatment of pertussis (Table 185-2); macrolide-resistant B. pertussis strains have been reported but are rare. Trimethoprim-sulfamethoxazole is recommended as an alternative for individuals allergic to macrolides.
|
ANTIMICROBIAL THERAPY FOR PERTUSSIS |
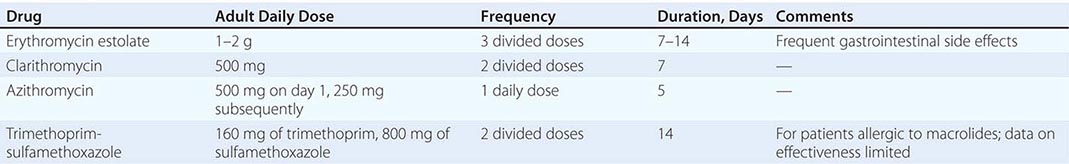
SUPPORTIVE CARE
Young infants have the highest rates of complication and death from pertussis; therefore, most infants (and older children with severe disease) should be hospitalized. A quiet environment may decrease the stimulation that can trigger paroxysmal episodes. Use of β-adrenergic agonists and/or glucocorticoids has been advocated by some authorities but has not been proven to be effective. Cough suppressants are not effective and play no role in the management of pertussis.
INFECTION CONTROL MEASURES
Hospitalized patients with pertussis should be placed in respiratory isolation, with the use of precautions appropriate for pathogens spread by large respiratory droplets. Isolation should continue for 5 days after initiation of macrolide therapy or, in untreated patients, for 3 weeks (i.e., until nasopharyngeal cultures are consistently negative).
PREVENTION
Chemoprophylaxis Because the risk of transmission of B. pertussis within households is high, chemoprophylaxis is widely recommended for household contacts of pertussis cases. The effectiveness of chemoprophylaxis, although unproven, is supported by several epidemiologic studies of institutional and community outbreaks. In the only randomized placebo-controlled study, erythromycin estolate (50 mg/kg per day in three divided doses; maximum dose, 1 g/d) was effective in reducing the incidence of bacteriologically confirmed pertussis by 67%; however, there was no decrease in the incidence of clinical disease. Despite these disappointing results, many authorities continue to recommend chemoprophylaxis, particularly in households with members at high risk of severe disease (children <1 year of age, pregnant women). Data are not available on use of the newer macrolides for chemoprophylaxis, but these drugs are commonly used because of their increased tolerability and their effectiveness.
Immunization (See also Chap. 148) The mainstay of pertussis prevention is active immunization. Pertussis vaccine became widely used in North America after 1940; the reported number of pertussis cases subsequently fell by >90%. Whole-cell pertussis vaccines are prepared through the heating, chemical inactivation, and purification of whole B. pertussis organisms. Despite their efficacy (average estimate, 85%; range for different products, 30–100%), whole-cell pertussis vaccines are associated with adverse events—both common (fever; injection-site pain, erythema, and swelling; irritability) and uncommon (febrile seizures, hypotonic hyporesponsive episodes). Alleged associations of whole-cell pertussis vaccine with encephalopathy, sudden infant death syndrome, and autism, although not substantiated, have spawned an active anti-immunization lobby. The development of acellular pertussis vaccines, which are effective and less reactogenic, has greatly alleviated concerns about the inclusion of pertussis vaccine in the combined infant immunization series.
![]() Although whole-cell vaccines are still used extensively in developing regions of the world, acellular pertussis vaccines are used exclusively for childhood immunization in much of the developed world. In North America, acellular pertussis vaccines for children are given as a three-dose primary series at 2, 4, and 6 months of age, with a reinforcing dose at 15–18 months of age and a booster dose at 4–6 years of age.
Although whole-cell vaccines are still used extensively in developing regions of the world, acellular pertussis vaccines are used exclusively for childhood immunization in much of the developed world. In North America, acellular pertussis vaccines for children are given as a three-dose primary series at 2, 4, and 6 months of age, with a reinforcing dose at 15–18 months of age and a booster dose at 4–6 years of age.
Although a wide variety of acellular pertussis vaccines were developed, only a few are still widely marketed; all contain pertussis toxoid and filamentous hemagglutinin. One acellular pertussis vaccine also contains pertactin, and another contains pertactin and two types of fimbriae. In light of phase 3 efficacy studies, most experts have concluded that two-component acellular pertussis vaccines are more effective than monocomponent vaccines and that the addition of pertactin increases efficacy still more. The further addition of fimbriae appears to enhance protective efficacy against milder disease. In two studies, protection conferred by pertussis vaccines correlated best with the production of antibody to pertactin, fimbriae, and pertussis toxin.
Adult formulations of acellular pertussis vaccines have been shown to be safe, immunogenic, and efficacious in clinical trials in adolescents and adults and are now recommended for routine immunization of these groups in several countries, including the United States. In this country, adolescents should receive a dose of the adult-formulation diphtheria–tetanus–acellular pertussis vaccine at the preadolescence physician’s visit, and all unvaccinated adults should receive a single dose of this combined vaccine. In addition, in the United States, pertussis immunization is specifically recommended for health care workers and for women during each pregnancy to increase passive transfer of maternal antibodies to the fetus. Pertussis vaccine coverage among U.S. adolescents was 78.2% in 2011, but coverage among adults is low (2.1% as of 2007). Further improvements in adult vaccine coverage may permit better control of pertussis across the age spectrum, with collateral protection of infants too young to be immunized. However, more effective vaccines with longer-lasting protection will ultimately be needed to control this disease.
186 |
Diseases Caused by Gram-Negative Enteric Bacilli |
GENERAL FEATURES AND PRINCIPLES
Escherichia coli, Klebsiella, Proteus, Enterobacter, Serratia, Citrobacter, Morganella, Providencia, Cronobacter, and Edwardsiella are gram-negative enteric bacilli that are members of the family Enterobacteriaceae. Salmonella, Shigella, and Yersinia, also in the family Enterobacteriaceae, are discussed in Chaps. 190, 191, and 196, respectively. These pathogens cause a wide variety of infections involving diverse anatomic sites in both healthy and compromised hosts. Increasing antimicrobial resistance in this group has put them at the forefront of an evolving public health crisis. In addition, new infectious syndromes have emerged. Therefore, a thorough knowledge of clinical presentations and appropriate therapeutic choices is necessary for optimal outcomes.
EPIDEMIOLOGY
![]() E. coli, Klebsiella, Proteus, Enterobacter, Serratia, Citrobacter, Morganella, Providencia, Cronobacter, and Edwardsiella are components of the normal animal and human colonic microbiota and/or the microbiota of a variety of environmental habitats, including long-term-care facilities (LTCFs) and hospitals. As a result, except for certain pathotypes of intestinal pathogenic E. coli, these genera are global pathogens. The incidence of infection due to these agents is increasing because of the combination of an aging population and increasing antimicrobial resistance. In healthy humans, E. coli is the predominant species of gram-negative bacilli (GNB) in the colonic flora; Klebsiella and Proteus are less prevalent. GNB (primarily E. coli, Klebsiella, and Proteus) only transiently colonize the oropharynx and skin of healthy individuals. In contrast, in LTCFs and hospital settings, a variety of GNB emerge as the dominant microbiota of both mucosal and skin surfaces, particularly in association with antimicrobial use, severe illness, and extended length of stay. LTCFs are emerging as an important reservoir for resistant GNB. This colonization may lead to subsequent infection; for example, oropharyngeal colonization may lead to pneumonia. Interestingly, the use of ampicillin or amoxicillin was associated with an increased risk of subsequent infection due to the hypervirulent variant of Klebsiella pneumoniae in Taiwan; this association suggests that changes in the quantity or prevalence of colonizing bacteria may be important. Serratia and Enterobacter infection may be acquired through a variety of infusates (e.g., medications, blood products). Edwardsiella infections are acquired through freshwater and marine environment exposures and are most common in Southeast Asia.
E. coli, Klebsiella, Proteus, Enterobacter, Serratia, Citrobacter, Morganella, Providencia, Cronobacter, and Edwardsiella are components of the normal animal and human colonic microbiota and/or the microbiota of a variety of environmental habitats, including long-term-care facilities (LTCFs) and hospitals. As a result, except for certain pathotypes of intestinal pathogenic E. coli, these genera are global pathogens. The incidence of infection due to these agents is increasing because of the combination of an aging population and increasing antimicrobial resistance. In healthy humans, E. coli is the predominant species of gram-negative bacilli (GNB) in the colonic flora; Klebsiella and Proteus are less prevalent. GNB (primarily E. coli, Klebsiella, and Proteus) only transiently colonize the oropharynx and skin of healthy individuals. In contrast, in LTCFs and hospital settings, a variety of GNB emerge as the dominant microbiota of both mucosal and skin surfaces, particularly in association with antimicrobial use, severe illness, and extended length of stay. LTCFs are emerging as an important reservoir for resistant GNB. This colonization may lead to subsequent infection; for example, oropharyngeal colonization may lead to pneumonia. Interestingly, the use of ampicillin or amoxicillin was associated with an increased risk of subsequent infection due to the hypervirulent variant of Klebsiella pneumoniae in Taiwan; this association suggests that changes in the quantity or prevalence of colonizing bacteria may be important. Serratia and Enterobacter infection may be acquired through a variety of infusates (e.g., medications, blood products). Edwardsiella infections are acquired through freshwater and marine environment exposures and are most common in Southeast Asia.
STRUCTURE AND FUNCTION
Enteric GNB possess an extracytoplasmic outer membrane, which consists of a lipid bilayer with associated proteins, lipoproteins, and polysaccharides (capsule, lipopolysaccharide). The outer membrane interfaces with the external environment, including the human host. A variety of components of the outer membrane are critical determinants in pathogenesis (e.g., capsule) and antimicrobial resistance (e.g., permeability barrier, efflux pumps).
PATHOGENESIS
Multiple bacterial virulence factors are required for the pathogenesis of infections caused by GNB. Possession of specialized virulence genes defines pathogens and enables them to infect the host efficiently. Hosts and their cognate pathogens have been co-adapting throughout evolutionary history. During the host-pathogen “chess match” over time, various and redundant strategies have emerged in both the pathogens and their hosts (Table 186-1).
|
INTERACTIONS OF EXTRAINTESTINAL PATHOGENIC ESCHERICHIA COLI WITH THE HUMAN HOST: A PARADIGM FOR EXTRACELLULAR, EXTRAINTESTINAL GRAM-NEGATIVE BACTERIAL PATHOGENS |
Intestinal pathogenic mechanisms are discussed below. The members of the Enterobacteriaceae family that cause extraintestinal infections are primarily extracellular pathogens and therefore share certain pathogenic features. Innate immunity (including the activities of complement, antimicrobial peptides, and professional phagocytes) and humoral immunity are the principal host defense components. Both susceptibility to and severity of infection are increased with dysfunction or deficiencies of these components. By contrast, the virulence traits of intestinal pathogenic E. coli—i.e., the distinctive strains that can cause diarrheal disease—are for the most part different from those of extraintestinal pathogenic E. coli (ExPEC) and other GNB that cause extraintestinal infections. This distinction reflects site-specific differences in host environments and defense mechanisms.
A given strain usually possesses multiple adhesins for binding to a variety of host cells (e.g., in E. coli: type 1, S, and F1C fimbriae; P pili). Nutrient acquisition (e.g., of iron via siderophores) requires many genes that are necessary but not sufficient for pathogenesis. The ability to resist the bactericidal activity of complement and phagocytes in the absence of antibody (e.g., as conferred by capsule or O antigen of lipopolysaccharide) is one of the defining traits of an extracellular pathogen. Tissue damage (e.g., as mediated by hemolysin in the case of E. coli) may facilitate spread within the host. Without doubt, many important virulence genes await identification (Chap. 145e).
The ability to induce septic shock is another defining feature of these genera. GNB are the most common causes of this potentially lethal syndrome. Pathogen-associated molecular pattern molecules (PAMPs; e.g., the lipid A moiety of lipopolysaccharide) stimulate a proinflammatory host response via pattern recognition receptors (e.g., Toll-like or C-type lectin receptors) that activate host defense signaling pathways; if overly exuberant, this response results in shock (Chap. 325). Direct bacterial damage of host tissue (e.g., by toxins) or collateral damage from the host response can result in the release of damage-associated molecular pattern molecules (DAMPs; e.g., HMGB1) that can propagate a detrimental proinflammatory host response.
Many antigenic variants (serotypes) exist in most genera of GNB. For example, E. coli has more than 150 O-specific antigens and more than 80 capsular antigens. This antigenic variability, which permits immune evasion and allows recurrent infection by different strains of the same species, has impeded vaccine development (Chap. 148).
INFECTIOUS SYNDROMES
E. coli can cause either intestinal or extraintestinal infection, depending on the particular pathotype, and Edwardsiella tarda can cause both intestinal and extraintestinal infection. Klebsiella primarily causes extraintestinal infection, but hemorrhagic colitis has been associated with a toxin-producing variant of Klebsiella oxytoca. Depending on both the host and the pathogen, nearly every organ or body cavity can be infected with GNB. E. coli and—to a lesser degree—Klebsiella account for most extraintestinal infections due to GNB and are the most virulent pathogens within this group; this virulence is demonstrated by the ability of E. coli and Klebsiella pneumoniae (primarily the hypervirulent variant) to cause severe infections in healthy, ambulatory hosts from the community. However, the other genera are also important, especially among LTCF residents and hospitalized patients, in large part because of the intrinsic or acquired antimicrobial resistance of these organisms and the increasing number of individuals with compromised host defenses. The mortality rate is substantial in many GNB infections and correlates with the severity of illness. Especially problematic are pneumonia and bacteremia (arising from any source), particularly when complicated by organ failure (severe sepsis) and/or shock, for which the associated mortality rates are 20–50%.
DIAGNOSIS
Isolation of GNB from sterile sites almost always implies infection, whereas their isolation from nonsterile sites, particularly from open soft-tissue wounds and the respiratory tract, requires clinical correlation to differentiate colonization from infection. Tentative laboratory identification based on lactose fermentation and indole production (described for each genus below), which usually is possible before final identification of the organism and determination of its antimicrobial susceptibilities, may help to guide empirical antimicrobial therapy.
|
TREATMENT |
INFECTIONS CAUSED BY GRAM-NEGATIVE ENTERIC ENTERIC BACILLI |
![]() (See also Chap. 170) Evidence indicates that initiation of appropriate empirical antimicrobial therapy early in the course of GNB infections (particularly serious infections) leads to improved outcomes. The ever-increasing prevalence of multidrug-resistant (MDR) and extensively drug-resistant (XDR) GNB; the lag between published (historical) and current resistance rates; and variations by species, geographic location, regional antimicrobial use, and hospital site (e.g., intensive care units [ICUs] versus wards) necessitate familiarity with evolving patterns of antimicrobial resistance for the selection of appropriate empirical therapy. Factors predictive of isolate resistance include recent antimicrobial use, a health care association (e.g., recent or ongoing hospitalization, dialysis, residence in an LTCF), or international travel (e.g., to Asia, Latin America, Africa, southern Europe). For appropriately selected patients, it may be prudent initially, while susceptibility results are awaited, to use two potentially active agents with the rationale that at least one agent will be active. If broad-spectrum treatment has been initiated, it is critical to switch to the most appropriate narrower-spectrum agent when information on antimicrobial susceptibility becomes available. Such responsible antimicrobial stewardship will slow down the ever-escalating cycle of selection for increasingly resistant bacteria, decrease the likelihood of Clostridium difficile infection, decrease costs, and maximize the useful longevity of available antimicrobial agents. Likewise, it is important to avoid treatment of patients who are colonized but not infected (e.g., who have a positive sputum culture without evidence of pneumonia). At present, the most reliably active agents against enteric GNB are the carbapenems (e.g., imipenem), the aminoglycoside amikacin, the fourth-generation cephalosporin cefepime, the β-lactam/β-lactamase inhibitor combination piperacillin-tazobactam, and the polymyxins (e.g., colistin or polymyxin B). The number of antimicrobials effective against certain Enterobacteriaceae is shrinking. Truly pan-resistant GNB exist, and it is unlikely that new agents will come to market in the short term. Accordingly, the presently available antimicrobials must be used judiciously.
(See also Chap. 170) Evidence indicates that initiation of appropriate empirical antimicrobial therapy early in the course of GNB infections (particularly serious infections) leads to improved outcomes. The ever-increasing prevalence of multidrug-resistant (MDR) and extensively drug-resistant (XDR) GNB; the lag between published (historical) and current resistance rates; and variations by species, geographic location, regional antimicrobial use, and hospital site (e.g., intensive care units [ICUs] versus wards) necessitate familiarity with evolving patterns of antimicrobial resistance for the selection of appropriate empirical therapy. Factors predictive of isolate resistance include recent antimicrobial use, a health care association (e.g., recent or ongoing hospitalization, dialysis, residence in an LTCF), or international travel (e.g., to Asia, Latin America, Africa, southern Europe). For appropriately selected patients, it may be prudent initially, while susceptibility results are awaited, to use two potentially active agents with the rationale that at least one agent will be active. If broad-spectrum treatment has been initiated, it is critical to switch to the most appropriate narrower-spectrum agent when information on antimicrobial susceptibility becomes available. Such responsible antimicrobial stewardship will slow down the ever-escalating cycle of selection for increasingly resistant bacteria, decrease the likelihood of Clostridium difficile infection, decrease costs, and maximize the useful longevity of available antimicrobial agents. Likewise, it is important to avoid treatment of patients who are colonized but not infected (e.g., who have a positive sputum culture without evidence of pneumonia). At present, the most reliably active agents against enteric GNB are the carbapenems (e.g., imipenem), the aminoglycoside amikacin, the fourth-generation cephalosporin cefepime, the β-lactam/β-lactamase inhibitor combination piperacillin-tazobactam, and the polymyxins (e.g., colistin or polymyxin B). The number of antimicrobials effective against certain Enterobacteriaceae is shrinking. Truly pan-resistant GNB exist, and it is unlikely that new agents will come to market in the short term. Accordingly, the presently available antimicrobials must be used judiciously.
β-Lactamases, which inactivate β-lactam agents, are the most important mediators of resistance to these drugs in GNB. Decreased permeability and/or active efflux of β-lactam agents, although less common, may occur alone or in combination with β-lactamase-mediated resistance.
Broad-spectrum β-lactamases (e.g., TEM, SHV), which mediate resistance to many penicillins and first-generation cephalosporins, are frequently expressed in enteric GNB. These enzymes are inhibited by β-lactamase inhibitors (e.g., clavulanate, sulbactam, tazobactam). They usually do not hydrolyze third- and fourth-generation cephalosporins or cephamycins (e.g., cefoxitin).
![]() Extended-spectrum β-lactamases (ESBLs; e.g., CTX-M, SHV, TEM) are modified broad-spectrum enzymes that confer resistance to the same drugs as well as to third-generation cephalosporins, aztreonam, and (in some instances) fourth-generation cephalosporins. GNB that express ESBLs may also possess porin mutations that result in decreased uptake of cephalosporins and β-lactam/β-lactamase inhibitor combinations. The prevalence of acquired ESBL production, particularly of CTX-M-type enzymes, is increasing in GNB worldwide, in large part due to the presence of the responsible genes on large transferable plasmids with linked or associated resistance to fluoroquinolones, trimethoprim-sulfamethoxazole (TMP-SMX), aminoglycosides, and tetracyclines. To date, ESBLs are most prevalent in K. pneumoniae, K. oxytoca, and E. coli but also occur (and are probably underrecognized) in Enterobacter, Citrobacter, Proteus, Serratia, and other enteric GNB. At present, the rough regional prevalence of ESBL-producing GNB is India > China > rest of Asia, Latin America, Africa, southern Europe > northern Europe > United States, Canada, and Australia. International travel to high-prevalence regions increases the likelihood of colonization with these strains. ESBL-producing GNB were initially described in hospitals (ICUs > wards) and LTCFs, where outbreaks occurred in association with extensive use of third-generation cephalosporins. However, over the last decade, the incidence of uncomplicated cystitis due to CTX-M ESBL-containing E. coli has increased worldwide (including in the United States) among healthy ambulatory women without health care or antimicrobial exposure. Antimicrobial use in food animals has also been implicated in the rise of ESBLs.
Extended-spectrum β-lactamases (ESBLs; e.g., CTX-M, SHV, TEM) are modified broad-spectrum enzymes that confer resistance to the same drugs as well as to third-generation cephalosporins, aztreonam, and (in some instances) fourth-generation cephalosporins. GNB that express ESBLs may also possess porin mutations that result in decreased uptake of cephalosporins and β-lactam/β-lactamase inhibitor combinations. The prevalence of acquired ESBL production, particularly of CTX-M-type enzymes, is increasing in GNB worldwide, in large part due to the presence of the responsible genes on large transferable plasmids with linked or associated resistance to fluoroquinolones, trimethoprim-sulfamethoxazole (TMP-SMX), aminoglycosides, and tetracyclines. To date, ESBLs are most prevalent in K. pneumoniae, K. oxytoca, and E. coli but also occur (and are probably underrecognized) in Enterobacter, Citrobacter, Proteus, Serratia, and other enteric GNB. At present, the rough regional prevalence of ESBL-producing GNB is India > China > rest of Asia, Latin America, Africa, southern Europe > northern Europe > United States, Canada, and Australia. International travel to high-prevalence regions increases the likelihood of colonization with these strains. ESBL-producing GNB were initially described in hospitals (ICUs > wards) and LTCFs, where outbreaks occurred in association with extensive use of third-generation cephalosporins. However, over the last decade, the incidence of uncomplicated cystitis due to CTX-M ESBL-containing E. coli has increased worldwide (including in the United States) among healthy ambulatory women without health care or antimicrobial exposure. Antimicrobial use in food animals has also been implicated in the rise of ESBLs.
The carbapenems are the most reliably active β-lactam agents against ESBL-expressing strains. Clinical experience with alternatives is more limited, but, for organisms susceptible to piperacillin-tazobactam (minimal inhibitory concentration [MIC], ≤4 μg/mL), this agent—at a dosage of 4.5 g q6h—may offer a carbapenem-sparing alternative, at least for E. coli. The role of tigecycline is unclear despite its excellent in vitro activity; Proteus, Morganella, and Providencia are inherently resistant, and attainable serum and urine levels are low. Therefore, caution appears to be prudent, especially with serious infections, until more clinical data become available. Oral options for the treatment of strains expressing CTX-M ESBLs are limited, with fosfomycin being the most reliably active agent (see section below on the treatment of extraintestinal E. coli infections).
AmpC β-lactamases, when induced or stably derepressed to high levels of expression, confer resistance to the same substrates as ESBLs plus the cephamycins (e.g., cefoxitin and cefotetan). The genes encoding these enzymes are primarily chromosomally located and therefore may not exhibit the linked or associated resistance to fluoroquinolones, TMP-SMX, aminoglycosides, and tetracyclines that is common with ESBLs. These enzymes are problematic for the clinician: resistance may develop during therapy with third-generation cephalosporins, resulting in clinical failure, particularly in the setting of bacteremia. Although chromosomal AmpC β-lactamases are present in nearly all members of the Enterobacteriaceae family, the risk of clinically significant induction of high expression levels or selection of stably derepressed mutants with cephalosporin treatment is greatest with Enterobacter cloacae and Enterobacter aerogenes, lower with Serratia marcescens and Citrobacter freundii, and lowest with Providencia and Morganella morganii. In addition, rare strains of E. coli, K. pneumoniae, and other Enterobacteriaceae have acquired plasmids containing inducible AmpC β-lactamase genes. Carbapenems are a viable treatment option. The fourth-generation cephalosporin cefepime may be an appropriate option if the concomitant presence of an ESBL can be excluded and source control is achieved. Other carbapenem-sparing alternatives to consider if isolates are susceptible in vitro are fluoroquinolones, piperacillin-tazobactam, TMP-SMX, tigecycline, and aminoglycosides, although clinical data are limited.
![]() Carbapenemases (e.g., KPC [class A]; NDM-1, VIM, and IMP [class B]; and OXA-48 [class D]) confer resistance to the same drugs as ESBLs and also to cephamycins and carbapenems. Similar to ESBLs, carbapenemases are usually encoded on large transferable plasmids, which often encode linked resistance to fluoroquinolones, TMP-SMX, tetracyclines, and aminoglycosides. Unfortunately, carbapenemase-producing Enterobacteriaceae are becoming increasingly common, particularly in Asia, and infection with these strains is associated with elevated mortality rates. This reality has prompted the Centers for Disease Control and Prevention (CDC) to categorize carbapenem-resistant Enterobacteriaceae as an “urgent threat” to health care. Carbapenemase production by Enterobacteriaceae is most prevalent in K. pneumoniae and E. coli but has been described in nearly all members of the family. Automated susceptibility systems may be unreliable for detection of carbapenemases. An elevated MIC or a diminished zone diameter for meropenem or imipenem should prompt genotypic confirmation, if available. Alternatively, the phenotype can be confirmed with a modified Hodge test (which detects classes A, B, and D, although results can be false positive) and/or inhibition tests with boronic acid (class A), EDTA (class B), or dipicolinic acid (class B). Carbapenem resistance may also occur in the absence of carbapenemase production and can be mediated by AmpC β-lactamase and ESBL production coupled with modifications in permeability/efflux.
Carbapenemases (e.g., KPC [class A]; NDM-1, VIM, and IMP [class B]; and OXA-48 [class D]) confer resistance to the same drugs as ESBLs and also to cephamycins and carbapenems. Similar to ESBLs, carbapenemases are usually encoded on large transferable plasmids, which often encode linked resistance to fluoroquinolones, TMP-SMX, tetracyclines, and aminoglycosides. Unfortunately, carbapenemase-producing Enterobacteriaceae are becoming increasingly common, particularly in Asia, and infection with these strains is associated with elevated mortality rates. This reality has prompted the Centers for Disease Control and Prevention (CDC) to categorize carbapenem-resistant Enterobacteriaceae as an “urgent threat” to health care. Carbapenemase production by Enterobacteriaceae is most prevalent in K. pneumoniae and E. coli but has been described in nearly all members of the family. Automated susceptibility systems may be unreliable for detection of carbapenemases. An elevated MIC or a diminished zone diameter for meropenem or imipenem should prompt genotypic confirmation, if available. Alternatively, the phenotype can be confirmed with a modified Hodge test (which detects classes A, B, and D, although results can be false positive) and/or inhibition tests with boronic acid (class A), EDTA (class B), or dipicolinic acid (class B). Carbapenem resistance may also occur in the absence of carbapenemase production and can be mediated by AmpC β-lactamase and ESBL production coupled with modifications in permeability/efflux.
For treatment of carbapenem-resistant Enterobacteriaceae, tigecycline and colistin are the parenteral agents with the most reliable in vitro activity. However, because tigecycline reaches only low serum and urine concentrations, caution is warranted in using it to treat bacteremia and perhaps urinary tract infection (UTI), although a few case reports describe some success with tigecycline therapy for UTI. Colistin has nephrotoxic and neurotoxic potential. Furthermore, increasing resistance has been described to both of these agents. Thus the clinician is left with few or no therapeutic options. Aminoglycosides may have some utility if active. Fosfomycin is often active in vitro, but clinical data are limited, concerns exist about the development of resistance with monotherapy, and no parenteral formulation is available in the United States. Although control data are lacking, combination therapy is being used in this setting with the goals of increasing efficacy and decreasing the emergence of resistance.
Resistance to fluoroquinolones usually is due to alterations of the target site (DNA gyrase and/or topoisomerase IV), with or without decreased permeability, active efflux, or protection of the target site. Resistance to this drug class is increasingly prevalent among GNB and is associated with resistance to other antimicrobial classes; for example, 20–80% of ESBL-producing enteric GNB are also resistant to fluoroquinolones. At present, quinolones should be considered unreliable as empirical therapy for infections due to GNB in critically ill patients.
In this era of increasing antimicrobial resistance, it is critical to culture the local site of infection before the initiation of antimicrobial therapy and, for systemically ill patients, to obtain blood samples for culture. Antimicrobial resistance may not always be identified by in vitro testing; therefore, it is important to assess the clinical response to treatment. Moreover, as discussed above, resistance may emerge during therapy through the induction or stable derepression of AmpC β-lactamases. In addition, drainage of abscesses, resection of necrotic tissue, and removal of infected foreign bodies are often required for cure. GNB are commonly involved in polymicrobial infections, in which the role of each individual pathogen is uncertain (Chap. 201). Although some GNB are more pathogenic than others, it is usually prudent, if possible, to design an antimicrobial regimen active against all of the GNB identified, because each is capable of pathogenicity in its own right. Lastly, for patients treated initially with a broad-spectrum empirical regimen, the regimen should be de-escalated as expeditiously as possible once susceptibility results are known and the patient has responded to therapy.
PREVENTION
(See also Chap. 168) Avoidance of inappropriate antimicrobial use is a key measure in preventing infections due to antimicrobial-resistant strains and the further development of antimicrobial resistance. Antimicrobial stewardship programs should be adopted to facilitate achievement of this goal. Diligent adherence to hand-hygiene protocols by health care personnel, cleaning/disinfection of objects that come into contact with patients (e.g., stethoscopes and blood pressure cuffs), and contact precautions should be implemented for patients colonized or infected with carbapenem-resistant (and perhaps other XDR) GNB. Avoidance of the use of indwelling devices (e.g., urinary and intravascular catheters, endotracheal tubes) and, when such devices are necessary, placement according to an appropriate protocol decrease infection risk. Likewise, protocols for daily use evaluation and removal as soon as possible should be implemented. Patient positioning (e.g., head of bed at ≥30°) and good oral hygiene decrease the incidence of pneumonia among ventilated patients. Increasing data support the implementation of universal decolonization to prevent infection in ICU patients.
ESCHERICHIA COLI INFECTIONS
![]() Strains of E. coli are united by a core genome of ~2000 genes. A strain’s ability to cause infections and the nature of such infections are defined by ancillary genes that encode various virulence factors. This experiment of nature is fluid and ongoing, as demonstrated by the recent evolution of Shiga toxin–producing enteroaggregative E. coli.
Strains of E. coli are united by a core genome of ~2000 genes. A strain’s ability to cause infections and the nature of such infections are defined by ancillary genes that encode various virulence factors. This experiment of nature is fluid and ongoing, as demonstrated by the recent evolution of Shiga toxin–producing enteroaggregative E. coli.
COMMENSAL STRAINS
For the most part, commensal E. coli variants, which constitute the bulk of the normal facultative intestinal flora in most humans, confer benefits to the host (e.g., resistance to colonization with pathogenic organisms). These strains generally lack the specialized virulence traits that enable extraintestinal and intestinal pathogenic E. coli strains to cause disease outside and within the gastrointestinal tract, respectively. However, even commensal E. coli strains can be involved in extraintestinal infections in the presence of an aggravating factor, such as a foreign body (e.g., a urinary catheter), host compromise (e.g., local anatomic or functional abnormalities, such as urinary or biliary tract obstruction or systemic immunocompromise), or an inoculum that is large or contains a mixture of bacterial species (e.g., fecal contamination of the peritoneal cavity).
EXTRAINTESTINAL PATHOGENIC STRAINS
![]() ExPEC strains are the most common enteric GNB to cause community-acquired and health care–associated bacterial infections. The emerging propensity of these strains to acquire new antimicrobial resistance mechanisms (e.g., ESBL and carbapenemase production) has posed challenges in managing ExPEC infection. One clonal group—ST131, the members of which are usually resistant to fluoroquinolones and increasingly express an ESBL (CTX-M)—has undergone global dissemination.
ExPEC strains are the most common enteric GNB to cause community-acquired and health care–associated bacterial infections. The emerging propensity of these strains to acquire new antimicrobial resistance mechanisms (e.g., ESBL and carbapenemase production) has posed challenges in managing ExPEC infection. One clonal group—ST131, the members of which are usually resistant to fluoroquinolones and increasingly express an ESBL (CTX-M)—has undergone global dissemination.
Like commensal E. coli (but in contrast to intestinal pathogenic E. coli), ExPEC strains are often found in the intestinal flora of healthy individuals and do not cause gastroenteritis in humans. Entry from their site of colonization (e.g., the colon, vagina, or oropharynx) into a normally sterile extraintestinal site (e.g., the urinary tract, peritoneal cavity, or lungs) is the rate-limiting step for infection. ExPEC strains have acquired genes encoding diverse extraintestinal virulence factors that enable the bacteria to cause infections outside the gastrointestinal tract in both normal and compromised hosts (Table 186-1). These virulence genes define ExPEC and, for the most part, are distinct from those that enable intestinal pathogenic strains to cause diarrheal disease (Table 186-2). All age groups, all types of hosts, and nearly all organs and anatomic sites are susceptible to infection by ExPEC. Even previously healthy hosts can become severely ill or die when infected with ExPEC; however, adverse outcomes are more common among hosts with comorbid illnesses and host defense abnormalities. The diversity and the medical and economic impact of ExPEC infections are evident from consideration of the following specific syndromes.
|
INTESTINAL PATHOGENIC E. COLI |
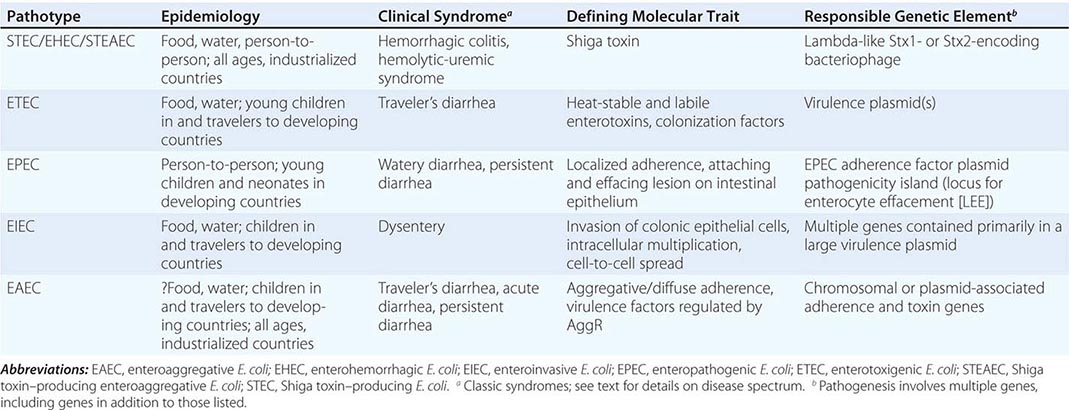
Extraintestinal Infectious Syndromes • URINARY TRACT INFECTION The urinary tract is the site most frequently infected by ExPEC. An exceedingly common infection among ambulatory patients, UTI accounts for 1% of ambulatory care visits in the United States and is second only to lower respiratory tract infection among infections responsible for hospitalization. UTIs are best considered by clinical syndrome (e.g., uncomplicated cystitis, pyelonephritis, and catheter-associated UTIs) and within the context of specific hosts (e.g., premenopausal women, compromised hosts; Chap. 162). E. coli is the single most common pathogen for all UTI syndrome/host group combinations. Each year in the United States, E. coli causes 80–90% of an estimated 6–8 million episodes of uncomplicated cystitis in premenopausal women. Furthermore, 20% of women with an initial cystitis episode develop frequent recurrences.
Uncomplicated cystitis, the most common acute UTI syndrome, is characterized by dysuria, urinary frequency, and suprapubic pain. Fever and/or back pain suggests progression to pyelonephritis. Even with appropriate treatment of pyelonephritis, fever may take 5–7 days to resolve completely. Persistently elevated or increasing fever and neutrophil counts should prompt evaluation for intrarenal or perinephric abscess and/or obstruction. Renal parenchymal damage and loss of renal function during pyelonephritis occur primarily with urinary obstruction, which can be preexisting or, rarely, occurs de novo in diabetic patients who develop renal papillary necrosis as a result of kidney infection. Pregnant women are at unusually high risk for developing pyelonephritis, which can adversely affect the outcome of pregnancy. As a result, prenatal screening for and treatment of asymptomatic bacteriuria are standard. Prostatic infection is a potential complication of UTI in men. The diagnosis and treatment of UTI, as detailed in Chap. 162, should be tailored to the individual host, the nature and site of infection, and local patterns of antimicrobial susceptibility.
ABDOMINAL AND PELVIC INFECTION The abdomen/pelvis is the second most common site of extraintestinal infection due to E. coli. A wide variety of clinical syndromes occur in this location, including acute peritonitis secondary to fecal contamination, spontaneous bacterial peritonitis, dialysis-associated peritonitis, diverticulitis, appendicitis, intraperitoneal or visceral abscesses (hepatic, pancreatic, splenic), infected pancreatic pseudocysts, and septic cholangitis and/or cholecystitis. In intraabdominal infections, E. coli can be isolated either alone or (as often occurs) in combination with other facultative and/or anaerobic members of the intestinal flora (Chap. 159).
PNEUMONIA E. coli is not usually considered a cause of pneumonia (Chap. 153). Indeed, enteric GNB account for only 1–3% of cases of community-acquired pneumonia, in part because these organisms only transiently colonize the oropharynx in a minority of healthy individuals. However, rates of oral colonization with E. coli and other GNB increase with severity of illness and antibiotic use. Consequently, GNB are a more common cause of pneumonia among residents of LTCFs and are the most common cause (60–70% of cases) of hospital-acquired pneumonia (Chap. 168), particularly among postoperative and ICU patients (e.g., ventilator-associated pneumonia). Pulmonary infection is usually acquired by small-volume aspiration but occasionally occurs via hematogenous spread, in which case multifocal nodular infiltrates can be seen. Tissue necrosis, probably due to bacterial cytotoxins, is common. Despite significant institutional variation, E. coli is generally the third or fourth most commonly isolated GNB in hospital-acquired pneumonia, accounting for 5–8% of episodes in both U.S.-based and Europe-based studies. Regardless of the host, pneumonia due to ExPEC is a serious disease, with high crude and attributable mortality rates (20–60% and 10–20%, respectively).
MENINGITIS (See also Chap. 164) E. coli is one of the two leading causes of neonatal meningitis, the other being group B Streptococcus. Most E. coli strains that cause neonatal meningitis possess the K1 capsular antigen and derive from a limited number of meningitis-associated clonal groups. Ventriculomegaly commonly occurs. After the first month of life, E. coli meningitis is uncommon, occurring predominantly in the setting of surgical or traumatic disruption of the meninges or in the presence of cirrhosis. In patients with cirrhosis who develop meningitis, the meninges are presumably seeded as a result of poor hepatic clearance of portal vein bacteremia.
CELLULITIS/MUSCULOSKELETAL INFECTION E. coli contributes frequently to infections of decubitus ulcers and occasionally to infections of ulcers and wounds of the lower extremity in diabetic patients and other hosts with neurovascular compromise. Osteomyelitis secondary to contiguous spread can occur in these settings. E. coli also causes cellulitis or infections of burn sites and surgical wounds (accounting for ~10% of surgical site infections), particularly when the infection originates close to the perineum. Hematogenously acquired osteomyelitis, especially of vertebral bodies, is more commonly caused by E. coli than is generally appreciated; this organism accounts for up to 10% of cases in some series (Chap. 158). E. coli occasionally causes orthopedic device–associated infection or septic arthritis and rarely causes hematogenous myositis. Upper-leg myositis or fasciitis due to E. coli should prompt an evaluation for an abdominal source with contiguous spread.
ENDOVASCULAR INFECTION Despite being one of the most common causes of bacteremia, E. coli rarely seeds native heart valves. When the organism does seed native valves, it usually does so in the setting of prior valvular disease. E. coli infections of aneurysms, the portal vein (pylephlebitis), and vascular grafts are quite uncommon.
MISCELLANEOUS INFECTIONS E. coli can cause infection in nearly every organ and anatomic site. It occasionally causes postoperative mediastinitis or complicated sinusitis and uncommonly causes endophthalmitis, ecthyma gangrenosum, or brain abscess.
BACTEREMIA E. COLI bacteremia can arise from primary infection at any extraintestinal site. In addition, primary E. coli bacteremia can arise from percutaneous intravascular devices or transrectal prostate biopsy or from the increased intestinal mucosal permeability seen in neonates and in the settings of neutropenia and chemotherapy-induced mucositis, trauma, and burns. Roughly equal proportions of E. coli bacteremia cases originate in the community and in health care settings. In most studies, E. coli and Staphylococcus aureus are the two most common blood isolates of clinical significance. Isolation of E. coli from the blood is almost always clinically significant and is typically accompanied by the sepsis syndrome, severe sepsis (sepsis-induced dysfunction of at least one organ or system), or septic shock (Chap. 325).
The urinary tract is the most common source of E. coli bacteremia, accounting for one-half to two-thirds of episodes. Bacteremia from a urinary tract source is particularly common among patients with pyelonephritis, urinary tract obstruction, or urinary instrumentation in the presence of infected urine. The abdomen is the second most common source, accounting for 25% of episodes. Although biliary obstruction (stones, tumor) and overt bowel disruption, which typically are readily apparent, are responsible for many of these cases, some abdominal sources (e.g., abscesses) are remarkably silent clinically and require identification via imaging studies (e.g., CT). Therefore, the physician should be cautious in designating the urinary tract as the source of E. coli bacteremia in the absence of characteristic signs and symptoms of UTI. Soft tissue, bone, pulmonary, and intravascular catheter infections are other sources of E. coli bacteremia.
Diagnosis Strains of E. coli that cause extraintestinal infections usually grow both aerobically and anaerobically within 24 h on standard diagnostic media and are easily identified by the clinical microbiology laboratory according to routine biochemical criteria. More than 90% of ExPEC strains are rapid lactose fermenters and are indole positive.
|
TREATMENT |
EXTRAINTESTINAL E. COLI INFECTIONS |
In the past, most E. coli isolates were highly susceptible to a broad range of antimicrobial agents. Unfortunately, this situation has changed. In general, the high prevalence of resistance precludes empirical use of ampicillin and amoxicillin-clavulanate, even for community-acquired infections. The prevalence of resistance to first-generation cephalosporins and TMP-SMX is increasing among community-acquired strains in the United States (with current rates of 10–40%) and is even higher outside North America. Until recently, TMP-SMX was the drug of choice for the treatment of uncomplicated cystitis in many locales. Although continued empirical use of TMP-SMX will predictably result in ever-diminishing cure rates, a wholesale switch to alternative agents (e.g., fluoroquinolones) will just as predictably accelerate the widespread emergence of resistance to these antimicrobial classes, as has already occurred in some areas. More than 90% of isolates that cause uncomplicated cystitis remain susceptible to nitrofurantoin and fosfomycin.
The prevalence of resistance to fluoroquinolones among E. coli isolates from U.S. outpatients has increased steadily over the last decade (i.e., from 3% in 2000 to 17.1% in 2010, according to one survey). Resistance rates are generally higher in the ambulatory setting outside the United States and are even higher in populations for which fluoroquinolone prophylaxis is used extensively (e.g., patients with leukemia, transplant recipients, and patients with cirrhosis) and among isolates from LTCFs and hospitals. For example, the National Healthcare Safety Network (NHSN) reported fluoroquinolone resistance in 41.8% of central line–associated bloodstream infection (CLABSI) E. coli isolates in 2009–2010, and the International Nosocomial Infection Control Consortium (INICC) reported that 53.4% of ICU E. coli isolates were resistant to quinolones in 2004–2009. Furthermore, the NHSN reported 19% resistance to third- and fourth-generation cephalosporins in CLABSI E. coli isolates, and the INICC found that 66.6% of ICU E. coli isolates were resistant to third-generation cephalosporins.
ESBL-producing strains are increasingly prevalent among both health care–associated (5–10%) and ambulatory isolates (region-dependent figures). An increasing number of reports describe community-acquired UTIs caused by E. coli strains that produce CTX-M ESBLs. Data suggest that acquisition of CTX-M-producing, fluoroquinolone-resistant strains may result from consumption of meat products from food animals treated with third- and fourth-generation cephalosporins and fluoroquinolones. Oral treatment options for such strains are limited; however, in vitro and limited clinical data indicate that, for cystitis, fosfomycin and nitrofurantoin appear to be useful options. Carbapenems and amikacin are the most predictably active agents overall, but carbapenemase-producing strains are on the rise (1–5% among health care–associated isolates in the United States and higher rates in many other countries). Tigecycline and the polymyxins, with or without a second agent, have been used most frequently against these extremely resistant isolates.
This evolving antimicrobial resistance—a source of serious concern—necessitates not only the increasing use of broad-spectrum agents but also the use of the most appropriate narrower-spectrum agent whenever possible and the avoidance of treatment of colonized but uninfected patients.
INTESTINAL PATHOGENIC STRAINS
Pathotypes Certain strains of E. coli are capable of causing diarrheal disease. Other important intestinal pathogens are discussed in Chaps. 160, 161, and 190–193. At least in the industrialized world, intestinal pathogenic strains of E. coli are rarely encountered in the fecal flora of healthy persons and instead appear to be essentially obligate pathogens. These strains have evolved a special ability to cause enteritis, enterocolitis, and colitis when ingested in sufficient quantities by a naive host. At least five distinct pathotypes of intestinal pathogenic E. coli exist: (1) Shiga toxin–producing E. coli (STEC), which includes the subsets of enterohemorrhagic E. coli (EHEC) and the recently evolved Shiga toxin–producing enteroaggregative E. coli (STEAEC); (2) enterotoxigenic E. coli (ETEC); (3) enteropathogenic E. coli (EPEC); (4) enteroinvasive E. coli (EIEC); and (5) enteroaggregative E. coli (EAEC). Diffusely adherent E. coli (DAEC) and cytodetaching E. coli are additional putative pathotypes. Lastly, a variant termed adherent invasive E. coli (AIEC) has been associated with Crohn’s disease (although a causal role remains unproven) but does not cause acute diarrheal disease. Transmission occurs predominantly via contaminated food and water for ETEC, STEC/EHEC/STEAEC, EIEC, and EAEC and by person-to-person spread for EPEC (and occasionally STEC/EHEC/STEAEC). Gastric acidity confers some protection against infection; therefore, persons with decreased stomach acid levels are especially susceptible. Humans are the major reservoir (except for STEC/EHEC, with regard to which bovines are the main concern); host range appears to be dictated by species-specific attachment factors. Although there is some overlap, each patho-type possesses a largely unique combination of virulence traits that results in a distinctive intestinal pathogenic mechanism (Table 186-2). These strains are largely incapable of causing disease outside the intestinal tract. Except in the cases of STEC/EHEC/STEAEC and EAEC, disease due to this group of pathogens occurs primarily in developing countries.
ENTEROHEMORRHAGIC E. COLI/SHIGA TOXIN–PRODUCING ENTEROAGGREGATIVE E. COLI STEC/EHEC/STEAEC strains constitute an emerging group of pathogens that can cause hemorrhagic colitis and the hemolytic-uremic syndrome (HUS). Several large outbreaks resulting from the consumption of fresh produce (e.g., lettuce, spinach, sprouts) and of undercooked ground beef have received significant attention in the media. An outbreak in central Europe in 2011 due to STEAEC (O104:H4) that was probably transmitted by sprouts, with some subsequent human-to-human transmission, resulted in more than 800 cases of HUS and 54 deaths. Within this group of organisms, O157:H7 is the most prominent serotype, but many other serotypes have also been associated with these syndromes, including O6, O26, O45, O55, O91, O103, O111, O113, O121, O145, and OX3.
The ability of STEC/EHEC/STEAEC to produce Shiga toxin (Stx2 and/or Stx1) or related toxins is a critical factor in the occurrence of clinical disease. Shigella dysenteriae strains that produce the closely related Shiga toxin Stx can cause the same syndrome. Stx2 and its Stx2C variant (which may be variably present in combination with Stx2 and/or Stx1) appear to be more important than Stx1 in the development of HUS. All Shiga toxins studied to date are multimers comprising one enzymatically active A subunit and five identical B subunits that mediate binding to globosyl ceramides, which are membrane-associated glycolipids expressed on certain host cells. As in ricin, the A subunit cleaves an adenine from the host cell’s 28S rRNA, thereby irreversibly inhibiting ribosomal function and potentially leading to apoptosis. Stx2-mediated activation of complement may also play a role in the development of HUS.
Additional properties, such as acid tolerance and epithelial cell adherence, are necessary for full pathogenicity among STEC strains. Most disease-causing isolates possess the chromosomal locus for enterocyte effacement (LEE). This pathogenicity island was first described in EPEC strains and contains genes that mediate adherence to intestinal epithelial cells and a system that subverts host cells by the translocation of bacterial proteins (type III secretion system). EHEC strains make up the subgroup of STEC strains that possess stx1 and/or stx2 as well as LEE. STEAEC (LEE-negative) evolved from EAEC via the acquisition of a number of genes, including those that encode Stx2, the Iha adhesin, tellurite resistance, a type VI secretion system, and the CTX-M-15 ESBL.
Domesticated ruminant animals, particularly cattle and young calves, serve as the major reservoir for STEC/EHEC. Ground beef—the most common food source of STEC/EHEC strains—is often contaminated during processing. Furthermore, manure from cattle or other animals (including that in the form of fertilizer) can contaminate produce (potatoes, lettuce, spinach, sprouts, fallen fruits, nuts, strawberries), and fecal runoff from this source can contaminate water systems. Dairy products and petting zoos are additional sources of infection. By contrast, humans appear to be the reservoir for STEAEC. It is estimated that <102 colony-forming units (CFU) of STEC/EHEC/STEAEC can cause disease. Therefore, not only can low levels of food or environmental contamination (e.g., in water swallowed while swimming) result in disease, but person-to-person transmission (e.g., at day-care centers and in institutions) is an important route for secondary spread. Laboratory-associated infections also occur. Illness due to this group of pathogens occurs both as outbreaks and as sporadic cases, with a peak incidence in the summer months.
![]() In contrast to other intestinal pathotypes, STEC/EHEC/STEAEC causes infections more frequently in industrialized countries than in developing regions. O157:H7 strains are the fourth most commonly reported cause of bacterial diarrhea in the United States (after Campylobacter, Salmonella, and Shigella). Colonization of the colon and perhaps the ileum results in symptoms after an incubation period of 3 or 4 days. Colonic edema and an initial nonbloody secretory diarrhea may develop into the STEC/EHEC/STEAEC hallmark syndrome of grossly bloody diarrhea (identified by history or examination) in >90% of cases. Significant abdominal pain and fecal leukocytes are common (70% of cases), whereas fever is not; absence of fever can incorrectly lead to consideration of noninfectious conditions (e.g., intussusception and inflammatory or ischemic bowel disease). Occasionally, infections caused by C. difficile, K. oxytoca (see “Klebsiella Infections,” below), Campylobacter, and Salmonella present in a similar fashion. STEC/EHEC disease is usually self-limited, lasting 5–10 days. An uncommon but feared complication of this infection is HUS, which occurs 2–14 days after diarrhea in 2–8% of cases, most often affecting very young or elderly patients. Distinctive features of STEAEC infection, as compared with classical STEC/EHEC disease, include a higher incidence among adults, especially young women, and a higher rate of HUS (~20%). It is estimated that >50% of all cases of HUS in the United States and 90% of HUS cases in children are caused by STEC/EHEC. This complication is mediated by the systemic translocation of Shiga toxins. Erythrocytes may serve as carriers of Stx to endothelial cells located in the small vessels of the kidney and brain. The subsequent development of thrombotic microangiopathy (perhaps with direct toxin-mediated effects on various nonendothelial cells) commonly produces some combination of fever, thrombocytopenia, renal failure, and encephalopathy. Although the mortality rate with dialysis support is <10%, residual renal and neurologic dysfunction may persist.
In contrast to other intestinal pathotypes, STEC/EHEC/STEAEC causes infections more frequently in industrialized countries than in developing regions. O157:H7 strains are the fourth most commonly reported cause of bacterial diarrhea in the United States (after Campylobacter, Salmonella, and Shigella). Colonization of the colon and perhaps the ileum results in symptoms after an incubation period of 3 or 4 days. Colonic edema and an initial nonbloody secretory diarrhea may develop into the STEC/EHEC/STEAEC hallmark syndrome of grossly bloody diarrhea (identified by history or examination) in >90% of cases. Significant abdominal pain and fecal leukocytes are common (70% of cases), whereas fever is not; absence of fever can incorrectly lead to consideration of noninfectious conditions (e.g., intussusception and inflammatory or ischemic bowel disease). Occasionally, infections caused by C. difficile, K. oxytoca (see “Klebsiella Infections,” below), Campylobacter, and Salmonella present in a similar fashion. STEC/EHEC disease is usually self-limited, lasting 5–10 days. An uncommon but feared complication of this infection is HUS, which occurs 2–14 days after diarrhea in 2–8% of cases, most often affecting very young or elderly patients. Distinctive features of STEAEC infection, as compared with classical STEC/EHEC disease, include a higher incidence among adults, especially young women, and a higher rate of HUS (~20%). It is estimated that >50% of all cases of HUS in the United States and 90% of HUS cases in children are caused by STEC/EHEC. This complication is mediated by the systemic translocation of Shiga toxins. Erythrocytes may serve as carriers of Stx to endothelial cells located in the small vessels of the kidney and brain. The subsequent development of thrombotic microangiopathy (perhaps with direct toxin-mediated effects on various nonendothelial cells) commonly produces some combination of fever, thrombocytopenia, renal failure, and encephalopathy. Although the mortality rate with dialysis support is <10%, residual renal and neurologic dysfunction may persist.
![]() ENTEROTOXIGENIC E. COLI In tropical or developing countries, ETEC is a major cause of endemic diarrhea. After weaning, children in these locales commonly experience several episodes of ETEC infection during the first 3 years of life. The incidence of disease diminishes with age, a pattern that correlates with the development of mucosal immunity to colonization factors (i.e., adhesins). In industrialized countries, infection usually follows travel to endemic areas, although occasional food-borne outbreaks occur. ETEC is the most common agent of traveler’s diarrhea, causing 25–75% of cases. The incidence of infection may be decreased by prudent avoidance of potentially contaminated fluids and foods, particularly items that are poorly cooked, unpeeled, or unrefrigerated (Chap. 149). ETEC infection is uncommon in the United States, but outbreaks secondary to consumption of food products imported from endemic areas have occurred. A large inoculum (106–1010 CFU) is needed to produce disease, which usually develops after an incubation period of 12–72 h.
ENTEROTOXIGENIC E. COLI In tropical or developing countries, ETEC is a major cause of endemic diarrhea. After weaning, children in these locales commonly experience several episodes of ETEC infection during the first 3 years of life. The incidence of disease diminishes with age, a pattern that correlates with the development of mucosal immunity to colonization factors (i.e., adhesins). In industrialized countries, infection usually follows travel to endemic areas, although occasional food-borne outbreaks occur. ETEC is the most common agent of traveler’s diarrhea, causing 25–75% of cases. The incidence of infection may be decreased by prudent avoidance of potentially contaminated fluids and foods, particularly items that are poorly cooked, unpeeled, or unrefrigerated (Chap. 149). ETEC infection is uncommon in the United States, but outbreaks secondary to consumption of food products imported from endemic areas have occurred. A large inoculum (106–1010 CFU) is needed to produce disease, which usually develops after an incubation period of 12–72 h.
After adherence of ETEC via colonization factors (e.g., CFA/I, CS1-6), disease is mediated primarily by a heat-labile toxin (LT-1) and/or a heat-stable toxin (STa) that causes net fluid secretion via activation of adenylate cyclase (LT-1) and/or guanylate cyclase (STa) in the jejunum and ileum. The result is watery diarrhea accompanied by cramps. LT-1 consists of an A and a B subunit and is structurally and functionally similar to cholera toxin. Strong binding of the B subunit to the GM1 ganglioside on intestinal epithelial cells leads to the intracellular translocation of the A subunit, which functions as an ADP-ribosyltransferase. Mature STa is an 18- or 19-amino-acid secreted peptide whose biologic activity is mediated by binding to the guanylate cyclase C found in the brush-border membrane of enterocytes and results in increased intracellular concentrations of cyclic GMP. Characteristically absent in ETEC-mediated disease are histopathologic changes within the small bowel; mucus, blood, and inflammatory cells in stool; and fever. The disease spectrum ranges from a mild illness to a life-threatening cholera-like syndrome. Although symptoms are usually self-limited (typically lasting for 3 days), infection may result in significant morbidity and mortality (mostly from profound volume depletion) when access to health care or suitable rehydration fluids is limited and when small and/or undernourished children are affected.
![]() ENTEROPATHOGENIC E. COLI EPEC causes disease primarily in young children, including neonates. The first E. coli pathotype recognized as an agent of diarrheal disease, EPEC was responsible for outbreaks of infantile diarrhea (including some outbreaks in hospital nurseries) in industrialized countries in the 1940s and 1950s. At present, EPEC infection is an uncommon cause of diarrhea in developed countries but is an important cause of diarrhea (both sporadic and epidemic) among infants in developing countries. Breast-feeding diminishes the incidence of EPEC infection. Rapid person-to-person spread may occur. Upon colonization of the small bowel, symptoms develop after a brief incubation period (1 or 2 days). Initial localized adherence via bundle-forming pili leads to a characteristic effacement of microvilli, with the formation of cuplike, actin-rich pedestals mediated by factors in the LEE. Diarrhea production is a complex and regulated process in which host cell modulation by a type III secretion system plays an important role. Strains lacking bundle-forming pili have been categorized as atypical EPEC (aEPEC); increasing data support a role for these strains as intestinal pathogens. Diarrheal stool often contains mucus but not blood. Although EPEC diarrhea is usually self-limited (lasting 5–15 days), it may persist for weeks.
ENTEROPATHOGENIC E. COLI EPEC causes disease primarily in young children, including neonates. The first E. coli pathotype recognized as an agent of diarrheal disease, EPEC was responsible for outbreaks of infantile diarrhea (including some outbreaks in hospital nurseries) in industrialized countries in the 1940s and 1950s. At present, EPEC infection is an uncommon cause of diarrhea in developed countries but is an important cause of diarrhea (both sporadic and epidemic) among infants in developing countries. Breast-feeding diminishes the incidence of EPEC infection. Rapid person-to-person spread may occur. Upon colonization of the small bowel, symptoms develop after a brief incubation period (1 or 2 days). Initial localized adherence via bundle-forming pili leads to a characteristic effacement of microvilli, with the formation of cuplike, actin-rich pedestals mediated by factors in the LEE. Diarrhea production is a complex and regulated process in which host cell modulation by a type III secretion system plays an important role. Strains lacking bundle-forming pili have been categorized as atypical EPEC (aEPEC); increasing data support a role for these strains as intestinal pathogens. Diarrheal stool often contains mucus but not blood. Although EPEC diarrhea is usually self-limited (lasting 5–15 days), it may persist for weeks.
![]() ENTEROINVASIVE E. COLI EIEC, a relatively uncommon cause of diarrhea, is rarely identified in the United States, although a few food-related outbreaks have been described. In developing countries, sporadic disease is infrequently recognized in children and travelers. EIEC shares many genetic and clinical features with Shigella, both of which evolved from a common ancestor. However, unlike Shigella, EIEC produces disease only with a large inoculum (108–1010 CFU), with onset generally following an incubation period of 1–3 days. Initially, enterotoxins are believed to induce secretory small-bowel diarrhea. Subsequently, colonization and invasion of the colonic mucosa, followed by replication therein and cell-to-cell spread, result in the development of inflammatory colitis characterized by fever, abdominal pain, tenesmus, and scant stool containing mucus, blood, and inflammatory cells. Symptoms are usually self-limited (7–10 days).
ENTEROINVASIVE E. COLI EIEC, a relatively uncommon cause of diarrhea, is rarely identified in the United States, although a few food-related outbreaks have been described. In developing countries, sporadic disease is infrequently recognized in children and travelers. EIEC shares many genetic and clinical features with Shigella, both of which evolved from a common ancestor. However, unlike Shigella, EIEC produces disease only with a large inoculum (108–1010 CFU), with onset generally following an incubation period of 1–3 days. Initially, enterotoxins are believed to induce secretory small-bowel diarrhea. Subsequently, colonization and invasion of the colonic mucosa, followed by replication therein and cell-to-cell spread, result in the development of inflammatory colitis characterized by fever, abdominal pain, tenesmus, and scant stool containing mucus, blood, and inflammatory cells. Symptoms are usually self-limited (7–10 days).
![]() ENTEROAGGREGATIVE AND DIFFUSELY ADHERENT E. COLI EAEC has been described primarily in developing countries and in young children. However, recent studies indicate that it may be a relatively common cause of diarrhea in all age groups in industrialized countries. EAEC has also been recognized increasingly as an important cause of traveler’s diarrhea. It is highly adapted to humans, the probable reservoir. A large inoculum is required for infection, which usually manifests as watery and sometimes persistent diarrhea in healthy, malnourished, and HIV-infected hosts. In vitro, the organisms exhibit a diffuse or “stacked-brick” pattern of adherence to small-intestine epithelial cells. Virulence factors that probably are necessary for disease are regulated in part by the transcriptional activator AggR and include the aggregative adherence fimbriae (AAF/I-III); the Hda adhesin; the mucinase Pic; the enterotoxins Pet, EAST-1, ShET1, and HlyE; and dispersin, an antiaggregation protein that promotes mucosal spread. Some strains of DAEC are capable of causing diarrheal disease, primarily in children 2–6 years of age in some developing countries, and may perhaps cause traveler’s diarrhea. The Afa/Dr adhesins may contribute to the pathogenesis of such infections.
ENTEROAGGREGATIVE AND DIFFUSELY ADHERENT E. COLI EAEC has been described primarily in developing countries and in young children. However, recent studies indicate that it may be a relatively common cause of diarrhea in all age groups in industrialized countries. EAEC has also been recognized increasingly as an important cause of traveler’s diarrhea. It is highly adapted to humans, the probable reservoir. A large inoculum is required for infection, which usually manifests as watery and sometimes persistent diarrhea in healthy, malnourished, and HIV-infected hosts. In vitro, the organisms exhibit a diffuse or “stacked-brick” pattern of adherence to small-intestine epithelial cells. Virulence factors that probably are necessary for disease are regulated in part by the transcriptional activator AggR and include the aggregative adherence fimbriae (AAF/I-III); the Hda adhesin; the mucinase Pic; the enterotoxins Pet, EAST-1, ShET1, and HlyE; and dispersin, an antiaggregation protein that promotes mucosal spread. Some strains of DAEC are capable of causing diarrheal disease, primarily in children 2–6 years of age in some developing countries, and may perhaps cause traveler’s diarrhea. The Afa/Dr adhesins may contribute to the pathogenesis of such infections.
Diagnosis A practical approach to the evaluation of diarrhea is to distinguish noninflammatory from inflammatory cases; the latter is suggested by grossly bloody or mucoid stool or a positive test for fecal leukocytes (Chap. 160). ETEC, EPEC, and DAEC cause noninflammatory diarrhea and are uncommon in the United States; in this country, the incidence of EAEC infection, which also causes noninflammatory diarrhea, may be underrecognized. The diagnosis of these infections requires specialized assays (e.g., polymerase chain reaction–based tests for pathotype-specific genes) that are not routinely available and are rarely needed because the diseases are self-limited. ETEC causes the majority and EAEC a minority of cases of noninflammatory traveler’s diarrhea. Definitive diagnosis generally is not necessary. Empirical antimicrobial (or symptom-based) treatment, along with rehydration therapy, is a reasonable approach. If diarrhea persists for >10 days despite treatment, Giardia or Cryptosporidium (or, in immunocompromised hosts, certain other microbial agents) should be sought. The diagnosis of infection with EIEC, a rare cause of inflammatory diarrhea in the United States, also requires specialized assays. The CDC now recommends that all patients with community-acquired diarrhea, whether inflammatory or not, be evaluated for STEC/EHEC/STEAEC infection by simultaneous culture (which is important for outbreak detection and control) and assay for the detection of Shiga toxin or its associated genes. The reasons for this recommendation are that bloody stool is not always present and detection of fecal white blood cells is not optimally sensitive for the diagnosis of STEC/EHEC/STEAEC infection. The use of both tests increases the rate of identification of infection over rates obtained with either test alone. O157 STEC/EHEC may be identified via culture by screening for E. coli strains that do not ferment sorbitol, with subsequent serotyping and testing for Shiga toxin. Selective or screening media are not available for the culture of non-O157 strains. Detection of Shiga toxins or toxin genes via DNA-based, enzyme-linked immunosorbent, and cytotoxicity assays offers the advantages of rapidity plus detection of non-O157 STEC/EHEC/STEAEC strains. Specimens positive for toxin but culture-negative for O157 should be forwarded to the local or state public health laboratory.
|
TREATMENT |
INTESTINAL E. COLI INFECTIONS |
(See also Chap. 128) The mainstay of treatment for all diarrheal syndromes is replacement of water and electrolytes. This measure is especially important for STEC/EHEC/STEAEC infection because appropriate volume expansion may decrease renal damage and improve outcome. The use of prophylactic antibiotics to prevent traveler’s diarrhea generally should be discouraged, especially in light of high rates of antimicrobial resistance. However, in selected patients (e.g., those who cannot afford a brief illness or are predisposed to infection), the use of rifaximin, which is nonabsorbable and is well tolerated, is reasonable. When stools are free of mucus and blood, early patient-initiated treatment of traveler’s diarrhea with a fluoroquinolone or azithromycin decreases the duration of illness, and the use of loperamide may halt symptoms within a few hours. Although dysentery caused by EIEC is self-limited, treatment hastens the resolution of symptoms, particularly in severe cases. In contrast, antimicrobial therapy for STEC/EHEC/STEAEC infection (the presence of which is suggested by grossly bloody diarrhea without fever) should be avoided because antibiotics may increase the incidence of HUS (possibly via increased production/release of Stx). The role of plasmapheresis and inhibition of C5 (eculizumab) in the treatment of HUS is unresolved.
KLEBSIELLA INFECTIONS
![]() K. pneumoniae is the most important Klebsiella species from a medical standpoint, causing community-acquired, LTCF-acquired, and nosocomial infections. K. oxytoca is primarily a pathogen in LTCF and hospital settings. Klebsiella species are broadly prevalent in the environment and colonize mucosal surfaces of mammals. In healthy humans, the prevalence of K. pneumoniae colonization is 5–35% in the colon and 1–5% in the oropharynx; the skin is usually colonized only transiently. Person-to-person spread is the predominant mode of acquisition. Most Klebsiella infections in Western countries are caused by “classic” K. pneumoniae (cKP) and occur in hospitals and LTCFs. The most common clinical syndromes due to cKP are pneumonia, UTI, abdominal infection, intravascular device infection, surgical site infection, soft tissue infection, and subsequent bacteremia. cKP strains have gained notoriety because their propensity for acquiring antimicrobial resistance determinants makes treatment challenging. Clonal group ST258, many members of which produce the KPC carbapenemase, is undergoing international dissemination. The spread of NDM-1 carbapenemase-producing strains from India in association with medical tourism has captured the attention of physicians and the lay press.
K. pneumoniae is the most important Klebsiella species from a medical standpoint, causing community-acquired, LTCF-acquired, and nosocomial infections. K. oxytoca is primarily a pathogen in LTCF and hospital settings. Klebsiella species are broadly prevalent in the environment and colonize mucosal surfaces of mammals. In healthy humans, the prevalence of K. pneumoniae colonization is 5–35% in the colon and 1–5% in the oropharynx; the skin is usually colonized only transiently. Person-to-person spread is the predominant mode of acquisition. Most Klebsiella infections in Western countries are caused by “classic” K. pneumoniae (cKP) and occur in hospitals and LTCFs. The most common clinical syndromes due to cKP are pneumonia, UTI, abdominal infection, intravascular device infection, surgical site infection, soft tissue infection, and subsequent bacteremia. cKP strains have gained notoriety because their propensity for acquiring antimicrobial resistance determinants makes treatment challenging. Clonal group ST258, many members of which produce the KPC carbapenemase, is undergoing international dissemination. The spread of NDM-1 carbapenemase-producing strains from India in association with medical tourism has captured the attention of physicians and the lay press.
cKP strains appear to be phenotypically and clinically distinct from hypervirulent K. pneumoniae (hvKP), an emerging variant that was first recognized in Taiwan in 1986. Although hvKP infections have occurred globally in all ethnic groups, the majority have been reported in the Asian Pacific Rim. This concentration of cases raises the question of whether a geo-specific distribution of the organism or increased susceptibility of Asian hosts is responsible. In contrast to the usual health care–associated venue for cKP infections in the West, hvKP causes serious life- and organ-threatening infections in younger, healthy individuals from the community and can spread metastatically from primary sites of infection. hvKP infection initially was characterized and distinguished from traditional infections due to cKP by (1) presentation as community-acquired pyogenic liver abscess (Fig. 186-1, top), (2) occurrence in patients lacking a history of hepatobiliary disease, and (3) a propensity for metastatic spread to distant sites (e.g., eyes, central nervous system, lungs), which occurred in 11–80% of cases. More recently, this variant has been recognized as the cause of a variety of serious community-acquired extrahepatic abscesses/infections in the absence of liver involvement, including pneumonia, meningitis, endophthalmitis (Fig. 186-1, middle), splenic abscess, and necrotizing fasciitis. The affected individuals often have diabetes mellitus and are of Asian ethnicity; however, nondiabetics and all ethnic groups can be affected. Survivors often suffer catastrophic morbidity, such as loss of vision and neurologic sequelae.
FIGURE 186-1 New hypervirulent variant of K. pneumoniae (hvKP). Top: Abdominal CT scan of a previously healthy 24-year-old Vietnamese man shows a primary liver abscess (red arrow) with metastatic spread to the spleen (black arrow). (Courtesy of Drs. Chiu-Bin Hsaio and Diana Pomakova.) Middle: A previously healthy 33-year-old Chinese man presented with endophthalmitis. (From Virulence 4:2, 1-12 Feb. 15, 2013.) Bottom: A hypermucoviscous phenotype (which does not necessarily equate with a mucoid phenotype) has been associated with hvKP strains. This phenotype has been semiquantitatively defined by a positive “string test” (formation of a viscous string >5 mm long when bacterial colonies on an agar plate are stretched by an inoculation loop). (Courtesy of Dr. Russo.)
K. pneumoniae subspecies rhinoscleromatis is the causative agent of rhinoscleroma, a granulomatous mucosal upper respiratory infection that progresses slowly (over months or years) and causes necrosis and occasionally obstruction of the nasal passages. K. pneumoniae subspecies ozaenae has been implicated as a cause of chronic atrophic rhinitis and rarely of invasive disease in compromised hosts. These two K. pneumoniae subspecies are usually isolated from patients in tropical climates and are genomically distinct from both cKP and hvKP.
INFECTIOUS SYNDROMES
![]() Pneumonia Although cKP accounts for only a small proportion of cases of community-acquired pneumonia in Western countries (Chap. 153), cKP and K. oxytoca are common causes of pneumonia among LTCF residents and hospitalized patients because of increased rates of oropharyngeal colonization. Mechanical ventilation is an important risk factor. In Asia and South Africa, community-acquired pneumonia due to hvKP is becoming increasingly common and often occurs in younger patients with no underlying disease. Klebsiella is also a common cause of pneumonia in severely malnourished children in developing countries.
Pneumonia Although cKP accounts for only a small proportion of cases of community-acquired pneumonia in Western countries (Chap. 153), cKP and K. oxytoca are common causes of pneumonia among LTCF residents and hospitalized patients because of increased rates of oropharyngeal colonization. Mechanical ventilation is an important risk factor. In Asia and South Africa, community-acquired pneumonia due to hvKP is becoming increasingly common and often occurs in younger patients with no underlying disease. Klebsiella is also a common cause of pneumonia in severely malnourished children in developing countries.
As in all pneumonias due to enteric GNB, production of purulent sputum and evidence of airspace disease are typical. Presentation with earlier, less extensive infection is now more common than that with the classically described lobar infiltrate and bulging fissure. Pulmonary infection due to hvKP that has spread metastatically (e.g., from a hepatic abscess) usually includes nodular bilateral densities, more commonly in the lower lobes. Pulmonary necrosis, pleural effusion, and empyema can occur with disease progression.
UTI cKP accounts for only 1–2% of UTI episodes among otherwise healthy adults but for 5–17% of episodes of complicated UTI, including infections associated with indwelling urinary catheters. UTI due to hvKP presents more commonly as renal or prostatic abscess due to bacteremic spread than as ascending infection.
![]() Abdominal Infection cKP causes a spectrum of abdominal infections similar to that caused by E. coli but is less frequently isolated from these infections. hvKP is a common cause of monomicrobial community-acquired pyogenic liver abscess and in the Asian Pacific Rim has been recovered with steadily increasing frequency over the past two decades, replacing E. coli as the most common pathogen causing this syndrome. hvKP is increasingly described as a cause of spontaneous bacterial peritonitis and splenic abscess.
Abdominal Infection cKP causes a spectrum of abdominal infections similar to that caused by E. coli but is less frequently isolated from these infections. hvKP is a common cause of monomicrobial community-acquired pyogenic liver abscess and in the Asian Pacific Rim has been recovered with steadily increasing frequency over the past two decades, replacing E. coli as the most common pathogen causing this syndrome. hvKP is increasingly described as a cause of spontaneous bacterial peritonitis and splenic abscess.
![]() Other Infections cKP- and K. oxytoca–mediated cellulitis or soft tissue infection most frequently affects devitalized tissue (e.g., decubitus and diabetic ulcers, burn sites) and immunocompromised hosts. cKP and K. oxytoca cause some cases of surgical site infection and nosocomial sinusitis in addition to occasional cases of osteomyelitis contiguous to soft tissue infection, nontropical myositis, and meningitis (both during the neonatal period and after neurosurgery). By contrast, hvKP has become an important cause of community-acquired monomicrobial necrotizing fasciitis; meningitis; brain, subdural, and epidural abscess; and endophthalmitis (Fig. 186-1, middle), particularly in the Asian Pacific Rim but also globally. Cytotoxin-producing strains of K. oxytoca have been implicated as a cause of hemorrhagic antibiotic-associated non–C. difficile colitis.
Other Infections cKP- and K. oxytoca–mediated cellulitis or soft tissue infection most frequently affects devitalized tissue (e.g., decubitus and diabetic ulcers, burn sites) and immunocompromised hosts. cKP and K. oxytoca cause some cases of surgical site infection and nosocomial sinusitis in addition to occasional cases of osteomyelitis contiguous to soft tissue infection, nontropical myositis, and meningitis (both during the neonatal period and after neurosurgery). By contrast, hvKP has become an important cause of community-acquired monomicrobial necrotizing fasciitis; meningitis; brain, subdural, and epidural abscess; and endophthalmitis (Fig. 186-1, middle), particularly in the Asian Pacific Rim but also globally. Cytotoxin-producing strains of K. oxytoca have been implicated as a cause of hemorrhagic antibiotic-associated non–C. difficile colitis.
![]() Bacteremia Klebsiella infection at any site can produce bacteremia. Infections of the urinary tract, respiratory tract, and abdomen (especially hepatic abscess) each account for 15–30% of episodes of Klebsiella bacteremia. Intravascular device–related infections account for another 5–15% of episodes, and surgical site and miscellaneous infections account for the rest. Klebsiella is a cause of sepsis in neonates and of bacteremia in neutropenic patients. Like enteric GNB in general, Klebsiella rarely causes endocarditis or endovascular infection.
Bacteremia Klebsiella infection at any site can produce bacteremia. Infections of the urinary tract, respiratory tract, and abdomen (especially hepatic abscess) each account for 15–30% of episodes of Klebsiella bacteremia. Intravascular device–related infections account for another 5–15% of episodes, and surgical site and miscellaneous infections account for the rest. Klebsiella is a cause of sepsis in neonates and of bacteremia in neutropenic patients. Like enteric GNB in general, Klebsiella rarely causes endocarditis or endovascular infection.
DIAGNOSIS
Klebsiellae are readily isolated and identified in the laboratory. These organisms usually ferment lactose, although the subspecies rhinoscleromatis and ozaenae are nonfermenters and are indole negative. hvKP usually possesses a hypermucoviscous phenotype (Fig. 186-1, bottom), although the sensitivity and specificity of this test are undefined and probably less than optimal. A better diagnostic test for hvKP is desirable.
|
TREATMENT |
KLEBSIELLA INFECTIONS |
![]() cKP and K. oxytoca have similar antibiotic resistance profiles. These species are intrinsically resistant to ampicillin and ticarcillin, and nitrofurantoin is inconsistently active against them. NHSN data for 2009–2010 documented resistance to third- and fourth-generation cephalosporins in 28.9% of CLABSI isolates of cKP and K. oxytoca, and INICC data for 2004–2009 identified resistance to third-generation cephalosporins in 76.3% of ICU isolates of K. pneumoniae. This increasing resistance is mediated primarily by plasmid-encoded ESBLs. In addition, such plasmids usually encode resistance to aminoglycosides, tetracyclines, and TMP-SMX. Furthermore, isolates of cKP that produce CTX-M ESBLs have been obtained from ambulatory patients with no recent health care contact (see the section on the treatment of extraintestinal E. coli infections for treatment considerations). Resistance to β-lactam/β-lactamase inhibitor combinations and cephamycins independent of ESBL-encoding plasmids has also been described with increasing frequency, particularly in Latin America. The prevalence of fluoroquinolone resistance is 15–20% overall and is 50% among ESBL-containing strains. Given both the undesirability of treating the latter strains with penicillins or cephalosporins and the fluoroquinolone resistance often associated with ESBLs, empirical treatment of serious or health care–associated cKP and K. oxytoca infections with amikacin or carbapenems is prudent, as dictated by local susceptibilities. Predictably, however, the ESBL-driven use of carbapenems has selected for strains of cKP and K. oxytoca that express carbapenemases. NHSN data for 2009–2010 documented resistance to carbapenems in 12.8% of CLABSI isolates of cKP and K. oxytoca. Treatment of infections due to strains that produce carbapenemases is highly challenging; increasingly, these strains are nearly pan-resistant. The optimal choice for therapy is unclear. Tigecycline and the polymyxins (e.g., colistin) are the most active agents in vitro and are used most frequently. However, resistance to these agents is already emerging, and strains of cKP resistant to all known antimicrobial agents have been described in the United States and globally. Combination therapy is often used in this setting.
cKP and K. oxytoca have similar antibiotic resistance profiles. These species are intrinsically resistant to ampicillin and ticarcillin, and nitrofurantoin is inconsistently active against them. NHSN data for 2009–2010 documented resistance to third- and fourth-generation cephalosporins in 28.9% of CLABSI isolates of cKP and K. oxytoca, and INICC data for 2004–2009 identified resistance to third-generation cephalosporins in 76.3% of ICU isolates of K. pneumoniae. This increasing resistance is mediated primarily by plasmid-encoded ESBLs. In addition, such plasmids usually encode resistance to aminoglycosides, tetracyclines, and TMP-SMX. Furthermore, isolates of cKP that produce CTX-M ESBLs have been obtained from ambulatory patients with no recent health care contact (see the section on the treatment of extraintestinal E. coli infections for treatment considerations). Resistance to β-lactam/β-lactamase inhibitor combinations and cephamycins independent of ESBL-encoding plasmids has also been described with increasing frequency, particularly in Latin America. The prevalence of fluoroquinolone resistance is 15–20% overall and is 50% among ESBL-containing strains. Given both the undesirability of treating the latter strains with penicillins or cephalosporins and the fluoroquinolone resistance often associated with ESBLs, empirical treatment of serious or health care–associated cKP and K. oxytoca infections with amikacin or carbapenems is prudent, as dictated by local susceptibilities. Predictably, however, the ESBL-driven use of carbapenems has selected for strains of cKP and K. oxytoca that express carbapenemases. NHSN data for 2009–2010 documented resistance to carbapenems in 12.8% of CLABSI isolates of cKP and K. oxytoca. Treatment of infections due to strains that produce carbapenemases is highly challenging; increasingly, these strains are nearly pan-resistant. The optimal choice for therapy is unclear. Tigecycline and the polymyxins (e.g., colistin) are the most active agents in vitro and are used most frequently. However, resistance to these agents is already emerging, and strains of cKP resistant to all known antimicrobial agents have been described in the United States and globally. Combination therapy is often used in this setting.
PROTEUS INFECTIONS
Proteus mirabilis causes 90% of Proteus infections, which occur in the community, LTCFs, and hospitals. Proteus vulgaris and Proteus penneri are associated primarily with infections acquired in LTCFs or hospitals. Proteus species are part of the colonic flora of a wide variety of mammals, birds, fish, and reptiles. The ability of these GNB to generate histamine from contaminated fish has implicated them in the pathogenesis of scombroid (fish) poisoning (Chap. 474). P. mirabilis colonizes healthy humans (prevalence, 50%), whereas P. vulgaris and P. penneri are isolated primarily from individuals with underlying disease. The urinary tract is by far the most common site of Proteus infection, with adhesins, flagella, IgA-IgG protease, iron acquisition systems, and urease representing the principal known urovirulence factors. Proteus less commonly causes infection at a variety of other extraintestinal sites.
INFECTIOUS SYNDROMES
UTI Most Proteus infections arise from the urinary tract. P. mirabilis causes only 1–2% of UTIs in healthy women, and Proteus species collectively cause only 5% of hospital-acquired UTIs. However, Proteus is responsible for 10–15% of cases of complicated UTI, primarily those associated with catheterization; indeed, among UTI isolates from chronically catheterized patients, the prevalence of Proteus is 20–45%. This high prevalence is due in part to bacterial production of urease, which hydrolyzes urea to ammonia and results in alkalization of the urine. Alkalization of urine, in turn, leads to precipitation of organic and inorganic compounds, which contributes to formation of struvite and carbonate-apatite crystals, formation of biofilms on catheters, and/or development of frank calculi. Proteus becomes associated with the stones and biofilms; thereafter, it usually can be eradicated only by removal of the stones or the catheter. Over time, staghorn calculi may form within the renal pelvis and lead to obstruction and renal failure. Thus, urine samples with unexplained alkalinity should be cultured for Proteus, and identification of a Proteus species in urine should prompt consideration of an evaluation for urolithiasis.
Other Infections Proteus occasionally causes pneumonia (primarily in LTCF residents or hospitalized patients), nosocomial sinusitis, intraabdominal abscesses, biliary tract infection, surgical site infection, soft tissue infection (especially decubitus and diabetic ulcers), and osteomyelitis (primarily contiguous); in rare cases, it causes nontropical myositis. In addition, Proteus uncommonly causes neonatal meningitis, with the umbilicus frequently implicated as the source; this disease is often complicated by development of a cerebral abscess. Otogenic brain abscess also occurs.
Bacteremia The majority of Proteus bacteremia episodes originate from the urinary tract; however, any of the less common sites of infection as well as intravascular devices are also potential sources. Endovascular infection is rare. Proteus species are occasional agents of sepsis in neonates and of bacteremia in neutropenic patients.
DIAGNOSIS
Proteus is readily isolated and identified in the laboratory. Most strains are lactose negative, produce H2S, and demonstrate characteristic swarming motility on agar plates. P. mirabilis and P. penneri are indole negative, whereas P. vulgaris is indole positive. The inability to produce ornithine decarboxylase differentiates P. penneri from P. mirabilis.
|
TREATMENT |
PROTEUS INFECTIONS |
P. mirabilis is usually susceptible to most antimicrobial agents except tetracycline, nitrofurantoin, the polymyxins, and tigecycline. Resistance to ampicillin and first-generation cephalosporins has been acquired by 10–50% of strains. Overall, 10–15% of P. mirabilis isolates are resistant to fluoroquinolones; 5% of isolates in the United States now produce ESBLs. Furthermore, isolates of P. mirabilis that produce CTX-M ESBLs have been recovered from ambulatory patients with no recent health care contact (see the section on the treatment of extraintestinal E. coli infections for treatment considerations). P. vulgaris and P. penneri exhibit more extensive drug resistance than does P. mirabilis. Resistance to ampicillin and first-generation cephalosporins is the rule, and 30–40% of isolates are resistant to fluoroquinolones. Induction or selection of variants with stable derepression of chromosomal AmpC β-lactamase may occur with P. vulgaris isolates. Carbapenems, fourth-generation cephalosporins (e.g., cefepime), amikacin, TMP-SMX, and fosfomycin display excellent activity against Proteus species (90–100% of isolates susceptible).
ENTEROBACTER AND CRONOBACTER INFECTIONS
E. cloacae and E. aerogenes are responsible for most Enterobacter infections (65–75% and 15–25%, respectively); Cronobacter sakazakii (formerly Enterobacter sakazakii) and Enterobacter gergoviae are less commonly isolated (1% for each). Enterobacter species cause primarily health care–related infections. The organisms are widely prevalent in foods, environmental sources (including equipment at health care facilities), and a variety of animals. Few healthy humans are colonized, but the percentage increases significantly with LTCF residence or hospitalization. Although colonization is an important prelude to infection, direct introduction via IV lines (e.g., contaminated IV fluids or pressure monitors) also occurs. Extensive antibiotic resistance has developed in Enterobacter species and probably has contributed to the emergence of the organisms as prominent nosocomial pathogens. Individuals who have previously received antibiotic treatment, have comorbid disease, and are ICU residents are at greatest risk for infection. Enterobacter causes a spectrum of extraintestinal infections similar to that described for other GNB.
INFECTIOUS SYNDROMES
Pneumonia, UTI (particularly catheter-related), intravascular device–related infection, surgical site infection, and abdominal infection (primarily postoperative or related to devices such as biliary stents) are the most common syndromes encountered. Nosocomial sinusitis, meningitis related to neurosurgical procedures (including use of intracranial pressure monitors), osteomyelitis, and endophthalmitis after eye surgery are less frequent. C. sakazakii is associated with neonatal bacteremia, necrotizing enterocolitis, and meningitis (which is often complicated by brain abscess or ventriculitis); contaminated formula has been implicated as a source for such infections. Enterobacter bacteremia can result from infection at any anatomic site. In bacteremia of unclear origin, the contamination of IV fluids or medications, blood components or plasma derivatives, catheter-flushing fluids, pressure monitors, and dialysis equipment should be considered, particularly in an outbreak setting. Enterobacter can also cause bacteremia in neutropenic patients. Enterobacter endocarditis is rare, occurring primarily in association with illicit IV drug use or prosthetic valves.
DIAGNOSIS
Enterobacter is readily isolated and identified in the laboratory. Most strains are lactose positive and indole negative.
|
TREATMENT |
ENTEROBACTER INFECTIONS |
![]() Significant antimicrobial resistance exists among Enterobacter strains. Ampicillin and first- and second-generation cephalosporins have little or no activity. Extensive use of third-generation cephalosporins can induce or select for variants with stable derepression of AmpC β-lactamase, which confers resistance to these agents as well as monobactams (e.g., aztreonam) and—in many cases—β-lactam/β-lactamase inhibitor combinations. Resistance may emerge during therapy; in one study, this phenomenon was documented in 20% of clinical isolates. De novo resistance should be considered when clinical deterioration follows initial improvement, and third-generation cephalosporins should be avoided in the treatment of serious Enterobacter infections. Cefepime is stable in the presence of AmpC β-lactamases; thus, it is a suitable option for treatment of Enterobacter infections so long as no coexistent ESBL is present. Detection of ESBLs in Enterobacter is difficult because of the presence of AmpC β-lactamase; nonetheless, their prevalence (particularly in E. cloacae) is known to be variable worldwide but is generally increasing and is now 5–50% overall. This increase is evidenced by NHSN data, which documented resistance to third- and fourth-generation cephalosporins in 37.4% of CLABSI Enterobacter isolates in the United States; fortunately, carbapenems, amikacin, and tigecycline have generally retained excellent activity (90–99% susceptibility) and fluoroquinolones have good activity (85–95% susceptibility). Once susceptibility data become available, it is critical to de-escalate the antimicrobial regimen whenever possible.
Significant antimicrobial resistance exists among Enterobacter strains. Ampicillin and first- and second-generation cephalosporins have little or no activity. Extensive use of third-generation cephalosporins can induce or select for variants with stable derepression of AmpC β-lactamase, which confers resistance to these agents as well as monobactams (e.g., aztreonam) and—in many cases—β-lactam/β-lactamase inhibitor combinations. Resistance may emerge during therapy; in one study, this phenomenon was documented in 20% of clinical isolates. De novo resistance should be considered when clinical deterioration follows initial improvement, and third-generation cephalosporins should be avoided in the treatment of serious Enterobacter infections. Cefepime is stable in the presence of AmpC β-lactamases; thus, it is a suitable option for treatment of Enterobacter infections so long as no coexistent ESBL is present. Detection of ESBLs in Enterobacter is difficult because of the presence of AmpC β-lactamase; nonetheless, their prevalence (particularly in E. cloacae) is known to be variable worldwide but is generally increasing and is now 5–50% overall. This increase is evidenced by NHSN data, which documented resistance to third- and fourth-generation cephalosporins in 37.4% of CLABSI Enterobacter isolates in the United States; fortunately, carbapenems, amikacin, and tigecycline have generally retained excellent activity (90–99% susceptibility) and fluoroquinolones have good activity (85–95% susceptibility). Once susceptibility data become available, it is critical to de-escalate the antimicrobial regimen whenever possible.
SERRATIA INFECTIONS
![]() S. marcescens causes the majority (>90%) of Serratia infections; Serratia liquefaciens, Serratia rubidaea, Serratia fonticola, Serratia grimesii, Serratia plymuthica, and Serratia odorifera are isolated occasionally. Serratiae are found primarily in the environment (including in health care institutions), particularly in moist settings. Serratiae have been isolated from a variety of animals, insects, and plants, but healthy humans are rarely colonized. In LTCFs or hospitals, reservoirs for the organisms include the hands and fingernails of health care personnel, food, milk (on neonatal units), sinks, respiratory and other medical equipment or devices, pressure monitors, IV solutions or parenteral medications (particularly those generated by compounding pharmacies), prefilled syringes and multiple-access medication vials (e.g., heparin, saline), blood products (e.g., platelets), hand soaps and lotions, irrigation solutions, and even disinfectants. Infection results from either direct inoculation (e.g., via IV fluid) or colonization (primarily of the respiratory tract). Sporadic infection is most common, but epidemics (often involving MDR strains in adult and neonatal ICUs) and common-source outbreaks also occur. The spectrum of extraintestinal infections caused by Serratia is similar to that for other GNB. Serratia species are usually considered causative agents of health care–associated infection and account for 1–3% of hospital-acquired infections. However, population-based laboratory surveillance studies in Canada and Australia demonstrated that community-acquired infections occur more commonly than was previously appreciated.
S. marcescens causes the majority (>90%) of Serratia infections; Serratia liquefaciens, Serratia rubidaea, Serratia fonticola, Serratia grimesii, Serratia plymuthica, and Serratia odorifera are isolated occasionally. Serratiae are found primarily in the environment (including in health care institutions), particularly in moist settings. Serratiae have been isolated from a variety of animals, insects, and plants, but healthy humans are rarely colonized. In LTCFs or hospitals, reservoirs for the organisms include the hands and fingernails of health care personnel, food, milk (on neonatal units), sinks, respiratory and other medical equipment or devices, pressure monitors, IV solutions or parenteral medications (particularly those generated by compounding pharmacies), prefilled syringes and multiple-access medication vials (e.g., heparin, saline), blood products (e.g., platelets), hand soaps and lotions, irrigation solutions, and even disinfectants. Infection results from either direct inoculation (e.g., via IV fluid) or colonization (primarily of the respiratory tract). Sporadic infection is most common, but epidemics (often involving MDR strains in adult and neonatal ICUs) and common-source outbreaks also occur. The spectrum of extraintestinal infections caused by Serratia is similar to that for other GNB. Serratia species are usually considered causative agents of health care–associated infection and account for 1–3% of hospital-acquired infections. However, population-based laboratory surveillance studies in Canada and Australia demonstrated that community-acquired infections occur more commonly than was previously appreciated.
INFECTIOUS SYNDROMES
The respiratory tract, the genitourinary tract, intravascular devices, the eye (contact lens–associated keratitis and other ocular infections), surgical wounds, and the bloodstream (from contaminated infusions) are the most common sites of Serratia infection; the former five sites are the most common sources of Serratia bacteremia. Soft tissue infections (including myositis, fasciitis, mastitis), osteomyelitis, abdominal and biliary tract infection (postprocedural), and septic arthritis (primarily from intraarticular injections) occur less commonly. Serratiae are uncommon causes of neonatal or postsurgical meningitis and of bacteremia in neutropenic patients. Endocarditis is rare.
DIAGNOSIS
Serratiae are readily cultured and identified by the laboratory and are usually lactose and indole negative. Some S. marcescens strains and S. rubidaea are red pigmented.
|
TREATMENT |
SERRATIA INFECTIONS |
![]() Most Serratia strains (>80%) are resistant to ampicillin, amoxicillin-clavulanate, ampicillin-sulbactam, first-generation cephalosporins, cephamycins, nitrofurantoin, and colistin. In general, >90% of Serratia isolates are susceptible to other antibiotics appropriate for use against GNB. Induction or selection of variants with stable derepression of chromosomal AmpC β-lactamases may develop during therapy. Both in the United States and globally, the prevalence of ESBL-producing isolates is generally low (<5%), but rates of 20–30% have been reported in Asia and Latin America. Acquisition of carbapenemase-encoding genes is uncommon but increasing.
Most Serratia strains (>80%) are resistant to ampicillin, amoxicillin-clavulanate, ampicillin-sulbactam, first-generation cephalosporins, cephamycins, nitrofurantoin, and colistin. In general, >90% of Serratia isolates are susceptible to other antibiotics appropriate for use against GNB. Induction or selection of variants with stable derepression of chromosomal AmpC β-lactamases may develop during therapy. Both in the United States and globally, the prevalence of ESBL-producing isolates is generally low (<5%), but rates of 20–30% have been reported in Asia and Latin America. Acquisition of carbapenemase-encoding genes is uncommon but increasing.
CITROBACTER INFECTIONS
C. freundii and Citrobacter koseri cause most human Citrobacter infections, which are epidemiologically and clinically similar to Enterobacter infections. Citrobacter species are commonly present in water, food, soil, and certain animals. Citrobacter is part of the normal fecal flora in a minority of healthy humans, but colonization rates are higher in LTCFs and hospitals—the settings in which nearly all Citrobacter infections occur. Citrobacter species account for 1–2% of nosocomial infections. The affected hosts are usually immunocompromised or have comorbid disease. Citrobacter causes extraintestinal infections similar to those described for other GNB.
INFECTIOUS SYNDROMES
The urinary tract accounts for 40–50% of Citrobacter infections. Less commonly involved sites include the biliary tree (particularly with stones or obstruction), the respiratory tract, surgical sites, soft tissue (e.g., decubitus ulcers), the peritoneum, and intravascular devices. Osteomyelitis (usually from a contiguous focus), adult central nervous system infection (from neurosurgical or other types of meningeal disruption), and myositis occur rarely. Citrobacter (primarily C. koseri) also causes 1–2% of neonatal meningitis cases, of which 50–80% are complicated by brain abscess. Further, case reports in adults suggest that C. koseri infection has a predilection for abscess formation. Bacteremia is most often due to UTI, biliary/abdominal infection, or intravascular device infection. Citrobacter occasionally causes bacteremia in neutropenic patients. Endocarditis and endovascular infections are rare.
DIAGNOSIS
Citrobacter species are readily isolated and identified; 35–50% of isolates are lactose positive, and 100% are oxidase negative. C. freundii is indole negative, whereas C. koseri is indole positive.
|
TREATMENT |
CITROBACTER INFECTIONS |
C. freundii is more extensively resistant to antibiotics than is C. koseri. More than 90% of isolates are resistant to ampicillin and first- and second-generation cephalosporins. Citrobacter species (except C. koseri) possess AmpC β-lactamases; induction or selection of variants with stable derepression may develop during therapy. Resistance to anti-pseudomonal penicillins, aztreonam, fluoroquinolones, gentamicin, and third-generation cephalosporins is variable but increasing. The prevalence of ESBL-producing isolates is <5%. Carbapenems, amikacin, cefepime, tigecycline (with which clinical experience is limited), fosfomycin (which is available in the United States only as an oral formulation), and colistin (which is an agent of last resort because of potential toxicities) are most active, with >90% of strains susceptible.
MORGANELLA AND PROVIDENCIA INFECTIONS
M. morganii, Providencia stuartii, and (less frequently) Providencia rettgeri are the members of their respective genera that cause human infections. The epidemiologic associations, pathogenic properties, and clinical manifestations of these organisms resemble those of Proteus species. However, Morganella and Providencia occur more commonly among LTCF residents; to a lesser degree, they affect hospitalized patients. In settings with extensive use of polymyxins and tigecycline, these organisms may become increasingly common because of their intrinsic resistance to these agents.
INFECTIOUS SYNDROMES
These species are primarily urinary tract pathogens, causing UTIs that are most often associated with long-term (>30-day) catheterization. Such infections commonly lead to biofilm formation and catheter encrustation (sometimes causing catheter obstruction) or to the development of struvite bladder or renal stones (sometimes causing renal obstruction and serving as foci for relapse). Morganella is also commonly isolated from snakebite infection. Other, less common infectious syndromes include surgical site infection, soft tissue infection (primarily involving decubitus and diabetic ulcers), burn site infection, pneumonia (particularly ventilator-associated), intravascular device infection, and intraabdominal infection. Rarely, the other extraintestinal infections described for GNB also occur. Bacteremia is uncommon; any infected site can serve as the source, but the urinary tract accounts for most cases, with the next most common sources being surgical site, soft tissue, and hepatobiliary infections.