Diplopia
Perspective
Diplopia, or double vision, comes in two varieties—monocular and binocular—and constitutes approximately 1.4% of ophthalmologic emergencies. For patients who visit the emergency department (ED) with diplopia, the majority of cases are binocular, with cranial nerve (CN; especially sixth nerve) palsies being among the most common causes.1 The remainder (approximately 15%) are monocular.
Diagnostic Approach
The refinement of the differential diagnosis for the ED patient with diplopia involves parsing out the exact nature of the diplopia, determining the functional location of the defect, and screening for associated symptoms and findings that may suggest the underlying cause. The majority of this diagnostic resolution is done at the bedside, followed by targeted neuro-ophthalmologic imaging as indicated. The causes of diplopia are myriad, with pathologies relatively benign to relatively fulminant, and the tactical approach to diplopia in the ED entails sorting out those that may result in rapid and profound morbidity from those that are less acute. Table 21-1 outlines some key causes of diplopia prioritized by immediate acuity, with mechanism and distinguishing features.
Table 21-1
| DIPLOPIA-CAUSING ENTITY | MECHANISM AND MORTALITY | DISTINGUISHING FEATURES |
| Tier 1—Critical | ||
| Basilar artery thrombosis | Acute thrombosis of the basilar artery with brainstem ischemia; untreated, mortality 70-90% | Vertigo, dysarthria, other cranial nerve involvement; risk factors for stroke |
| Botulism | Toxin inhibits of release of acetylcholine (ACh) at cholinergic synapses and presynaptic myoneural junctions; untreated, mortality 60% | Dysarthria, dysphagia, autonomic dysreflexia, pupillary dysfunction |
| Basilar meningitis | Infection; untreated, mortality close to 100% if bacterial (25-40% if treated) | Headache, meningismus, fever |
| Aneurysm | Enlarging aneurysm directly compresses cranial nerve; untreated, rupture risk is 1% per year; mortality 26-50% per rupture | CN III palsy with pupillary involvement |
| Tier 2—Emergent | ||
| Vertebral dissection | Dissection causes vertebrobasilar ischemia; acute untreated, mortality 28% (2-5% if neurologically asymptomatic) | Neck pain, vertigo; risk factors for vertebral dissection |
| Myasthenia gravis | Autoantibodies develop against ACh nicotinic postsynaptic receptors; untreated, crisis mortality 42% (5% if treated) | Fluctuating muscle weakness, ptosis, and diplopia worsen with activity and improve with rest |
| Wernicke’s encephalopathy | Thiamine-dependent metabolic failure and tissue injury; untreated, mortality 20% | Nystagmus, ataxia, altered mental status, and ophthalmoplegia; risk factors and nutritional deficiency |
| Orbital apex syndrome, cavernous sinus process | Inflammation or infection in the orbital apex or cavernous sinus directly affects oculomotor cranial nerves; acute mortality low unless infectious and complicated by meningitis | A combination of palsies of CN III, IV, or VI, with retro-orbital pain, conjunctival injection, and possible periorbital or facial numbness |
| Tier 3—Urgent | ||
| Brainstem tumor | Tumor involvement at the supranuclear level; acute mortality low (long-term mortality variable) | Skew deviation vertical diplopia, internuclear ophthalmoplegia |
| Miller-Fisher syndrome | Autoantibodies develop to a cranial nerve ganglioside, GQ1b; acute mortality low if fully differentiated from GBS; mortality 2-12% if GBS | Ophthalmoplegia, ataxia, areflexia |
| Multiple sclerosis | Demyelinating lesions; acute mortality low | Internuclear ophthalmoplegia |
| Thyroid myopathy (Graves’ disease) | Autoimmune myopathy; acute mortality low with regard to ocular complaints | Proptosis, restriction of elevation and abduction of the eye, signs of Graves’ disease |
| Ophthalmoplegic migraine | Inflammatory cranial neuropathy; low mortality—self-limited disease | Ipsilateral headache, CN (usually III) palsy |
| Ischemic neuropathy | Microvascular ischemia; mortality low—self-limited disease | Isolated CN palsy (pupil-sparing if CN III) |
| Orbital myositis, pseudotumor | Autoimmune or idiopathic myositis; acute mortality low with regard to ocular complaints | Eye pain, restriction of movement, periorbital edema; exophthalmos and chemosis when more severe |
| Orbital apex mass | A tumor, infiltration, or mass effect in the orbital apex or cavernous sinus directly compresses oculomotor cranial nerves; acute mortality low | A combination of palsies of CN III, IV, or VI, and possible periorbital or facial numbness, with retro-orbital pain, proptosis, signs of venous congestion |
Important Historical Elements
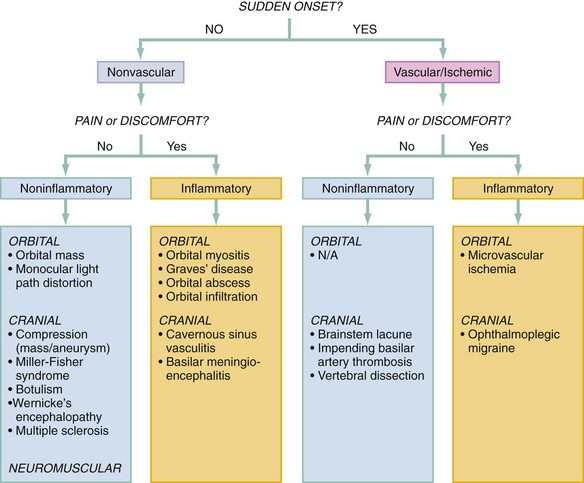
There are four aspects of questioning that guide the formulation of the differential diagnosis: (1) the cadence of onset and symptoms, (2) the directionality and orientation of the diplopia, (3) the presence of pain, and (4) the presence of other associated symptoms.2,3 In terms of the cadence of onset, a truly sudden onset suggests an ischemic cause, either cerebrovascular or microvascular, especially if the intensity or degree of diplopia was maximal at onset. A fluctuation of symptoms over time may suggest transient ischemic attacks or an impending stroke but also generally implies a neuromuscular disease.2,3 Regarding the directionality of the diplopia, the directions of gaze that elicit or worsen the diplopia, and the general orientation of that diplopia—that is, horizontal, vertical, or torsional—should be carefully determined in order to localize the problem. Finally, symptoms associated with the diplopia (such as pain or neurologic or neuromuscular symptoms) are critical to forming a differential diagnosis, if not to making the diagnosis. The presence of pain suggests an inflammatory or infectious process and narrows the differential significantly.
The Examination
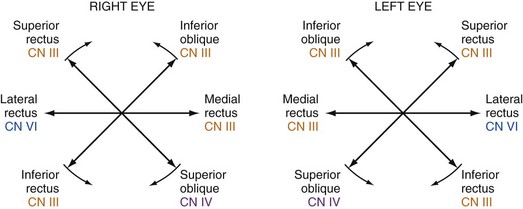
The patient complaining of diplopia should undergo a thorough neurologic examination with attention to the CNs and an evaluation of the six cardinal movements of gaze. Each extraocular muscle (and the nerve that supplies it) has a maximal action in a specific direction, and the evaluation of gaze should therefore specifically follow the configuration of a six-limbed asterisk, or an H2 (Fig. 21-1). The patient should also undergo a careful pupillary and facial examination for signs of pupillary asymmetry, ptosis, lid lag, conjunctival injection or chemosis, periorbital swelling, or proptosis2 and assessment of overall head positioning.
The Diagnostic Approach
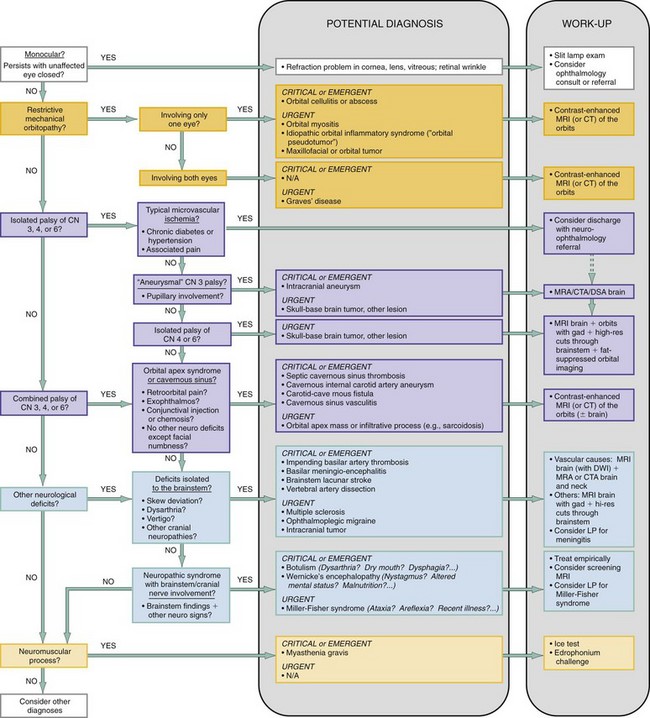
The acuity of onset and presence or absence of pain can be used to prestratify diagnostic possibilities as shown in Figure 21-2, especially with regard to vascular, potentially ischemic events. Through use of the lines of questioning and examination elements outlined previously, the differential diagnosis can then be rapidly winnowed down by means of a phased, systematic approach that incorporates the following queries (reflected in the algorithm in Figure 21-3):
2. Is the binocular diplopia a result of a restrictive, mechanical orbitopathy?
3. Is the binocular diplopia a result of a palsy of the oculomotor CNs (III, IV, or VI) in a single eye?
4. Is the binocular diplopia a result of a neuroaxial process involving the brainstem and related CNs?
5. Is the binocular diplopia a result of a neuromuscular disorder?
1 Is the Diplopia Monocular?
The first key assessment is to determine if the diplopia is purely monocular. Monocular diplopia is an ophthalmologic problem related to distortions in the light path from dry eyes, a corneal irregularity, cataract, or lens dislocation,3,4 or rarely to retinal wrinkles involving the macula.5 The patient with monocular diplopia may require a slit-lamp, funduscopic examination and evaluation by an ophthalmologist and will not typically require an extensive neuro-ophthalmologic workup.
2 Is the Binocular Diplopia a Result of a Restrictive, Mechanical Orbitopathy?
If the diplopia is determined to be monocular, the evaluation essentially ends with the previous question. If the diplopia is determined to be binocular, on the other hand, the next question is whether or not there is a simple mechanical orbitopathy in one of the orbits causing the diplopia. This type of orbitopathy can be caused by orbital myositis, trauma, or infection (abscess) or by craniofacial masses, any of which can cause direct restriction of the movement of a single eye. Orbital myositis may involve as little as a single extraocular muscle and may be a manifestation of a variety of steroid-responsive conditions, such as Wegener’s granulomatosis, giant cell arteritis, systemic lupus erythematosus, dermatomyositis, sarcoidosis, rheumatoid arthritis, or idiopathic orbital inflammatory syndrome (orbital pseudotumor).7
If both eyes are involved, then thyroid disease (Graves’ orbitopathy) is a consideration. It is the most common cause of ocular myopathy in older adults and is bilateral in at least 85% of cases.9 The patient with thyroid-related diplopia will likely have a preexisting diagnosis of Graves’ disease but may have isolated diplopia before the onset of systemic symptoms (and diagnosis).9
Symptoms.: A structural restriction of motion of a single eye, typically gradual in onset, may cause diplopia in a single or multiple directions of gaze, depending on the type and extent of muscular involvement. A sensation of mass effect, discomfort, or pain in the culprit eye is a characteristic symptom. If the cause is infectious, the patient may describe subjective fever. Diplopia that is worse in the morning suggests Graves’ myopathy, which is presumably worsened owing to the venous congestion of the ocular muscle associated with being supine.2
Signs.: Signs of a structural orbitopathy or myositis include proptosis, periorbital swelling, edema, conjunctival or scleral hyperemia, or palpebral swelling involving a single eye.2 Although findings may mimic a neurogenic palsy to some extent, the signs induced on testing extraocular eye movements will not reflect the deficits typical of palsies of the oculomotor CNs (discussed subsequently). Ocular myositis can be distinguished from a neurogenic palsy in that it abruptly restricts eye movement away from the muscle, whereas a CN palsy smoothly and progressively impairs movement toward the weakened muscle.10
The signs of Graves’ disease include lid lag,2 diffuse conjunctival edema and vascular injection,9 and—because it classically affects the inferior and medial recti muscles first—restriction of elevation and abduction of the eye.2 Patients with thyroid-related diplopia may tilt the head back to accommodate for the restriction of the inferior rectus muscle.2,3
3 Is the Binocular Diplopia a Result of a Palsy of the Oculomotor Cranial Nerves (III, IV, or VI) in a Single Eye?
If there is no obvious mechanical restriction evident, the next question is whether or not there is a neuropathic cause of the diplopia. The first consideration in this regard is whether there is a unilateral palsy in one or more of the oculomotor nerves. The oculomotor (CN III), trochlear (CN IV), and abducens (CN VI) CNs innervate the muscles that move the eye. CN VI is the most commonly affected, followed by CN III and then CN IV.3,11 The clinical distinction to be made at this point is whether the oculomotor palsy is caused by an isolated mononeuropathy of single nerve or by a unilateral simultaneous palsy involving multiple oculomotor nerves.
An isolated, simple mononeuropathy in CN III, IV, or VI may be from a demyelinating process (such as multiple sclerosis12), hypertensive or diabetic vasculopathy, or compression. Although in a significant proportion of cases the cause of the palsy is not determinable,11 each nerve has characteristic predilections to which it is vulnerable. In adults, CN III is most commonly affected by diabetic or hypertensive vasculopathy.11 Aneurysms in the posterior communicating, basilar, superior cerebellar, posterior cerebral, and cavernous internal carotid arteries are a close second. CN IV is most commonly affected by trauma from abutment against the tentorium (typically not an isolated symptom or finding, however), followed by vascular causes.11 CN VI is the most common nerve to be affected by tumor and elevated intracranial pressure.
A unilateral simultaneous palsy of more than one oculomotor nerve (but with no other CN involvement or other neurologic signs beyond possible involvement of the upper branches of the trigeminal nerve) suggests a process in the posterior orbit (orbital apex) or cavernous sinus. A cavernous sinus infection, mass, or vasculitis may affect CNs III, IV, and VI simultaneously but typically affects CN VI first because it traverses through the cavernous sinus as opposed to within its wall as do CNs III and VI.6 Causes include carotid-cavernous fistula, inflammatory vasculitides such as giant cell arteritis, Tolosa-Hunt syndrome (a rare idiopathic vasculitis), or tumor or infiltration (e.g., sarcoidosis) in the orbital apex.13 A complex palsy in the cavernous sinus may also be iatrogenic, from an intraoral dental anesthetic nerve block, presumably because of intravascular injection or diffusion of anesthetic along tissue planes into the pterygoid venous plexus.14
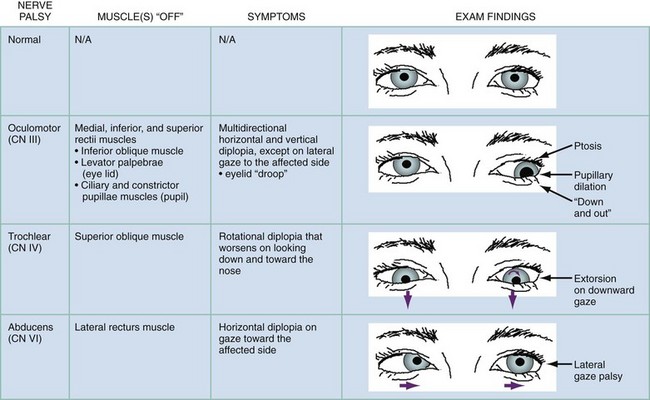
Symptoms.: An isolated oculomotor nerve palsy has characteristic symptoms. The patient with a CN III palsy typically reports diplopia in all directions of gaze except on lateral gaze to the affected side.6 A rotational diplopia that worsens on looking down and toward the nose implies a superior oblique (CN IV) palsy. A CN IV palsy makes descending stairs, reading, and watching television in bed difficult.2 Diplopia that worsens on lateral gaze to one direction implies an issue with CN VI on that side.3 A patient with diplopia from an isolated palsy of CN III, IV, or VI will typically not have associated symptoms beyond the diplopia. Pain and speed of onset are differentiators; a sudden isolated CN III, IV, or VI palsy associated with orbital discomfort in a patient with chronic diabetes or hypertension strongly suggests that microvascular ischemia is the cause.2,13
The diplopia from a cavernous sinus problem, unlike an isolated mononeuropathy, may manifest as a combination of any of the gaze abnormalities noted previously, given that more than one oculomotor nerve may be involved. It may be gradual in onset and associated with retro-orbital pain or blurred vision as a result of venous congestion.13 Because branches of the trigeminal nerve travel though the orbital apex, the patient may have associated ipsilateral periorbital facial numbness or dysesthesia.13
Signs.: Palsies from an isolated mononeuropathy in each oculomotor nerve manifest with typical findings as outlined in Figure 21-4. CN III also innervates the levator palpebrae superioris muscle, which lifts the upper eyelid and provides parasympathetic innervation to two intrinsic ocular muscles, the ciliary and constrictor pupillae muscles, which constrict the pupil.2,6 A CN III palsy, when complete, therefore results in an eye that is deviated “down and out” with a dilated pupil and ptosis.2 Diplopia caused by microvascular ischemia, typically seen in older patients with vascular risk factors such as diabetes and hypertension, may manifest with an isolated palsy associated with pain in CN III, CN IV, or CN VI. A CN III palsy from this process classically spares the pupil, whereas a CN III palsy that develops from compression (i.e., from an aneurysm) does not and typically results in pupillary mydriasis owing to compression of pupillomotor parasympathetic fibers that run in the peripheral and superomedial surface of the nerve.15 The “rule of the pupil” (more of a guideline than a rule) is that an otherwise complete CN III palsy (complete ptosis, completely “down and out”) with normal pupillary size and reactivity essentially rules out compression as the source.15,16
In contrast to a mononeuropathy, the combination of ipsilateral palsies of CN III, IV, and VI from an orbital apex syndrome or cavernous sinus process will typically manifest with additional findings of exophthalmos, chemosis, and injection.6,13 Sensory deficits corresponding to the ophthalmic (V1) and maxillary (V2) divisions of the trigeminal nerve may be present, given their course through the orbital apex.13
4 Is the Binocular Diplopia a Result of a Neuroaxial Process Involving the Brainstem and Related Cranial Nerves?
A focal brainstem lesion may be from multiple sclerosis (as a so-called “clinically isolated syndrome,” of which 68% manifest as diplopia).12 A more diffuse but localized brainstem process may be caused by brainstem tumor, a brainstem lacunar stroke,3 an impending basilar artery thrombosis or a vertebral artery dissection,17,18 or an ophthalmoplegic migraine.6,19 A vertebral artery dissection may manifest with diplopia alone,17 as can an impending basilar artery thrombosis, which can then go on to acute cause coma.18
A more diffuse process involving the brainstem and/or CNs III, IV, and VI may be infectious, autoimmune, neurotoxic, or metabolic and may involve other neurologic structures, resulting in additional symptoms and signs. Possibilities include basilar meningio-encephalitis, botulism,20 an autoimmune syndrome such as Miller-Fisher or Guillain-Barré syndrome,21,22 and Wernicke’s encephalopathy syndrome, in which the ophthalmologic manifestations are a result of metabolically induced lesions in the pontine tegmentum, abducens nucleus, and oculomotor nucleus.24
Symptoms.: Double vision may be the most prominent symptom (and therefore the presenting complaint) in a neurologic syndrome in which it is one of many components, making it important to actively screen for those other symptoms and signs. A focal brainstem lesion (as in multiple sclerosis) may result in an isolated diplopia, but localized brainstem lesions such as those from mass effect or ischemia typically result in so-called “neighborhood” symptoms and signs (from anatomically contiguous involvement). A brainstem lacunar stroke may cause sudden diplopia and symptoms of other CN involvement, such as facial weakness. Nausea, vertigo, or slurred speech raises concern for an impending basilar artery occlusion, especially if symptoms are sudden in onset, are painless, and fluctuate or may represent a brainstem mass if gradual in onset and progressive. A young person with a history of migraine headaches who has an ophthalmoplegic migraine may have a presentation similar to that of someone with a brainstem stroke but will typically develop an associated ipsilateral headache.
Diplopia from a more diffuse neurologic syndrome that happens to involve the brainstem and CNs is usually gradual in onset and manifests with a variety of more discordant symptoms. A gradually evolving combination of double vision, slurred speech, and problems swallowing suggests food-borne botulism20,23 especially if additional symptoms of dry mouth, nausea, and diffuse muscle weakness are present. Double vision, clumsiness, and altered mentation in a patient with chronic alcoholism, malnutrition, or a history of bariatric surgery should raise the possibility of Wernicke’s encephalopathy.24 Diplopia and other CN symptoms, together with headache, photophobia, stiff neck, or fever, should raise suspicion regarding basilar meningio-encephalitis.
Signs.: Vertical diplopia without the torsional component seen with CN IV palsy, called a vertical skew deviation, suggests a brainstem lesion. An internuclear ophthalmoplegia, suggested by an ability to adduct the eye on one side in the contralateral direction during lateral gaze that resolves during convergence, implicates a lesion in the medial longitudinal fasciculus (MLF), such as that classically found in patients with multiple sclerosis.3 In multiple sclerosis, diplopia may appear alone as a clinically isolated syndrome12 or may be associated with a host of additional heterogeneous neurologic findings that typify this disorder, such as nystagmus, optic neuritis (with blurred vision and eye pain), or focal motor or sensory abnormalities. A brainstem lacunar stroke may manifest as any of a number of identifiable syndromes (Box 21-1). An impending basilar occlusion may cause additional symptoms of nystagmus, dysmetria, gait ataxia, and dysarthria.
A brainstem or cranial neuropathy that is part of a more diffuse neurologic syndrome may cause a stereotypical assortment of additional associated deficits. With food-borne botulism, patients have a descending flaccid paralysis that begins with multiple CN palsies. There may also be autonomic signs, such as dry mouth, ileus, postural hypotension, respiratory muscle weakness, and pupillary abnormalities.20,24 Patients with Miller-Fisher syndrome may have an isolated ophthalmoplegia,25 considered a forme fruste of the disease, but more typically have the classic triad of ophthalmoplegia, ataxia, and areflexia.22 Muscle weakness should not be present21; if it is, the case may be better classified as Guillain-Barré syndrome with ophthalmoplegia.21,22 Almost a third of patients with Wernicke’s encephalopathy have ocular abnormalities including nystagmus and ophthalmoplegia, typically associated with the classic triad of nystagmus, altered mental status, and truncal and gait ataxia. Other findings might include sluggish pupillary reaction to light, anisocoria, papilledema, limb ataxia and dysarthria, as well as hypothermia resulting from involvement of the posterior hypothalamic regions.24 On the other hand, a temperature elevation or fever suggests the possibility of an infectious process such as basilar meningio-encephalitis.
5 Is the Binocular Diplopia a Result of a Neuromuscular Disorder?
If none of the previous considerations seem applicable, and specifically if there are no anatomically distinct neurologic deficits evident, then a neuromuscular disorder should be considered. Although clinical entities such as snake envenomation and tick paralysis can on rare occasion cause isolated diplopia, the prototypical neuromuscular cause of diplopia is myasthenia gravis.26 Unlike other causes of diplopia, myasthenia will typically manifest with diplopia in a setting of a preestablished diagnosis, which facilitates a determination, if not immediate recognition, of the cause.
Symptoms.: Diplopia that is variably triggered in multiple directions (without a distinct structural or neuropathic cause evident) implies a neuromuscular cause such as myasthenia gravis. Milder versions of each of these may result in a diplopia isolated to one direction, however. Diplopia from neuromuscular disease generally fluctuates over time.2,3 The diplopia of myasthenia gravis typically gets worse as the patient fatigues and improves with rest.26 Associated symptoms of proximal muscle weakness (e.g., difficulty holding arms above the head or climbing stairs), shortness of breath, or difficulty swallowing imply a systemic neuromuscular disease.2
Signs.: The signs of neuromuscular disease, such as muscle atrophy or weakness, may be readily apparent on inspection of the patient.2 Patients with myasthenia gravis may have unilateral or bilateral ptosis, weakness on forced eyelid closure, and generalized muscle weakness, but with normal reflexes and no sensory abnormalities11; about half have isolated ocular abnormalities.26,27 The diplopia may represent a myasthenic crisis, possibly associated with occult respiratory muscle weakness and ventilatory insufficiency.28
If myasthenia gravis is suspected, a bedside test that can be performed is the “ice test.” An ice-filled glove or bag is applied to the patient’s closed eye or eyes, held there for about 5 minutes, and withdrawn; any improvement in ptosis (typically about 5 mm29) or diplopia is noted. Cold is believed to mitigate the effect of myasthenia-related acetylcholine-receptor blockade by decreasing cholinesterase activity and by promoting efficiency of acetylcholine at the end plate.30 An edrophonium (Tensilon) challenge can also be performed if the drug is available (see Chapter 108).
Diagnostics
The diagnostics applicable to each of the differential considerations in the previous sections are outlined in Figure 21-3. Most patients with undifferentiated binocular diplopia will need imaging, the mainstay of the workup. Imaging may be deferred in the older diabetic or hypertensive patient with a clear-cut, isolated microvascular ischemic CN III palsy, although it may be helpful to confirm the diagnosis. Routine laboratory studies, except for specialized tests such as a thyroid panel to screen for thyroid disease or a lumbar puncture when meningitis or Miller-Fisher syndrome is considered, are of little diagnostic utility. Furthermore, routine noncontrast computed tomography (CT) of the brain may yield little information when compared with CT angiography (CTA) or magnetic resonance angiography (MRA) of the cerebral circulation, and contrast-enhanced magnetic resonance imaging (MRI) of the orbits, brain, and brainstem.
In the patient with a suspected or evident mechanical orbitopathy, an MRI of the orbits with gadolinium can allow an assessment for enlargement or enhancement in extraocular muscles and orbital structures,2 although a contrast-enhanced cranial CT scan with fine cuts through the orbit can be used as a second-line option in this case.8 The same imaging paradigm applies to a localization of the process within the cavernous sinus or orbital apex, because it will highlight infiltrative, inflammatory, or compressive pathology.8
For an isolated neuropathy of CN III, IV, or VI without evidence of an aneurysm, the most optimal study is MRI of the brain and orbits with gadolinium, high-resolution cuts through the brainstem, and fat-suppressed orbital imaging to assess for inflammation, neoplasm, or demyelination along the course of the nerves.31a If an aneurysm is suspected, the imaging chosen should be standard for that required to assess for an aneurysm and is detailed in other chapters specifically devoted to the topic. MRA and CTA are now considered first-line alternatives to the traditional cerebral angiogram, but because the sensitivity of MRA decreases with aneurysm sizes less than 5 mm, MRA should be used cautiously as the definitive screening test in cases in which the likelihood of aneurysm is high.31
For a presentation suggestive of a subarachnoid space (e.g., cerebellopontine angle) or brainstem process, an MRI with and without gadolinium is highly recommended.31a If acute brainstem ischemia is a consideration, then adding an MRI diffusion-weighted imaging protocol may be helpful.
Empirical Management
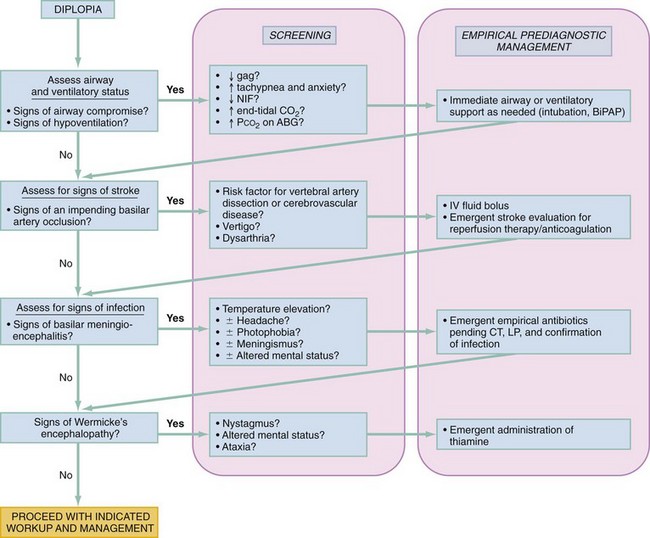
Because the treatment of diplopia depends entirely on the cause, there is little in the way of a primary treatment for diplopia in the ED beyond addressing whatever secondary disorder is causing it (approaches to which are outlined in individual chapters in this text). Certain emergent therapeutic measures may be indicated in the context of potentially serious underlying causes, as outlined in the algorithm in Figure 21-5. The priority is to consider imminent threats to CNS tissue viability, such as an impending basilar artery thrombosis, and then consider rapidly evolving threats to CNS tissue viability, such as meningio-encephalitis or Wernicke’s encephalopathy, and empirically institute indicated treatments as the workup gets underway.
The patient with diplopia will typically require an admission for further workup and treatment of the underlying disorder, unless diagnosed with a low-acuity condition such as microvascular ischemia. A CN III or CN VI palsy from microvascular ischemia is generally self-limited; the pain usually resolves after a few days, and complete spontaneous resolution is the norm, occurring in up to 95% of patients.32 These patients can typically be discharged home with close outpatient follow-up.
References
1. Comer, RM, Dawson, E, Plant, G, Acheson, JF, Lee, JP. Causes and outcomes for patients presenting with diplopia to an eye casualty department. Eye. 2007;21:413–418.
2. Rucker, JC, Tomsak, RL. Binocular diplopia. A practical approach. Neurologist. 2005;11:98–110.
3. Friedman, DI. Pearls: Diplopia. Semin Neurol. 2010;30:54–65.
4. Coffeen, P, Guyton, DL. Monocular diplopia accompanying ordinary refractive errors. Am J Ophthalmol. 1988;105:451.
5. Barton, JJ. “Retinal diplopia” associated with macular wrinkling. Neurology. 2004;63:925–927.
6. Duong, DK, Leo, MM, Mitchell, EL. Neuro-ophthalmology. Emerg Med Clin North Am. 2008;26:137–180.
7. Lutt, JR, Lim, LL, Phal, PM, Rosenbaum, JT. Orbital inflammatory disease. Semin Arthritis Rheum. 2008;37:207–222.
8. Reference deleted in proofs.
9. Lee, HB, Rodgers, IR, Woog, JJ. Evaluation and management of Graves’ orbitopathy. Otolaryngol Clin North Am. 2006;39:923–942.
10. Danchaivijitr, C, Kennard, C. Diplopia and eye movement disorders. J Neurol Neurosurg Psychiatry. 2004;75(Suppl 4):iv.
11. Richards, BW, Jones, FR, Jr., Younge, BR. Causes and prognosis in 4278 cases of paralysis of the oculomotor, trochlear, and abducens cranial nerves. Am J Ophthalmol. 1992;113:489–496.
12. Jacobs, DA, Galetta, SL. Multiple sclerosis and the visual system. Ophthalmol Clin North Am. 2004;17:265–273.
13. La Mantia, L, Erbetta, A, Bussone, G. Painful ophthalmoplegia: An unresolved clinical problem. Neurol Sci. 2005;26(Suppl 2):s79–s82.
14. Choi, EH, Seo, JY, Jung, BY, Park, W. Diplopia after inferior alveolar nerve block anesthesia: Report of 2 cases and literature review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:e21–e24.
15. Purvin, VA. Neuro-ophthalmic aspects of aneurysms. Int Ophthalmol Clin. 2009;49:119–132.
16. Trobe, JD. Third nerve palsy and the pupil. Footnotes to the rule. Arch Ophthalmol. 1988;106:601–602.
17. Jickling, G, et al. Left vertebral artery dissection causing bilateral internuclear ophthalmoplegia. CJEM. 2008;10:485–487.
18. Mennel, S, Hergan, K, Peter, S. Skew deviation: A precursor to basilar artery thrombosis. Clin Experiment Ophthalmol. 2005;33:536–538.
19. Lal, V, Sahota, P, Singh, P, Gupta, A, Prabhakar, S. Ophthalmoplegia with migraine in adults: Is it ophthalmoplegic migraine? Headache. 2009;49:838–850.
20. Kongsaengdao, S, et al. An outbreak of botulism in Thailand: Clinical manifestations and management of severe respiratory failure. Clin Infect Dis. 2006;43:1247–1256.
21. Bushra, JS. Miller Fisher syndrome: An uncommon acute neuropathy. J Emerg Med. 2000;18:427–430.
22. Overell, JR, Willison, HJ. Recent developments in Miller Fisher syndrome and related disorders. Curr Opin Neurol. 2005;18:562–566.
23. Ruthman, JC, Hendricksen, DK, Bonefeld, R. ED presentation of type A botulism. Am J Emerg Med. 1985;3:203–205.
24. Sechi, G, Serra, A. Wernicke’s encephalopathy: New clinical settings and recent advances in diagnosis and management. Lancet Neurol. 2007;6:442–455.
25. Yuki, N, Odaka, M, Hirata, K. Acute ophthalmoparesis (without ataxia) associated with anti-GQ1b IgG antibody: Clinical features. Ophthalmology. 2001;108:196–200.
26. Kusner, LL, Puwanant, A, Kaminski, HJ. Ocular myasthenia: Diagnosis, treatment, and pathogenesis. Neurologist. 2006;12:231–239.
27. Barton, JJ, Fouladvand, M. Ocular aspects of myasthenia gravis. Semin Neurol. 2000;20:7–20.
28. Lacomis, D. Myasthenic crisis. Neurocrit Care. 2005;3:189–194.
29. Kubis, KC, Danesh-Meyer, HV, Savino, PJ, Sergott, RC. The ice test versus the rest test in myasthenia gravis. Ophthalmology. 2000;107:1995–1998.
30. Hubbard, JI, Jones, SF, Landau, EM. The effect of temperature change upon transmitter release, facilitation and post-tetanic potentiation. J Physiol. 1971;216:591–609.
31. Vaphiades, MS, Cure, J, Kline, L. Management of intracranial aneurysm causing a third cranial nerve palsy: MRA, CTA or DSA? Semin Ophthalmol. 2008;23:143–150.
31a. Wippold, FJ, II., et al. ACR Appropriateness Criteria orbits, vision and visual loss. Reston, Va: American College of Radiology (ACR). www.guideline.gov, 2009.
32. Sanders, SK, Kawasaki, A, Purvin, VA. Long-term prognosis in patients with vasculopathic sixth nerve palsy. Am J Ophthalmol. 2002;134:81–84.
33. Lewandowski, CA, Rao, CP, Silver, B. Transient ischemic attack: Definitions and clinical presentations. Ann Emerg Med. 2008;52:S7–S16.