CHAPTER 131 Disc Replacement
INTRODUCTION
Maintaining motion has long been the quest in the treatment of painful spinal disorders. Movement of the neck and trunk is a large part of everyday life. Limitations due to pain or surgical intervention are frustrating for patients with back pain and can be related to significant loss of work and reduced quality of life. Beginning in the 1980s, clinically useful lumbar disc replacements were introduced. Today, there are several such devices available for use. Although these devices hold great promise for improving the long-term care of patients with back pain, they are certainly not a cure-all, and the basics of any spine surgery still apply with respect to the importance of proper patient selection.
HISTORY
The first patent for disc replacement was issued in 1956 to van Steenbrugghe in France.1 This was just one of many devices described in his patent application. The first clinically useful artificial disc was simply stainless steel spheres implanted into the disc space following discectomy.2,3 This procedure was first performed in 19624 and reported in 1966 by Fernström of Sweden, who implanted the spheres into 133 patients, 125 lumbar disc levels and eight cervical disc levels. Among the lumbar implants, in follow-up of 6–30 months, there were only two complications: one sphere displaced into the epidural space and one case of temporary paresis of the peroneus. Clinically, Fernström noted that patients receiving the sphere had a better result than patients undergoing discectomy alone. In 1971, Fernström reported the 4–8-year follow-up on 142 patients.4 He found that among the patients operated for disc prolapse, 65% had no pain and were able to work full duty. An additional 28% had reduced pain and were able to work in some capacity. These results were more favorable than those from a series of control patients undergoing discectomy without the placement of a sphere. In 1995, McKenzie reported follow-up of 10–20 years on 67 patients who had received the spheres described by Fernström.5 He noted a high success rate, 83% among patients with disc herniations and 75% among those with degenerative disc. Prosthesis removal was required in only one of 155 patients who received the implant. The author reported that although more than 90% of patients were not working prior to surgery, 95% were working after receiving the sphere. The author also reported that 95% of the patients felt that the procedure was worthwhile.
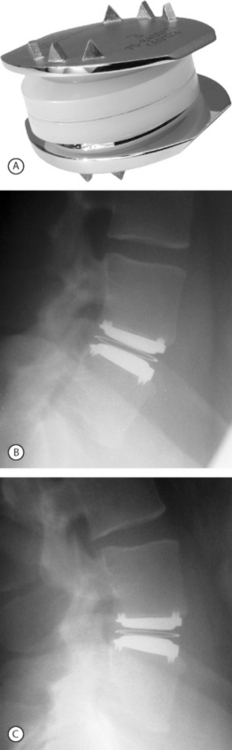
In 1982, Drs. Kurt Schellnack and Karin Büttner-Janz in East Germany at the Charité Hospital began the development of an artificial disc based on careful biomechanical analysis of the motion and properties of a normal lumbar disc. The design was a three-piece implant consisting of two metallic endplates and a sliding polyethylene core. The design concept was tested in the laboratory, and the first SB Charité artificial disc prosthesis was implanted in September 1984 in Berlin.6 There were problems with subsidence with this device. In 1985, a second design was introduced that had ‘wings’ on two sides of the circular endplates to increase the contact surface area between the metallic endplates and the vertebral body. This design had problems with fracture between the core and the wings. A third design was introduced in 1987. This design proved to be reliable and remains in use today (Fig. 131.1).
In the late 1980s, Thierry Marnay in France designed the ProDisc. It also allows motion through articulation between a concave and convex surface. The original design was anchored by two keels on each of the superior and inferior metallic endplates. The current design of this device has one keel in the center of each of the endplates (Fig. 131.2). In the last several years there have been numerous other designs, including metal-on-metal discs such as the Maverick (Medtronic-Sofamor-Danek) and the FlexiCore (Stryker). These four discs are currently being studied in IDE FDA studies with the Charité having achieved FDA clearance October 26, 2004. There are two additional devices that are being used outside of the US called the Mobidisc (LDR Medical), consisting of metal and polyethylene, and SpinalMotion’s Kineflex™, which is of similar design.
Indications
As with any surgical procedure, much of the success of total disc replacement is dependent upon the selection of patients being treated. There are indications that apply to any elective spine surgery procedure. These include the failure to achieve acceptable pain relief after an appropriate nonoperative course of treatment. In general, appropriate nonoperative management should include medication, active physical rehabilitation, education, activity modification, and often injections with at least 6 months of nonoperative care. As with any patient, careful and comprehensive history and physical examination are crucial to patient evaluation. These findings are then reviewed with respect to image findings. Most patients who have failed nonoperative management have had a magnetic resonance imaging (MRI) scan. This is reviewed to evaluate the disc, facets, bony structures, and to rule out pathologies such as spinal tumor. In most patients being evaluated for symptomatic disc degeneration, it is recommended that further evaluation be undertaken using discography. This evaluation is used as a confirmatory test to determine if the disc(s) appearing as abnormal on an MRI is the source of the patient’s symptoms. This is particularly important in view of the reports of the high rates of disc abnormalities seen on MRI scans made on individuals with no back pain.7 Also, the discogram can be used to better assess the condition of the discs adjacent to the suspect level. Discography should be undertaken with the patient awake and responsive. If the patient is too heavily sedated the pain response cannot be adequately evaluated. The patient should be asked the location of the pain provoked, if any, its location with respect to the location of their usual symptoms, and the intensity of the pain. If pain is provoked that is not concordant to the usual symptoms, the discogram is not considered to be a positive test.
Another important aspect in the evaluation of possible surgical candidates is psychological screening. While imaging studies are closely related to anatomical findings during surgery, psychological testing is more strongly related to surgical outcome.8 The presurgical psychosocial screening instrument has been found to have a high correlation to surgical outcome.9,10 Patients with a significant psychological component to their pain experience are likely to do poorly following surgery and should generally not undergo an elective procedure. Another important component to achieving a favorable surgical outcome is the establishment of realistic expectations. This may be addressed during the psychological screening. It can also be addressed during preoperative patient education. Patients must understand that results are not guaranteed and there is a very good chance that they will continue to have some level of pain or painful flare-ups following any spine surgery. The goal is to significantly reduce their pain and allow for improved function. Being totally and permanently pain free is not a realistic goal. Patients also need to understand that they play a major role in accomplishing these goals and must be willing to comply with a postoperative rehabilitation plan.
TOTAL DISC REPLACEMENT IN THE LUMBAR SPINE
The primary indication for total disc replacement is symptomatic disc degeneration or disruption at one or two disc levels unresponsive to nonoperative management. This condition is best diagnosed by the combined use of MRI and discography. Other diagnostic observations include disc space narrowing. The patient may have complaints of back pain with or without leg pain.
Contraindications
The contraindications for total disc replacement are very similar to those traditionally applied to anterior lumbar interbody fusion since the approach to the spine is the same. Details of inclusion and exclusion criteria have been discussed.11 One should screen patients for the number of types of previous abdominal surgery. If there have been several procedures, or surgery in the immediate vicinity of the painful disc, the patient may not be a good candidate. There is a risk of significant vascular injury related to scarring from previous surgery. One must evaluate the preoperative imaging studies to rule out patients with significant calcification of the vessels. This could result in significant vascular complications.
Diseases affecting bone quality, such as osteoporosis, Paget’s disease, and osteomalacia, are contraindications for total disc replacement. There is the risk of the device subsiding into osteoporotic bone, particularly if the positioning and size of the implant used are less than ideal. There may also be an increased risk of fracturing the vertebral body during, or after, surgery if the patient is osteoporotic. Poor bone quality may also negatively influence the anchoring of the device to the vertebral bodies. Vertebral body fracture following implantation of a total disc replacement into a patient with osteopenia has been reported.12
Bertagnoli and Kumar described what they defined as an ideal candidate for total disc replacement.13 Such patients have single-level disc degeneration, a disc space height of at least 4 mm, no facet joint changes, intact posterior elements, and no degeneration at the adjacent segments.
Procedure
The surgical approach for total disc replacement is the same as for anterior lumbar interbody fusion. The mini ALIF retroperitoneal approach has been described in detail elsewhere.14,15 In this chapter, we will provide an overview of the general approach to the anterior lumbar spine. The exact approach may vary by surgeon preference or design of the device to be implanted. In our practice, a general surgeon initiates the procedure to provide access to the spine and remains available in the event of a vascular injury or other difficulty. A radiolucent table is necessary, since imaging is needed during the surgery. It is helpful if a table is used that allows the spine to be put into a slightly extended position to aid during device implantation. Another alternative is to have an inflatable device under the level to be operated that can be inflated to create extension when desired during the implantation. During the rest of the procedure, this positioning is not needed.
Once access to the spine has been safely achieved, the midline of the disc space is marked with a small screw into the vertebral body or an osteotome is used to mark the vertebral endplate, depending on the type of prosthesis being implanted. The disc tissue is removed. The spine may be placed into a slightly extended position to widen the access to the disc space. Preoperative templating can be used to estimate the size of the endplates and polyethylene core needed. However, the final determination of device size is made intraoperatively. The prosthesis size should be selected to fit within the disc space but cover as much of the vertebral body endplates as possible. This will help prevent subsidence. Obliquely-shaped endplates are selected to match the lordosis of the spine at the level to be implanted. If the vertebral endplates have a relatively posterior lip, it may be beneficial to remove them with a Kerrison ronguer or burr to increase the amount of contact area between the vertebrae and the metallic endplates of the device, allowing for a firmer fit. The disc space is temporarily distracted to make space for the device. Prior to finalizing the device placement, the spine is returned to a neutral position, rather than the slightly extended position.
Once the device is implanted, images are taken to make sure that the prosthesis is properly positioned. In the anteroposterior (AP) view, the prosthesis should be centered in the disc space. On the lateral view, the prosthesis should be centered in the disc space or placed approximately 2 mm posteriorly for the Charité. Cinotti et al. have reported that optimal prosthetic sizing and positioning are related to outcome.16 Coverage of at least 80% of the vertebral body endplates by the device endplates was related to the amount of motion at the operated segment at follow-up. These authors also found that prostheses positioned anteriorly to midline were related to decreased motion at follow-up. Lemaire reported that implantation of the prosthesis of more than 4 mm anterior to the center was related to posterior facet pain.17 After verification of proper device positioning, the retractors are removed and the layers of fascia are closed with absorbable sutures. The patient is usually hospitalized for 1–3 days. A light brace or corset is worn for 2–6 weeks.
Although the indications and surgical approach for total disc replacement are similar to those for anterior lumbar interbody fusion, the postoperative rehabilitation is quite different. There is no need to have a period of significantly reduced activities to allow the formation of bone to create a solid fusion. With respect to activities, one must keep in mind that many of these patients have become deconditioned during the months of symptoms leading to surgical intervention. After surgery, rehabilitation can be helpful in increasing strength, through core stabilization exercises, and general fitness. Also, these programs will hopefully help modulate symptoms and prevent future episodes of back pain. A description of postoperative rehabilitation following total disc replacement has been provided by Keller.18 During the first 3 weeks after total disc replacement, activities are oriented toward symptom management and not stressing the area of the surgical incision. The patient is encouraged to walk frequently. Other activities are oriented toward gentle range of motion exercises, excluding extension. After 3 weeks, the patient is progressed to rotation and side-bending, as well as stabilization exercises. Controlled resistance training is also introduced at this time. After 6 weeks, the patient is progressed to more strenuous exercises, and activities involving extension of the spine are introduced.
Reported outcomes
Charité
In 1988, Büttner-Janz et al. reported on the first 62 patients to receive the Charité implant.19 This group included patients receiving all three designs of the device. A high level of satisfaction was reported by 54% of patients with an additional 29% being classified as improved. There were device-related complications with the first two designs of the device, including migration and breakage of the metallic endplates. These problems have not been reported with the use of the third, and current, design of the implant.
A retrospective review of 93 patients receiving the Charité device at several sites in Europe was reported with a mean follow-up of approximately 12 months.20 It reported significant improvement in visual analog scale (VAS) scores assessing back and leg pain. Walking distance was also reported to have improved postoperatively.
Cinotti et al. from Italy retrospectively reported results with a minimum 2-year follow-up after implantation of the Charité disc replacement in a series of 46 patients receiving 56 prostheses.16 The diagnoses in the group were symptomatic disc degeneration and pain in patients who had previously undergone a lumbar discectomy. Excellent or good results were noted in 63% of patients. Great benefit was reported by 67% of patients. The results followed a pattern similar to that frequently seen in lumbar fusion studies. Patient satisfaction was greater among those undergoing single-level implantation compared to those receiving prostheses at two levels (69% versus 40%). Also similar to fusion studies, patients with a history of previous spine surgery had less satisfactory results than did those with no previous spine surgery, 50% compared to 77%.
Other authors have also reported favorable results in 70–75% of patients.21,22 Long-term follow-up after total disc replacement with the Charité device was reported by Lemaire et al.23 Their study included 100 patients with a minimum follow-up of 10 years. Single-level replacement was performed in 54 patients, two-level replacement in 45 patients, and one patient underwent a three-level replacement. They reported that 62% of patients had excellent results with an additional 28% having good results. The return to work rate was 91.5%.
Zeegars et al. reported on a series of 50 patients with a minimum of 2-year follow-ups.24 The authors found that 70% of patients had satisfactory outcomes, and surgery at multiple levels was not associated with a less desirable outcome compared to patients receiving single-level replacement. However, they did find that patients with previous surgery or other lumbar degenerative pathology had less desirable results than those without such factors present (81% versus 66% satisfactory outcomes).
Recently, Blumenthal et al. reported the results of a multicenter FDA trial.25 They found that at most of the follow-up periods the results of Charité artificial disc were superior to anterior lumbar interbody fusion based on Oswestry and VAS scores. A significantly greater percentage of patients in the Charité group indicated that they would choose the same treatment again compared to the fusion group.
ProDisc
The device in use for the second longest period of time is the ProDisc, invented in France by Marnay.26 It is composed of three components: two cobalt chromium alloy endplates and a domed polyethylene core. Motion occurs through articulation between the domed core and the concave superior metallic endplate. Each metallic endplate is anchored to the vertebral body by a keel in the center of the device. Marnay implanted the devices into 64 patients and has reported the 7–11-year follow-up of 58 of these patients (three died for unrelated reasons).27 All of the devices were functioning with no cases of device fracture. The back pain scores improved significantly from a preoperative mean of 8.5 to 3.0 postoperatively. The leg pain scores also improved significantly. At follow-up, 65% of the patients reported themselves to be ‘entirely satisfied,’ 28% ‘satisfied,’ and only 7% ‘not satisfied.’
Mayer et al. in Germany reported their early results on a series of 34 patients.28 With a mean follow-up of 5.8 months, the pain VAS scores improved from 6.3 to 3.9. The change in Oswestry scores was much less, improving from 19.1 to 11.5. Patient satisfaction was high with 82.6% of patients reporting that they were either satisfied or completed satisfied with the results of their surgery.
In a study with highly variable length of follow-up of 3–24 months, Bertagnoli and Kumar reported on 108 patients.13 Excellent results were noted in 90.8% of patients.
Zigler et al. presented the results of one center’s experience with the ProDisc implant with 24 month (n=116) to 36 month (n=40) follow-up.29 In this prospective, randomized study, arthroplasty was compared to combined anteroposterior lumbar fusion. There were significant improvements in pain (measured by VAS), and function, measured by the Oswestry Disability Questionnaire. At some follow-up periods the ProDisc scores were significantly less than the fusion scores.
Tropiano et al. reported their experience with 53 patients with a minimum follow-up of one year.12 They reported significant improvements in the VAS and Oswestry scores. Although they had only 13 patients receiving the device at multiple levels, these patients’ outcomes were similar to patients undergoing implantation at a single level.
Motion of the operated segment
Two of the early Charité studies reported on the motion at the operated level. The results in the two studies were similar. Cinotti et al. reported 16° at a replaced L4–5 level and 9° at L5–S1.16 Lemaire et al. reported very similar results with 14° at a replaced L4–5 disc level and 9.5° at L5–S1.17 The study by Cinotti et al. was the first to carefully analyze the motion at the implanted disc levels.16 They found that motion was significantly greater at levels in which the prosthesis was implanted centrally or slightly posterior to central. The motion among such levels was 12° compared to only 5° measured at levels with the device implanted more anteriorly. The authors also noted the great influence of postoperative activities following implantation in future motion of the prosthesis. Patients who began exercising 1 week after surgery had 11° of motion at the implanted level compared to only 6° among those who wore a corset for 3 months. These authors also noted that patients in whom the metallic endplates covered 80% or more of the vertebral body endplate had significantly greater motion than patients with less coverage (13° versus 6°). In a recent prospective study, Guyer et al. reported that at the L4–5 and L5–S1 levels, at 24-month follow-up, the range of motion was the same as, or slightly greater than, the preoperative range of motion.30 These authors reported values that were less that those from the European studies, with range of motion of the implanted L4–5 level being 6.5° degrees and 3.9° at L5–S1. However, it should be noted that these patients also had less motion preoperatively than what was reported in the European studies.
Data from the FDA IDE clinical trial evaluating Charité provided information on the motion of the implants.31 The range of motion at the operated level decreased at 3-month follow-up, but then increased at subsequent follow-up periods and exceeded the preoperative value. The range of motion, as well as clinical outcome (VAS and Oswestry), were significantly better among patients in whom the device was ideally positioned compared to those in whom it was suboptimally or poorly positioned.
With respect to the ProDisc implant, little has been published on the range of motion achieved with the device. Huang et al. reported that the mean motion of disc levels receiving the ProDisc implant was 3.8° at a mean follow-up of 104 months.32 These authors found that female gender was related to having less than 2° of motion at the operated segment. An interesting finding in that study was regarding degeneration of the segment adjacent to the replaced disc level. Among nondegenerated adjacent segments, the range of motion of the implanted level was 4.7°. This was significantly greater than the 1.6° of motion noted at the operated level below a degenerated adjacent segment. Although this does not prove a causal relationship between motion and deterioration of an adjacent segment, it does provide support for a protective property of the prosthesis.
Tropiano et al. reported that at a replaced L4–5 level, the segmental motion was 10° and at L5–S1 was 8°. The authors did not provide data on the preoperative segmental motion at these levels.12
Complication of total disc replacement
David reported 10 complications in a series of 96 patients.21 These included one device removal and fusion, one secondary bone migration with fusion, and eight patients who underwent posterior fusion. Five patients had complete ossification around the prosthesis. While this compromised the function of the prosthesis, it basically produced a fusion and did not necessarily compromise the patient’s clinical outcome. In a series of only 14 patients reported by Sott and Harrison, five patients had a warm foot postoperatively due to interference with the left paravertebral sympathetic nerves.22 In one patient, 3 mm of subsidence was noted at 6 months; the patient’s follow-up at 30 months indicated no further subsidence, and the patient had a good clinical result. Zeegars reported 24 reoperations in his series of the first 50 patients.24 There were 11 reoperations at levels other than the one with a prosthesis, six at the level of the prosthesis, and seven reoperations in three patients for the treatment of complications.
Sachs et al. reported on a group of 147 patients with a minimum 2-year follow-up enrolled in randomized studies comparing total disc replacement to fusion.33 Overall occurrence of complications were similar in the disc replacement group and the fusion group. The most frequent complication was radiculopathy. Prosthesis subsidence occurred in 7% of disc replacement cases. There were no cases of deep infection or device failure.
In a study of 53 ProDisc patients, Tropiano et al. reported five complications (9%).12 The complications were one case of postoperative vertebral body fracture occurring 3 weeks after surgery, one due to implantation into a patient with osteopenia, two cases with malpositioned implants, and two cases of patients with ongoing radicular pain in the absence of neural compression. The patient with the fractured vertebra required reoperation for removal of the device and an anterior fusion. Both patients with malpositioned devices were reoperated 6–8 weeks after surgery. The patients with radicular pain were managed successfully with medication.
van Ooij et al. reported on 27 patients seen over a period of 8 years, presenting with various complications following total disc replacement.34 The authors did not provide a complication rate because the initial surgeries were performed at another center. However, the type of complications that arose is notable. In two patients, the prosthesis dislocated anteriorly. Disc degeneration at another level was seen in 12 patients. In seven of these patients, disc degeneration could be seen on radiographs made prior to the disc prosthesis surgery. In 11 patients, facet joint arthrosis was identified at the level of the prosthesis or an adjacent segment. In the eight patients who underwent a posterior fusion, a hypertrophic facet joint was visualized. Device subsidence was seen in 18 patients. In 10 of these cases, a prosthesis that was too small had been implanted. In one patient, the polyethylene core subluxed anteriorly. In two patients, there was slow anterior migration of the implant. One of these patients experienced good results for 10 years and then began having symptoms. Removal of the device revealed that it was loose in fibrotic tissue and had adhered onto the great vessels. The patient underwent anterior interbody fusion followed later by posterior fusion. In one patient, CT imaging revealed cysts, as well as sclerosis and fragmentation of bone around the prosthesis. The authors associated these findings with wear; however, this could not be confirmed as the patient refused further surgery. In two patients, the wire around the polyethylene core was broken. In three patients, there was hyperlordosis of the implanted segment, resulting in opening of the facet joints.
To date, there has been one case of possible wear debris reported by van Ooji et al.; however, there was no tissue confirmation of this.34
Reported results of reoperation following arthroplasty
Cinotti et al. reported that among 17 patients who had unsatisfactory results following total disc replacement with the Charité device, seven underwent reoperation.16 Salvage surgery consisted of posterior spinal fusion, leaving the prosthesis in place. Only three of the seven had satisfactory results following the posterior fusion.
Other lumbar total disc replacements
There have been a few reports on the use of other total disc replacements used in small numbers of patients. In 1993, Enker et al. reported on a series of six patients in whom an Acroflex disc (Acromed Corp; Cleveland, OH) was implanted.35 This device had a polyolefin rubber core vulcanized to two titanium endplates. The rubber core fractured in one patient, requiring reoperation. Three patients had good to excellent results, one had a fair outcome, and two patients had poor outcomes. Modifications of this device are still being pursued.
TOTAL CERVICAL DISC REPLACEMENTS
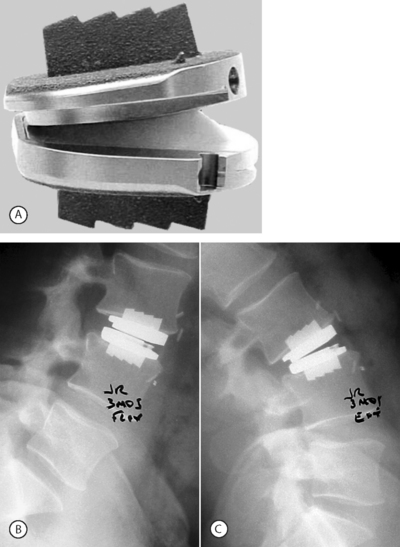
As in the lumbar spine, total cervical disc replacements are designed with the concept to allow motion of the spine. Although the end goal is the same, the motion patterns and loads borne by the cervical spine are very different than in the lumbar region. These differences may allow for significantly different designs of implants. However, there is benefit in using the technology that has been applied and tested in lumbar total disc replacements. Several cervical total disc replacement devices (Bryant Cervical Disc, Prestige, ProDisc-C (Fig. 131.3), PCM, and Mobi-C) are currently being evaluated in FDA-regulated clinical trials, and others are near beginning their trials.

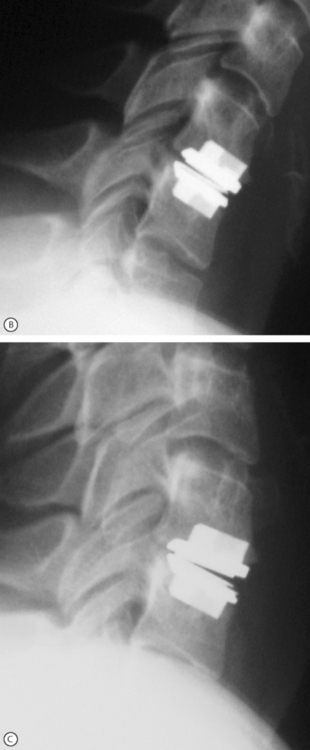
Fig. 131.3 Model and radiographic images of the ProDisc-C cervical device (A).
Motion at the implanted level can be seen when comparing the flexion (B) and extension (C) views.
Clinical outcomes
As in the lumbar spine, the first cervical disc replacements were metallic spheres implanted by Fernström.2 However, in his publication he did not report the results of these devices since the follow-up was less than 12 months.
In 1998, Cummins et al. reported on the use of a two-piece stainless steel metal-on-metal device, allowing motion through articulation of concave and convex surfaces.36 The device is anchored by screws attaching the device to the anterior surface of the vertebral bodies adjacent to the implanted level. This implant has been called the Cummins device. They used the device in 20 patients between 1991 and 1996. The Cummins device was redesigned and the name changed to the Frenchay implant.37 In 2002, Wigfield et al. reported on a series of 15 patients receiving this implant.37 At 24-month follow-up, the implanted disc levels demonstrated a mean of 6.5° of motion in flexion–extension. Pain scores improved 45% and scores on a disability questionnaire improved by 31%.
Pointillart published on what he termed the first failures of cervical disc prosthesis.38 The implant uses a one-piece L-shaped titanium device with a carbon convex surface upon which the vertebral body superior to the implant can glide. Good clinical results were achieved initially in all ten patients. In eight of the ten patients, a spontaneous fusion occurred at the operated segment. The remaining two patients later reported the onset of neck pain. In one of these patients, the device was removed and a fusion performed.
The results of the Bryan Cervical Disc prosthesis have been reported.39 This device has a polyurethane nucleus encased by, and articulated within, a shell made of titanium plates. There is a flexible membrane between the perimeters of the plates. The device has been implanted into 97 patients, of whom 49 had reached 12-month follow-up and 10 had reached 24-month follow-up. At 12 months, 70% of patients were classified as having an excellent outcome and 4% as having a good outcome.
Complications of cervical disc replacement
In the Wigfield study using the Frenchay device, 10 patients experience adverse events postoperatively.37 These included the following: recurrence of brachialgia 2 months after surgery requiring foraminotomy at the adjacent level; two screws broke in one patient by the 6-month follow-up period with the patient developing pain on full extension; pain on extension (2 patients, one occurring after a car collision); progression of myelopathy; transient hoarseness in two patients (resolved in 3 and 6 months); recurrent brachialgia at 6 months after surgery (resolved by 12 months after surgery); and in one patient the device was removed and a fusion performed due to pain during extension; however, the patient’s pain persisted.
Complications in the study of 97 patients reported by Bryan included: one patient with temporary dysphonia, one patient with pain related to an osteophyte that was not addressed during the device implantation (this was removed during a foraminotomy, with no negative effects on the disc prosthesis), two patients had pain in the shoulder region not requiring further surgical intervention, and one patient required drainage of a hematoma that formed due to the loosening of a postoperative drain.39 There were no device failures in the series.
1 van Steenbrugghe H. Perfectionnements aux prothèses articulaires. French Patent FR1122634, 1956.
2 Fernström U. Arthroplasty with intercorporal endoprostheses in herniated disc and in painful disc. Acta Chir Scand. 1966;357:S154-S159.
3 Reitz H, Joubert MJ. Intractable headache and cervicobrachialgia treated by complete replacement of cervical intervertebral disc with a metal prosthesis. S Afr Med J. 1964;38:881-889.
4 Fernström U. Disk replacement with maintenance of mobility. Presented at the 7th working session of the Association for Spinal Column Research. Nov., 1971; Bad Homberg, Germany.
5 McKenzie AH. Fernström intervertebral disc arthroplasty: A long-term evaluation. Orthop International. 1995;3:313-324.
6 Büttner-Janz K. History. In: Büttner-Janz K, Hochschuler SH, McAfee PC, editors. The artificial disc. Berlin: Springer Verlag; 2003:1-10.
7 Boos N, Rieder R, Schade V, et al. The diagnostic accuracy of magnetic resonance imaging, work perception, and psychological factors in identifying symptomatic disc herniations. Spine. 1995;20:2613-2625.
8 Spengler DM, Ouelette EA, Battie M, et al. Elective discectomy for herniation of a lumbar disc. J Bone Joint Surg [Am]. 1990;72:230-237.
9 Block AR, Ohnmeiss DD, Guyer RD, et al. The use of presurgical psychological screening to predict the outcome of spine surgery. Spine J. 2001;1:274-282.
10 Block AR, Gatchel RJ, Deardorff WW, et al. The psychology of spine surgery. Washington, DC: American Psychological Association, 2003.
11 Sachs BL, Clawson JJ. Indications and contra-indications for total disc replacement. In: Guyer RD, Zigler JE, editors. Spinal arthroplasty: a new era in spine care. St. Louis, MO: Quality Medical Publishers; 2005:39-46.
12 Tropiano PJ, Huang R, Marnay T. Lumbar disc replacement: Preliminary results with ProDisc II after a minimum follow-up of one year. J Spin Disord Tech. 2003;16:362-368.
13 Bertagnoli R, Kumar S. Indications for full prosthetic disc arthroplasty: a correlation of clinical outcome against a variety of indications. Eur Spine J. 2002;11(Suppl 2):S131-S136.
14 Büttner-Janz K. Surgical Approach. In: Büttner-Janz K, Hochschuler SH, McAfee PC, editors. The artificial disc. Berlin: Springer Verlag; 2003:103-114.
15 Brau SA. Mini-open approach to the lumbar spine for anterior lumbar interbody fusion: description of the procedure, results and complications. Spine J. 2002;2:216-223.
16 Cinotti G, David T, Postacchini F. Results of disc prosthesis after a minimum follow-up of 2 years. Spine. 1996;21:995-1000.
17 Lemaire JP, Skalli W, Lavaste F, et al. Intervertebral disc prosthesis – Results and prospects for the year 2000. Clin Orthop. 1997;337:64-76.
18 Keller J. Rehabilitation following total disc replacement surgery. In: Büttner-Janz K, Hochschuler SH, McAfee PC, editors. The artificial disc. Berlin: Springer Verlag; 2003:175-182.
19 Büttner-Janz K, Schellnack K, Zippel H, et al. Experience and results with the SB Charité lumbar intervertebral endoprostheses. Z Klin Med. 1988;43:1785-1789.
20 Griffith SL, Shelokov AP, Büttner-Janz K, et al. A multicenter retrospective study of the clinical results of the LINK SB Charité intervertebral prosthesis – The initial European experience. Spine. 1994;19:1842-1849.
21 David TJ. Lumbar disc prosthesis: five years follow-up study on 96 patients. Presented at the annual meeting of the North American Spine Society. 2000.
22 Sott AH, Harrison DJ. Increasing age does not affect good outcome after lumbar disc replacement. Int Orthop. 2000;24:50-53.
23 Lemaire JP, Carrier H, Sari E-H, et al. Clinical and radiological outcomes with the Chariteé™ Artificial Disc: A 10-year minimum follow-up. J Spinal Disord Tech. 2005;18:353-359.
24 Zeegers WS, Bohnen LM, Laaper M, et al. Artificial disc replacement with the modular type SB Charité III: 2-year results in 50 prospectively studied patients. Eur Spine J. 1999;8:210-217.
25 Blumenthal S, McAfee P, Guyer R, et al. A prospective, randomized, multicenter Food and Drug Administration Investigational Device Exemptions study of lumbar total disc replacement with the Chariteé™ Artificial Disc versus lumbar fusion. Part I: Evaluation of clinical outcomes. Spine. 2005;30:1565-1575.
26 Marnay T. L’arthroplasty intervertebrale lombaire. Med Orthop. 1991;25:48-55.
27 Marnay T. Lumbar disc replacement: 7 to 11-year results with Prodisc. Spine J. 2002;2:94S.
28 Mayer HM, Wiechert K, Korge A, et al. Minimally invasive total disc replacement: surgical technique and preliminary clinical results. Eur Spine J. 2002;11(Suppl 2):S124-S130.
29 Zigler JE, Sachs BL, Rashbaum RF, et al. Lumbar disc replacement with ProDisc: 24 to 36-month results of a prospective randomized comparison to fusion. Presented at the 3rd Trans Atlantic Spine Congress. November, 2005; Dallas, Texas.
30 Guyer RD, Sohn JM, Blumenthal SL, et al. Range of motion analysis of the lumbar spine after total disc replacement: a prospective two-year follow-up study. Presented at the annual meeting of the Spinal Arthroplasty Society. May, 2004; Vienna, Austria.
31 McAfee PC, Cunningham B, Holtsapple G, et al. A prospective, randomized, multi-center FDA IDE study of the Charité™ Artificial Disc: A radiographic outcomes analysis, correlation of surgical technique accuracy with clinical outcomes, and evaluation of the learning curve. Spine. 2005;30:1576-1583.
32 Huang R, Girardi F, Cammisa FJr, et al. Long-term flexion–extension range of motion of the ProDisc total disc replacement. J Spinal Disord Tech. 2003;16:435-440.
33 Sachs BL, Gottlieb J, Guyer RD, et al. Comparison of complications associated with total disc replacement versus lumbar fusion at two-year follow-up. Presented at the annual meeting of the Spinal Arthroplasty Society. May, 2004; Vienna, Austria.
34 van Ooij A, Oner FC, Verbout AJ. Complications of artificial disc replacement: a report of 27 patients with the SB Charité disc. J Spinal Disord Tech. 2003;16:369-383.
35 Enker P, Steffee A, McMillin C, et al. Artificial disc replacement – Preliminary report with a 3-year minimum follow-up. Spine. 1993;18:1061-1070.
36 Cummins BH, Robertson JT, Gill SS. Surgical experience with an improved artificial cervical joint. J Neurosurg. 1998;88:943-948.
37 Wigfield CC, Gill SS, Nelson RJ, et al. The new Frenchay artificial cervical joint: Results from a two-year pilot study. Spine. 2002;27:2446-2452.
38 Pointillart V. Cervical disc prosthesis in humans: First failure. Spine. 2001;26:E90-E92.
39 Bryan VEJr. Cervical motion segment replacement. Eur Spine J. 2002;11(Suppl 2):S92-S97.