PYREXIA OF UNKNOWN ORIGIN
Case vignette
A 66-year-old man of Greek descent has been investigated in hospital for a period of 1 week for a swinging fever of 1 month’s duration and associated headache, weight loss, arthralgia, myalgia and lethargy. He complains of drenching night sweats and rigors. The patient denies any localising symptoms. He has a background history of myocardial infarction treated with angioplasty and stent 1 year ago. He is currently on metoprolol 25 mg twice daily and aspirin 150 mg daily. He denies any allergies or family history of significant illness. He has recently travelled to the west coast of the United States for a family reunion. On examination his pulse rate is 100 bpm and his temperature is 38°C. His temperature chart shows a regular pattern of temperature spikes up to 39°C. No other significant signs are evident.
On further enquiry the patient reveals that he noticed an expanding erythematous maculopapular rash, which disappeared spontaneously. The lesion was painless. According to the detailed description, the lesion had a clear central core and a well-defined border.
Approach to the patient
Pyrexia of unknown origin is defined as intermittent or continuous fever for more than 3 weeks in a patient in whom the cause of the fever has not been identified in spite of repeated investigations for more than a week.
History
Ask about:
• the onset and duration of the illness
• any treatment received so far
• other associated symptoms that may give some clues to the likely aetiology of the fever—such as weight loss, cough, rashes
• symptoms that may help in the localisation of the focus
• the temporal pattern of the febrile episodes
• past medical history—focusing on infectious conditions, connective tissue diseases, vasculitic conditions and malignancies
• detailed medication history—looking particularly for drugs that may cause fever
• history of alcohol intake and recreational drug use
• pets, farm animals, hobbies, sexual activity
• travel—be familiar with region-specific rare infections such as kala-azar, Lassa fever, Q fever, Lyme disease
• specific features of rather rare diseases such as erythema migrans of Lyme disease (as was the case in the above case vignette) when there is a suspicion
• detailed family history—important in excluding diseases such as familial Mediterranean fever.
Examination
The condition could be due to multiple causes, both infective and non-infective (see box). A detailed physical examination should be carried out, looking first for a focus of sepsis. Look for areas of inflammation in the skin and check for lymphadenopathy and/or hepatosplenomegaly. Look for rashes, inflamed joints, painless mass lesions etc. Study the temperature chart. Perform the examination with a particular focus on the most likely body system to be involved, based on the overall clinical picture.
Some of the commonly encountered causes of undiagnosed persistent fever are occult abscesses in the abdomen, tuberculosis, cytomegalovirus (CMV) infection, HIV infection, haematological malignancy, solid cancer, pulmonary embolism, Wegener’s granulomatosis, undiagnosed vasculitis (especially polymyalgia rheumatica and giant cell arteritis), granulomatous conditions such as sarcoidosis, Still’s disease and drugs. The remainder of the physical examination should be focused on looking for features of non-infective causes of fever.
Causes of pyrexia of unknown origin
• Bacterial sepsis—e.g. bacterial endocarditis, subphrenic abscess, typhoid fever, brucellosis
• Viral sepsis—e.g. HIV, Epstein-Barr virus, parvovirus, Ross River virus, Barmah Forest virus
• Fungal sepsis
• Rickettsial sepsis—e.g. Q fever
• Mycobacterial sepsis—tuberculosis/atypical varieties
• Systemic vasculitis—e.g. Wegener’s granulomatosis, polyarteritis nodosa, Behçet’s disease
• Granulomatous conditions—e.g. sarcoidosis, Crohn’s disease
• Neoplasms—e.g. sarcoma, renal carcinoma, Hodgkin’s disease
• Drug fever—sulfa drugs, vancomycin, hydralazine, methyldopa
• Pulmonary embolism
• Haematoma
• Gout
• Dressler’s syndrome
• Alcoholic hepatitis
• Hypothalamic lesions—impaired thermoregulation
• Factitious fever
Investigations
Initial investigations should be aimed at excluding infection, and a standard septic work-up may already have been done. If the results are inconclusive, it is most appropriate to repeat the septic work-up. The standard septic work-up includes:
1. Full blood count and differential white cell count, to exclude leucocytosis or leucopenia
2. ESR and C-reactive protein (CRP), looking for persisting inflammation
3. At least three sets of blood cultures from different sites of the body at different intervals
4. Alerting the microbiology lab to the need for prolonged cultures for fungi and fastidious organisms
5. Chest X-ray
6. Urine analysis and midstream urine for microscopy and culture
7. Sputum microscopy and culture
8. Stool culture, for Salmonella
9. Lumbar puncture if neurological features are present.
If these investigations are non-diagnostic and the patient is still febrile, further investigations should be ordered, looking for specific diagnostic clues. These include:
1. CT of the thorax, abdomen and pelvis—looking for abscesses and unsuspected lymph node enlargement
2. Transoesophageal echocardiography—looking for valvular vegetations, to exclude subacute infective endocarditis
3. Gallium scan, looking for areas of active inflammation or lymphoma
4. Indium-labelled white cell scan, looking for foci of sepsis
5. Three-phase bone scan (or bone first gallium record)—looking for osteomyelitis or other bony lesions (inflammation or metastatic deposits)
6. Swabs as clinically indicated (e.g. cannula site)
7. Serology for CMV and HIV
8. Autoimmune serology
10. Temporal artery biopsy if there is clinical suggestion of temporal arteritis
11. Liver biopsy
Management
Very ill patients should be treated as for septicaemia, with broad-spectrum, potent parenteral antibiotic combinations. Otherwise, therapy should be guided by the clinical circumstances and the above investigational results.
THE IMMUNOCOMPROMISED HOST
Approach to the patient
There is a high likelihood that a patient presented as a long case will have some form of immunodeficiency, either as the primary presentation or as a comorbidity. Immunodeficiency could be granulocytopenia, cellular immunodeficiency (see box) or humoral immunodeficiency (see box).
Causes of cellular immunodeficiency
• Lymphoma
• Chronic lymphocytic leukaemia
• High-dose corticosteroid therapy
• Cytotoxic therapy
• HIV/AIDS
• Immunosuppression associated with solid organ or bone marrow transplantation. (Viral infections due to agents such as herpes simplex, varicella zoster and cytomegalovirus are common in this group of patients. In the setting of solid organ transplantation, the most common causative organism of opportunistic sepsis is cytomegalovirus—with the exception of heart transplantation, where it is toxoplasmosis. Other opportunistic infections of importance are Pneumocystis carinii pneumonia and reactivation of tuberculosis.)
Causes of humoral immunodeficiency
Therapy for humoral immunodeficiency may include IV infusion of immunoglobulins at regular intervals. Encapsulated bacteria such as Streptococcus pneumoniae and Haemophilus influenzae usually cause sepsis in this setting. In terminal component complement and properdin deficiency, Neisseria meningitidis is the organism that causes the most sepsis.
History
Ask about:
• the current symptoms the patient presents with, to ascertain the focus of sepsis
• recurrent infections and, if known, investigations performed so far
• other medical conditions—diabetes mellitus, renal failure, HIV, haematological malignancy
• details of any relevant family history
• medications consumed—immunosuppressive agents
• alcohol abuse, recreational drug use
• sexual behaviour.
Examination
Search for the focus of sepsis. You should carry out a detailed examination of the systems involved. Check the temperature chart to ascertain the temporal pattern of the fevers. Look for clues to the predisposing condition, such as stigmata of liver disease or chronic renal failure, venepuncture marks of injecting drug use, lymphadenopathy, hepatosplenomegaly, splenectomy scar and central venous catheters.
The following is an outline of some of the different forms of immunodeficiency that can be expected in the long case setting and some information that may be useful in approaching the management of such patients.
Granulocytopenia
A patient is absolutely granulocytopenic by the classic definition when the neutrophil count goes below 0.5 × 109/L. The risk of sepsis is increased in this setting.
If a granulocytopenic patient is febrile, possible foci of sepsis are: oropharynx, lung, distal oesophagus, colon, perianal skin, intravenous cannula site and the urinary tract.
Management
Empiric therapy should generally be commenced with a beta-lactamase inhibitor agent with anti-pseudomonal activity together with an aminoglycoside. If the patient fails to respond to the Gram-negative regimen or there is suspected peripheral or central-line sepsis, or if there are Gram-positive organisms identified in the blood microscopy, add vancomycin to the antibiotic combination. If the patient does not defervesce after 3–7 days of antibiotic therapy, consider commencing empiric antifungal therapy with an agent such as IV amphotericin-B or fluconazole. Remember to ask the microbiology lab to perform prolonged cultures to identify fungal organisms. Administration of granulocyte colony-stimulating factor or granulocyte-macrophage colony-stimulating factor would help shorten the duration of neutropenia, but its use in the setting of sepsis has not been proved to be of any benefit.
Metabolic acidosis
This is seen in conditions such as diabetes mellitus, acute myeloid leukaemia and renal failure. Acidosis impairs the optimal functioning of the granulocytes and the complement system. The causative organisms of infections include Pseudomonas aeruginosa and fungi.
Fungal infections
In a septic patient, if the following risk factors are present, consider the possibility of candidaemia:
Febrile neutropenia
This condition is common among patients treated with cytotoxic chemotherapy as well as those suffering from aggressive haematological malignancies. There is a high likelihood that the discussion of a long case with the above pathology will also involve the management of febrile neutropenia. Examiners expect the candidate to be confident with the management of such life-threatening conditions.
Febrile neutropenia is defined as a body temperature of more than 38°C, in a patient whose white cell count is less than 1 × 109/L. The causative organism is more often a Gram-positive than a Gram-negative bacterium; however, if the agent is Pseudomonas aeruginosa, mortality is expected to be extremely high, so the empiric antibiotic regimen should include agents that would be active also against Pseudomonas sp.
Management
The therapeutic regimen is usually determined according to the data on causative organisms of sepsis in patients at that particular institution. A standard regimen in febrile neutropenia is gentamicin 5 mg/kg together with ceftazadime 1 g three times a day, or ticarcillin and clavulanic acid 3 g four times a day. Addition of vancomycin should be strongly considered if the patient is known to be colonised with methicillin-resistant Staphylococcus aureus, if the patient is in septic shock, or if the fever continues despite the above antibiotic therapy.
BACTERIAL ENDOCARDITIS
Case vignette
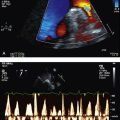
A 41-year-old man presents with severe dyspnoea, orthopnoea with fevers and chills progressive with associated arthralgia over a period of 2 weeks. He reports feeling extremely lethargic prior to the onset of fevers. He also reports significant weight loss over a period of about 1 month. He has been healthy otherwise. He denies any intravenous drug use, smoking or alcohol excess. He reports allergy to penicillin. On examination the patient is saturating at 95% on 100% oxygen via the non-rebreather mask. He is febrile at 39°C. He has splinter haemorrhages in the upper extremities. His JVP is significantly elevated with a prominent V wave. There is a harsh pansystolic murmur audible across the precordium. There are coarse crepitations in all lung fields bilaterally.
1. What investigations would you perform to work up this patient further?
2. Discuss your plan of management, focusing on the immediate and long-term objectives.
3. Discuss the possible predisposing factors in this man for endocarditis.
4. What are the possible causative organisms in this case and what are your therapeutic options?
Approach to the patient
History
Ask about the presenting symptoms and their duration. Ask about previous cardiac surgery, valvular repair or replacement, and any past history of a known cardiac murmur. Check what investigations have been performed so far (transoesophageal echocardiogram should not escape a patient’s memory!). Ask about the treatment received and any side effects. Enquire about predisposing conditions, such as previous valvular heart disease, rheumatic fever during childhood, injecting drug use, and recent dental work or invasive procedures. Ask about other medical conditions such as gastrointestinal malignancy etc. Streptococcus bovis infection may be associated with colonic malignancy.
Examination
Check the temperature chart and look for peripheral stigmata (petechiae, finger clubbing, splinter haemorrhages, Osler’s nodes (tender nodules in the finger pads) and Janeway lesions (non-tender macular lesions on the palms and soles)). Look in the fundus for Roth spots. Perform a detailed cardiovascular examination, looking for signs of valve pathology and congestive cardiac failure. A sternotomy scar would testify to previous cardiac surgery. Check for neurological deficit and palpate the abdomen for splenomegaly. Don’t forget to look for ports or percutaneous indwelling central venous catheter (PICC) lines meant for chronic parenteral antibiotic therapy. Inflammation or cellulitis around such foreign bodies is of major concern.
Investigations
Investigation of suspected subacute bacterial endocarditis should include:
1. Three sets of blood cultures taken from different sites of the body at different intervals (of > 1 hour apart) within the first 24 hours of presentation. Negative cultures should be maintained for 3–4 weeks to facilitate the detection of fastidious and slow-growing HACEK (Haemophilus, Actinobacillus sp., Cardiobacterium hominis, Eikenella, Kingella) organisms. Unusual organisms that should be considered in this setting include Coxiella burnetii, Chlamydia sp., Legionella sp., Bartonella sp. and fungi.
3. ESR and CRP
4. Urine analysis—looking for haematuria
5. Transthoracic echocardiogram—looking for valvular regurgitation, ventricular septal defect (VSD), large vegetations and cardiac failure. Transoesophageal echocardiography—looking for valvular vegetations and/or cardiac abscess.
6. Chest X-ray—looking for features of cardiac failure.
Remember Duke’s criteria for the diagnosis of bacterial endocarditis:
For a conclusive diagnosis of endocarditis, one should have a combination of two major criteria or one major criterion with three minor criteria.
Management of infective endocarditis
1. Sensitive streptococcal endocarditis (minimal inhibitory concentration (MIC) < 0.1) may be treated with 2 weeks of IV penicillin and gentamicin. Also high-dose ceftriaxone (2 g daily) can be used intravenously for 4 weeks. Patients with penicillin allergy can be treated with vancomycin for 4 weeks.
If the disease is complicated or the symptoms have been present for more than 3 months, the antibiotic regimen should be changed to 4 weeks of IV penicillin together with 2 weeks of IV gentamicin.
2. Enterococcal endocarditis can be treated with penicillin/gentamicin combination for 4–6 weeks or ampicillin/gentamicin combination for 4–6 weeks. Penicillin-allergic patients may be treated with vancomycin.
3. Staphylococcal endocarditis can be treated with flucloxacillin or nafcillin for 4–6 weeks. If the organism is methycillin resistant or if the patient is penicillin intolerant, vancomycin should be given for 6 weeks.
4. Treatment for prosthetic valve endocarditis caused by Staphylococcus aureus, S. epidermidis or diphtheroids will be guided by sensitivity testing. Methicillin-resistant S. aureus is treated with vancomycin combined with gentamicin for a total of 2 months.
Clinical progress is monitored with the observation of the temperature pattern, follow-up blood cultures, ESR and CRP, as well as urine analysis looking for resolution of haematuria.
5. Patients with significant haemodynamic instability, heart failure, significant valve damage, septic emboli or cardiac abscess need surgical intervention.
6. Patients with a previous history of endocarditis, known valve disease, mitral valve prolapse with regurgitation, prosthetic valves and intracardiac shunts need antibiotic prophylaxis (see box) prior to invasive procedures that can cause bacteraemia such as dental work, large bowel surgery or genitourinary surgery.
Antibiotic prophylaxis regimens
1. For oral, upper respiratory tract and oesophageal procedures:
2. For genitourinary and lower gastrointestinal procedures:
• Amoxycillin 2 g orally 1 hour before the procedure for low-risk patients
• Ampicillin 2 g intravenously or intramuscularly, together with gentamicin 1.5 mg/kg intravenously or intramuscularly within 30 minutes followed by ampicillin 1 g intravenously or intramuscularly 6 hours after the procedure (for high-risk patients)
• For penicillin-allergic patients vancomycin can be substituted.
(Adapted from Dajanis A S, Taubert K A, Wison W et al 1997 Prevention of bacterial endocarditis. Recommendations by the American Heart Association. Circulation 96(1):358–366)
(Adapted from Dajanis A S, Taubert K A, Wison W et al 1997 Prevention of bacterial endocarditis. Recommendations by the American Heart Association. Circulation 96(1):358–366)
THE HIV PATIENT
Human immunodeficiency virus (HIV) infection with its multiple opportunistic infections, drug interactions and adverse drug effects, together with numerous sociocultural and economic problems, is ideal for a long case at the examination. Although it is a complex disorder, there are certain basic HIV/AIDS concepts with which the candidate must be familiar.
Case vignette
A 35-year-old female is admitted with acute confusion. She has a history of haemophilia and multiple blood transfusions. She has also had transfusion-acquired HIV for the past 14 years. She was managed on lamivudine, zidovudine and the combination of ritonavir and lopinavir. Due to a recent rise in the viral load, her HAART regimen has been changed with the addition of efavirenz. She has not experienced any AIDS-defining illness in the recent past. She works as a shop assistant and has had a steady male partner for the past 3 months who is not known to have HIV. She is sexually active. On examination the patient is confused to place and time but not to person. Her vital signs are stable. There is evidence of treatment-related lipodystrophy and generalised lymphadenopathy. She has no overt signs of opportunistic infections or wasting.
1. What are the possible differential diagnoses for her confusion?
2. What additional information, including investigations, would you require to work this patient up?
3. What is your initial approach to the management of this patient?
4. Her urine beta-hCG is positive. What concerns do you have and how would her management be affected by this finding?
5. How would you monitor the response to therapy?
6. What options are available to manage treatment-resistant HIV-1?
Approach to the patient
History
Enquire about how and when the diagnosis was made, whether the patient experienced any seroconversion illness, the initial viral load and the initial CD4 cell count. Ask about all risk-prone behaviour, partner infection, and any deaths among previous or current partners due to AIDS. Ask about opportunistic infections and other infections, particularly candidiasis, varicella zoster, Mycobacterium avium complex (MAC), tuberculosis, and hepatitis A, B and C. Enquire about the symptoms, such as fever, weight loss, diarrhoea, night sweats, dyspnoea, cough, anorexia, depression and symptoms of neurological impairment. Other important aspects of the history are psychosocial problems, travel history, living circumstances, relationships, financial problems, employment and details of available community resources. It is important to learn about the various drugs that have been used to treat the patient and the complications encountered. The candidate has to gain a very good understanding of the patient’s insight into this serious disease condition and the associated prognosis.
Examination
Physical examination of the HIV patient needs to be extensive.
1. Check the oral cavity—looking at the general hygiene and for candidiasis, oral hairy leucoplakia, Kaposi’s sarcoma and herpes simplex infection.
2. Perform fundoscopy—looking for retinitis, generally due to HIV or CMV.
3. Check for lymphadenopathy.
4. Thoroughly examine the respiratory system, abdomen and pelvis—looking for evidence of opportunistic infection and malignancy.
5. Perform a Mini-Mental State Examination—looking for cognitive impairment.
6. Perform a neurological examination—looking especially for peripheral neuropathy.
Investigations
Diagnosis
Early detection, prior to seroconversion, can be made by serum assays of p24 antigen level or detection of HIV RNA in the patient’s blood by polymerase chain reaction (PCR) assay. Remember that there is a possibility of false-positive results with radioimmunoassays. Seroconversion takes place usually 1–3 weeks after infection and the serological test can be performed for the purpose of diagnosis thereafter. However, sometimes the antibody test will not yield a positive result for as long as 3 months. The standard enzyme-linked immunosorbent assay (ELISA) test is highly sensitive. A repeat ELISA test and/or a Western blot test may be carried out for confirmation. A positive Western blot result includes the standard determination of the number of viral components. Indeterminate results (positive ELISA test and indeterminate Western blot test) occur in about 4 in 1000 tests and are reviewed by repeat testing to see if progression to a positive Western blot has occurred. Rapid serological tests for HIV are now available. These tests are convenient to use. However, these test results need to be validated with a standard enzyme immunoassay (EIA) or by Western blot test. For the newly diagnosed HIV patient it is necessary to organise pre-test and post-test counselling, with partner notification and contact tracing.
On diagnosis of HIV, the viral load together with the CD4 lymphocyte subset count should be checked. Viral load and CD4 count should be checked three to four times a year unless the clinical course is very stable.
After diagnosis
Upon diagnosis, the basic investigational battery includes:
1. Full blood count—looking for anaemia, leucopenia and thrombocytopenia.
2. CD4 lymphocyte count (per mL) and the viral load (copies per mL), genotype testing for drug resistance. (The CD4 subset lymphocyte count in the blood gives an impression of the short-term prognosis of the patient and the viral load gives an idea of the patient’s long-term prognosis.)
3. Electrolyte profile and renal function indices
4. Liver function indices and amylase level
5. Serum cholesterol levels (preferably fasting)
6. Serology for syphilis, toxoplasmosis, CMV, hepatitis B and hepatitis C
7. Tuberculin skin test (Mantoux)—a positive test proves that the patient has had previous exposure or the BCG vaccination; however, false-negative tests are seen due to immunosuppression in AIDS
8. Pap smear—important in the female patient
9. Chest X-ray—as a baseline test
10. Testing for therapeutic drug resistance in patients who have a higher viral load (> 1000 copies/mL)
Management
1. All patients should be immunised against hepatitis A, hepatitis B, and annually against the influenza virus. A tuberculin reaction of 5 mm or more is an indication for isoniazid prophylaxis for 12 months.
2. An HIV-specific flowchart of clinical events should be commenced on every new case.
3. HIV infection is divided into stages depending on the patient’s CD4 count, and these divisions indicate what opportunistic infections the patient is prone to and what prophylactic therapy is indicated.
4. Objectives of therapy include suppression of HIV viral load, maintenance of immunocompetence and maintenance of quality of life.
5. A detailed review of the patient’s socioeconomic circumstances is vital in the planning of the management strategy. Extensive counselling is important, particularly about sexual activity and protection, drug compliance, surveillance for opportunistic infections and drug toxicity/interactions in those who are commenced on HAART regimens.
Stages of HIV
HIV is divided into several clinical stages from a management and prognostic point of view.
Intermediate stage
CD4 count of 200–500. The clinical associations include oral hairy leucoplakia, oral candidiasis, herpes simplex virus (HSV) infection, herpes zoster infection, seborrhoeic dermatitis, tuberculosis, Kaposi’s sarcoma (human herpesvirus 8), bacterial sinusitis, bronchitis and pneumonia.
Late stage
CD4 count of 50–200. Opportunistic infections with Pneumocystis carinii and chronic diarrhoea due to Cryptosporidium are likely, and prophylaxis with oral co-trimoxazole against P. carinii should be commenced.
Advanced stage
CD4 count of less than 50. The patient is susceptible to numerous opportunistic infections, such as MAC, Bartonella, histoplasmosis, aspergillosis, CMV infection, cryptococcal infection, toxoplasmosis, microsporidiosis, extrapulmonary tuberculosis, Epstein-Barr virus infection, disseminated Mycobacterium avium complex infection, disseminated herpes simplex or disseminated herpes zoster, and progressive multifocal leucoencephalopathy. At this stage the patient is also susceptible to primary central nervous system lymphoma as well as wasting and dementia due to HIV.
Terminal stage
Therapeutic decisions should be made with the aim of best palliation and psychological comfort. The change of classification to terminal stage from the advanced stage should be based on the patient’s overall clinical status, the patient’s perspective on his or her condition and expectations. A decision should be made regarding the continuation of active therapy. Strong consideration should be given to stopping active treatment if signs and symptoms of the disease are not controlled with available therapy or if therapy is not tolerated. At this stage the focus of management should shift to comfort care, with psychological support, family support and pain management.
Drug therapy in HIV
The current management approach to the HIV patient is to commence HAART early so as to prolong life expectancy and maintain good quality of life. There is still some debate as to exactly when the treatment should commence. It is important to be cognisant of other factors that would influence the decision whether to treat or not. These include the patient’s expectations, resistance, and tolerance to side effects, drug interactions, affordability and psychosocial factors that would affect compliance.
Recommendations: Current consensus is to commence antiretroviral therapy when the CD4 count is < 200 or the viral load is > 30000 copies per mL. Symptomatic HIV and/or AIDS-defining illnesses are additional indications for therapy with antiretroviral agents. A CD4 count of 200–350 is a grey area where patients may or may not benefit from HAART therapy. In this regard an individualised decision should be made based on the clinical merits. Pregnant women require therapy to mitigate vertical transmission.
Compliance is the critical issue in treatment. The therapeutic objective is to achieve an undetectable viral load. The candidate is expected to possess a good knowledge of the commonly used antiretroviral drugs and the compatible combinations. It is important to be able to identify adverse reactions associated with these therapeutic agents.
The four classes of drugs in common usage are: nucleoside/nucleotide reverse transcriptase inhibitors, non-nucleoside reverse transcriptase inhibitors, protease inhibitors and fusion inhibitors. These are discussed below.
Protease inhibitors
These are: indinavir, ritonavir, saquinavir (available in two forms: hard-gel and soft-gel formulation), nelfinavir, atazanavir, fosamprenavir and amprenavir.
Indinavir has the propensity to cause nephrolithiasis, so adequate hydration of the patient should be ensured. Ritonavir has significant side effects, including nausea, diarrhoea, circumoral paraesthesias and hepatitis. It is important to establish the baseline liver function prior to commencement of therapy and to follow up with regular liver function tests. Ritonavir can augment the activity of other protease inhibitors by blocking hepatic drug metabolism, and therefore it can be used in combination at low dose to boost the activity of another agent of the same class, which is an accepted practice.
The hard-gel formulation of sequinavir is well tolerated but has compromised efficacy due to poor gastrointestinal absorption; the soft-gel formulation has better absorption. Nelfinavir has the gastrointestinal side effects of nausea, vomiting and diarrhoea. Amprenavir causes rash and gastrointestinal discomfort.
Protease inhibitors cause a lipodystrophy in treated patients, with significant cosmetic implications. Redistribution of peripheral adiposity to the trunk, particularly to the dorsum of the neck and the abdominal region, is observed. There is potential for the acceleration of atherosclerosis; therefore, close supervision of the serum lipid profile is important.
Protease inhibitors have many serious interactions with other therapeutic agents, so it is important to be vigilant about polypharmacy in these patients. Rifampicin, astemizole, midazolam and cisapride should not be administered to patients who are being treated with protease inhibitors.
Nucleoside analogue reverse transcriptase inhibitors
These include: entecavir, emtricitabine, zidovudine, zalcitabine, stavudine, didanosine, lamivudine, tenofovir and abacavir.
Zidovudine can cause the side effects of headache, nausea, myelosuppression, myopathy and neuropathy. Zalcitabine is known to cause peripheral neuropathy. Stavudine, too, can cause peripheral neuropathy, in addition to fatty liver and lactic acidosis. Didanosine is another agent that causes peripheral neuropathy, and it can also cause pancreatitis.
Lamivudine is a relatively safe drug that has a minimal side-effect profile.
Abacavir is reported to cause serious hypersensitivity reactions, so the patient should be warned of early signs of hypersensitivity and the necessary steps to be taken in such an event.
Non-nucleoside reverse transcriptase inhibitors
These are nevirapine, delavirdine and efavirenz. These drugs cause a transient rash. They also have significant interactions with the hepatic cytochrome P-450 enzyme system.
Nevirapine has the highest incidence of Stevens-Johnson syndrome of any licensed drug and can also cause a severe (often fatal) hepatitis. Efavirenz can cause neurocognitive side effects. It is also teratogenic. Nevirapine is preferred in pregnancy.
Viral resistance testing should guide change of therapy if viral load rebound occurs. But it is important to check the level of compliance prior to attributing viral load rebound to drug resistance.
Fusion inhibitors
The approved agent is enfuvirtide, an amino acid synthetic peptide. This agent blocks the fusion of HIV with CD4 cells. It is indicated in HIV-1 treatment-experienced patients who have ongoing viral replication despite therapy with other agents. It is also indicated in treatment-experienced patients who are becoming intolerant to previous antiretroviral agents. No data are available on treatment-naive patients. Injection site reaction and pneumonia are known side effects.
Commonly used combinations
Two nucleoside reverse transcriptase inhibitors in combination with a protease inhibitor or with a non-nucleoside reverse transcriptase inhibitor are commonly used.
It should be noted that zidovudine and stavudine should not be used in combination because they antagonise each other’s effect; and it is contraindicated to give didanosine and zalcitabine in combination due to enhanced neurotoxicity.
Upon commencement of antiretroviral therapy, treatment response can be monitored by checking the CD4 count and viral load at regular intervals. Initial response can be checked at 4 weeks. Then monitoring should continue at increasing intervals to every 3 months until viral load is recorded as undetectable. Thereafter, monitoring every 3–4 months should be continued.
NON-SPECIFIC CLINICAL PRESENTATION WITH CONSTITUTIONAL SYMPTOMS
Case vignette
A 53-year-old HIV-positive man presents with severe lethargy, odynophagia and significant weight loss (11 kg) over a period of 2 months. He was initially diagnosed with HIV 9 years ago. He was treated with several antiretroviral combination agent regimens. His CD4 count has been 500 and viral load undetectable 4 months ago. In the past he has suffered from CMV retinitis, oral thrush and Pneumocystis carinii pneumonia. He is homosexual and currently has no steady partner. He is occasionally sexually active. On examination the patient has generalised lymphadenopathy and evidence of significant wasting. He is afebrile.
Approach to the patient
HIV can have various clinical presentations, and in each situation the candidate should judiciously estimate the clinical possibilities. A presentation with constitutional symptoms of fever, night sweats, weight loss and fatigue is common, and the possibilities that should be considered are:
Investigations
The battery of investigations that should be performed in this situation includes:
1. Full blood count
2. Electrolyte profile
3. Liver function tests
4. Multiple blood cultures (at least three), including cultures for MAC
5. Cultures for acid-fast bacilli
6. Serology for syphilis, cryptococcosis (also test for the cryptococcal antigen) and toxoplasmosis
7. CT of chest and abdomen.
If the investigation is still inconclusive, perform a gallium scan (looking for foci of inflammation), a total body MRI scan, bone marrow biopsy (looking for MAC infection), a lumbar puncture and, if necessary, a liver biopsy.
The HIV patient can complain of chronic fatigue, and this presentation could be due to multiple causes. Some possible causes are infection, medication side effect, malnutrition, chronic pain and depression. Management in this setting is dependent upon the investigational results. Management objectives include aggressive therapy to control opportunistic infections (if detected), aggressive HAART regimen, proper nourishment, and antidepressant or psychiatric help if the patient is depressed.