39 Directed Cement Flow Kyphoplasty for Treatment of Osteoporotic Vertebral Compression Fractures
KEY POINTS
Introduction
In an effort to achieve fracture reduction and restore sagittal balance, balloon kyphoplasty was introduced. This procedure has proven to be safe and efficacious, and its beneficial effects are sustained according to the most recent clinical studies.1 However, reproducible and clinically significant fracture reduction has not been clearly demonstrated in these same studies.2,3 The technique involves the use of inflatable bone tamps to create a cavity through compaction of bone and marrow, followed by high viscosity cement injection using bone filling cannulas. The cumulative volume required to fill the large voids requires the use of multiple cannulas, but provides the surgeon greater control of the cement injection rate and volume compared to vertebroplasty. Cement flow and interdigitation are limited to some extent by the compressed bone lining the cavity walls and by the cement viscosity. This technique is generally reliable and safe, provided cement viscosity is high and the operator includes careful fluoroscopic monitoring.
System Overview
The Shield system includes a set of single patient use disposable instruments for unipedicular percutaneous access and specially designed instruments for cavity creation, implant deployment, and cement injection, as shown in Figure 39-1. The unique curved design of the cavity creation instrument allows the surgeon to drill a curved path from one pedicle, crossing the sagittal midline, and stopping within the contralateral anterior quadrant of the vertebral body. The cavity creation instrument then converts to a reamer in situ, which is capable of creating a 10-mm diameter cylindrical cavity in the retrograde (proximal) direction that is matched to the implant size. The delivery system subsequently provides a means to insert and deploy the cement directing device within the cavity and facilitates cement injection with a high pressure injection system.
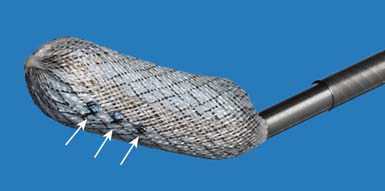
The Shield cement director is an elongated 10-mm diameter hollow structure fabricated from braided nitinol wire and other biocompatible textile and polymeric materials. The device is available in three lengths: 15 mm, 20 mm, and 25 mm, a range selected to approximate the anatomic distance between the medial pedicle borders in the thoracic and lumbar spine in the patient population with osteoporosis. The cylindrical wall of the implant is impermeable to bone cement with the exception of small holes located anteriorly-superiorly and anterior-inferiorly on the device, as shown in Figure 39-2. The implant is supplied preloaded onto a delivery device and collapsed within a sheath to facilitate placement into the cavity through the working channel. After placement, the sheath is retracted to deploy the self-expanding implant in the prepared cavity.
Contraindications
The Shield Kyphoplasty System should not be used when the following conditions are present:
Biomechanical Testing
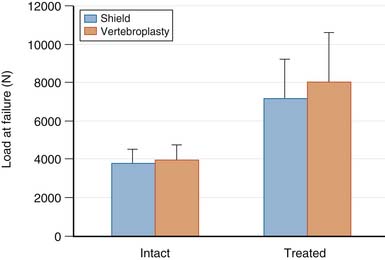
The mechanical behavior of fractured osteoporotic vertebral bodies treated with the Shield Kyphoplasty System has been studied under monotonic and cyclic loading conditions. Controlled, reproducible compression fractures were created by applying a uniaxial compressive load until the vertebral body experienced a 25% loss in height. The fractured vertebral bodies were subsequently treated with either the Shield Kyphoplasty System or bipedicular vertebroplasty, which was used as a comparative control. The failure strength of intact and treated vertebral bodies is shown in Figure 39-3. There were no statistically significant differences between the intact failure strengths or the treated failure strengths for both groups (p = .146). Treatment of vertebral compression fractures with Shield Kyphoplasty System, using a unipedicular approach, resulted in biomechanical performance that was equivalent to conventional bipedicular vertebroplasty. Furthermore, the presence of the cement director appears to have no detectable effect on the ability of the bone cement to interdigitate and reinforce the fractured vertebral body.
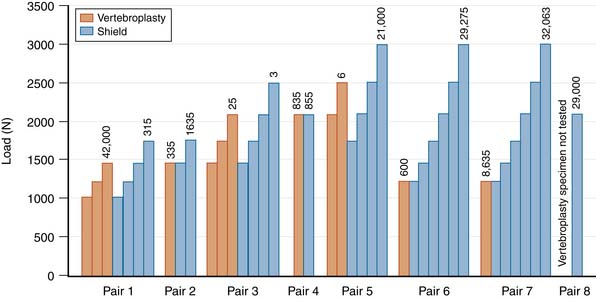
Treated vertebral bodies either failed during cyclic loading at the first load level (6 of 15 specimens), or they required multiple loading levels to fail (9 of 15 specimens). A compilation of all of the results from the cyclic testing of treated vertebral bodies is provided in Figure 39-4. For 6 of 8 specimen pairs, the specimen treated with the Shield Kyphoplasty System withstood a greater number of loading cycles and failed at a higher load than the specimen treated with conventional vertebroplasty. These results demonstrate that directed cement flow using the unipedicular Shield Kyphoplasty System can provide biomechanical reinforcement to fractured osteoporotic vertebral bodies that is equivalent to or better than conventional vertebroplasty, while reducing the risk of posterior leakage.
The Shield Kyphoplasty System Surgical Technique
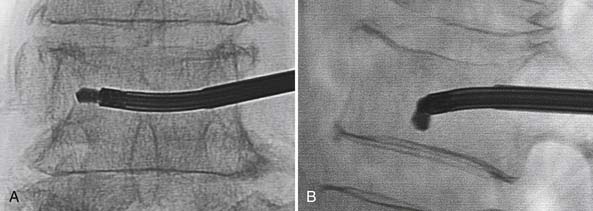
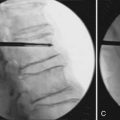
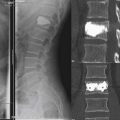
The percutaneous needle approach used for the Shield Kyphoplasty System is similar to the original kyphoplasty procedure widely used to treat vertebral compression fractures. However, only a single needle placement targeting the anterior sagittal midline is required for the Shield system for all levels of the spine. The approach angle and needle position is verified with anteroposterior (AP) and lateral fluoroscopy, as shown in Figure 39-5. Once angular orientation has been established, the needle is advanced into the vertebral body beyond the posterior cortical wall. The stylet of the needle is removed and replaced with a blunt tipped wire. The needle is then retracted leaving the wire in place. A 4-mm outer diameter working channel cannula is then placed over the wire to a depth of 5 mm anterior to the posterior cortical wall, as depicted in Figure 39-6. Proper placement of the working channel cannula includes orienting the key slot in the medial direction. This ensures that all subsequent invasive steps in the procedure involving bone cutting will have the proper medial orientation.
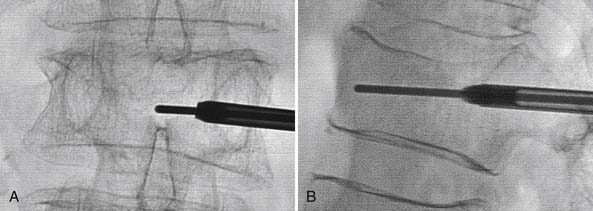
FIGURE 39-5 Blunt tip wire verifies medial needle placement in A/P (A) and lateral (B) fluoroscopic images.
(Images provided by Dr. G. C. Anselmetti, Institute for Cancer Research and Treatment, Torino, Italy.)
The Shield Kyphoplasty System cavity cutter is inserted within and locked to the working channel. The cutting device is designed to drill along a curved path projecting anteriorly and medially from the end of the working channel. The surgeon advances the drill by manually rotating in the clockwise direction while stabilizing the working channel with the other hand. It is recommended that drilling be ceased once the blade tip has reached the medial border of the contralateral pedicle, as shown in Figure 39-7. Once the path is complete, the blade is deflected to 10 mm. A cylindrical cavity is created as the blade rotates and translates along the original path in the proximal direction. After several turns, the blade is retracted at a predetermined point on the proximal end of the path to create a cavity that is the same length as the selected implant: 15 mm, 20 mm, or 25 mm.
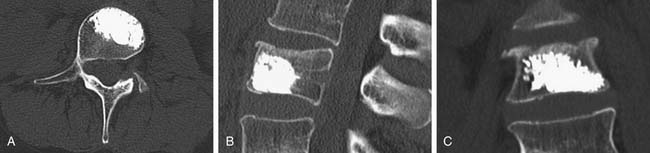
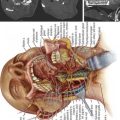
The appropriate size delivery system with a preloaded Shield implant is then inserted into the working channel. The delivery system is curved, allowing the Shield implant to be deployed precisely within the central cavity. Removal of an internal wire exposes a luer connector, which is the cement fill portal. The Shield Kyphoplasty System includes a bone cement mixing and injection device that mates with the delivery system. The injection system is capable of injecting high viscosity cement. Cement injection should be performed under biplanar fluoroscopic imaging. The materials used in the construction of the Shield cement director allow visualization of the cement while it fills the implant and flows into the anterior, superior, and inferior regions of the cancellous bone, as shown in Figure 39-8.
Clinical Outcomes
The clinical performance of the Shield Kyphoplasty System has been evaluated in a 1-year, multicenter, prospective, 2:1 randomized controlled study comparing pain relief, cement leakage rates, and leak locations for the Shield and conventional percutaneous vertebroplasty.4 The patient population for this study consisted of adults at least 50 years old with osteoporosis and painful benign vertebral compression fractures at one to three levels between T4 and L5. A total of 77 patients and 104 levels were treated, 49 patients (65 levels) with the Shield Kyphoplasty System and 28 patients (39 levels) with conventional vertebroplasty. The Shield procedure was performed using a unilateral transpedicular or extrapedicular approach, whereas the vertebroplasty procedure was performed using a standard bipedicular approach.
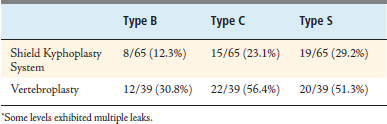
Pain was assessed using a 10-point visual analog scale (VAS) at 24 hours postoperatively, 3 months, and again at 1 year. To evaluate and compare cement leakage rates, plain radiographs (AP and lateral) and CT scans were obtained within 24 hours of the procedure. Cement leakage was assessed by the operating surgeon (radiographs) and an independent reviewer (CT images). The increased resolution of the CT images allowed small leaks to be identified that would have been overlooked on radiographic images. Cement leaks were classified as follows: Type B—through the basivertebral vein, Type C—through a cortical bone defect (including endplates), and Type S—through a segmental vein.5
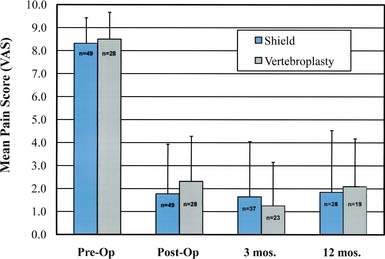
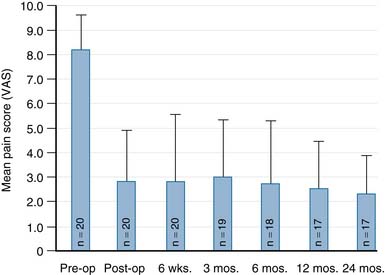
Significant pain relief was achieved immediately following treatment with both the Shield Kyphoplasty System and conventional vertebroplasty, as shown in Figure 39-9. Mean preoperative pain scores were 8.31 ± 1.12 and 8.49 ± 1.18 for the Shield and vertebroplasty groups, respectively. The mean pain scores decreased by more than 6 points for both groups at 24 hours post-op, and pain relief was sustained during the 12 month follow-up period. The results of a single arm, two-year pilot study (20 patients) further demonstrate that long-term pain relief is achieved in patients treated with the Shield Kyphoplasty System, as shown in Figure 39-10.
In the randomized clinical study, cement leaks were identified and classified from both plain radiographic images and CT reconstructions. Leakage rates reported in the literature are highly variable, ranging from 7% to 90% for vertebroplasty and 0% to 33% for kyphoplasty.6 This data is often difficult to interpret because different methods are used to assess leak rates, and the resolution can vary among the methods and among institutions. In general, studies that use CT imaging to quantify cement leaks report much greater leakage rates than studies that rely on routine radiographic interpretation. The overall leakage rate for patients treated with the Shield Kyphoplasty System was substantially lower than the leakage rate for patients treated with conventional vertebroplasty, as shown in Table 39-1. Eight levels in the Shield group and 14 levels in the control group exhibited multiple leaks and in these instances, each leak was counted and classified separately. The leakage rate for all types of leaks was decreased for the Shield treatment group, as compared to the vertebroplasty treatment group shown in Table 39-2. The Type B leakage rate, involving cement leakage into the basivertebral vein, was markedly decreased for patients treated with the Shield system and was the lowest leakage rate observed overall. This result confirms that anteriorly directed cement flow is effectively achieved by using the Shield implant.
| Radiographs | CT Images | |
|---|---|---|
| Shield Kyphoplasty System | 8/65 (12.3%) | 42/65 (64.6%) |
| Vertebroplasty | 10/39 (25.6%) | 54/39 (138.5%) |
1. Wardlaw D., Cummings S.R., Van Meirhaeghe J., Bastian L., Tillman J.B., Ranstam J., Eastell R., Shabe P., Talmadge K., Boonen S. Efficacy and safety of balloon kyphoplasty compared with non-surgical care for vertebral compression fracture (FREE): a randomised controlled trial. Lancet. 2009 Mar 21;373(9668):1016-1024.
2. Hiwatashi A., Sidhu R., Lee R.K., deGuzman R.R., Piekut D.T., Westesson P.L. Kyphoplasty versus vertebroplasty to increase vertebral body height: a cadaveric study. Radiology. 2005;237(3):1115-1119.
3. Pradhan B.B., Bae H.W., Kropf M.A., Patel V.V., Delamarter R.B. Kyphoplasty reduction of osteoporotic vertebral compression fractures: correction of local kyphosis versus overall sagittal alignment. Spine. 2006 Feb 15;31(4):435-441.
4. Pflugmacher R., Hierholzer J., Stender G., Hammerstingl R., Truumees E., Wakhloo A.K., Gounis M.J., Vogl T.J. Evaluation of leakage rates for a cement directing kyphoplasty system. San Francisco CA: Presented at the 25th Annual Meeting of the North American Spine Society; Nov. 10–14, 2009.
5. Yeom J.S., Kim W.J., Choy W.S., Lee C.K., Chang B.S., Kang J.W. Leakage of cement in percutaneous transpedicular vertebroplasty for painful osteoporotic compression fractures. J. Bone Joint. Surg. Br.. 2003 Jan;85(1):83-89.
6. Hulme P.A., Krebs J., Ferguson S.J., Berlemann U. Vertebroplasty and kyphoplasty: a systematic review of 69 clinical studies. Spine. 2006 Aug 1;31(17):1983-2001.