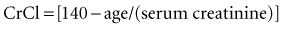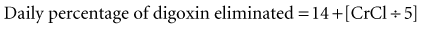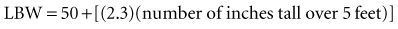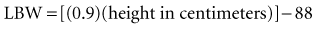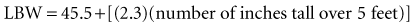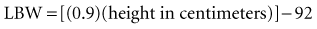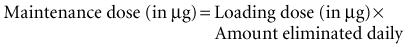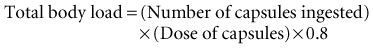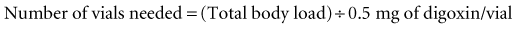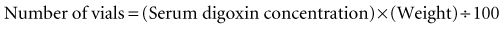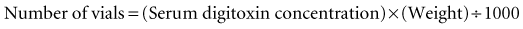177 Digitalis
 Therapeutic Indications
Therapeutic Indications
Digoxin is indicated for the treatment of mild to moderate congestive heart failure (CHF) and for the control of ventricular response rates in patients with chronic atrial fibrillation.1 Digoxin improves left ventricular ejection fraction, improves exercise tolerance, ameliorates CHF-related symptoms, and decreases CHF-related hospitalizations and emergency care. But treatment with digoxin has not been shown to improve survival in patients with systolic left ventricular dysfunction.2–4 However, digoxin is not indicated as primary treatment for stabilization of acutely decompensated heart failure.5
In the critical care setting, digoxin may be used to treat atrial arrhythmias, predominantly atrial fibrillation.6 In chronic atrial fibrillation, digoxin is useful for controlling the ventricular rate in patients with left ventricular systolic dysfunction.5 Rate control occurs in a linear dose-response fashion over a range of digoxin doses from 0.25 to 0.75 mg/d for adults,1 but the drug may not consistently control ventricular rate in dysfunctional states associated with increased sympathetic tone, such as exercise- or emotional stress–induced tachycardia.6–9 In acute atrial fibrillation, digoxin provides effective ventricular rate control and represents a useful therapy for rate control, especially if left ventricular function is compromised.1,10 The agent does not restore normal sinus rhythm, although occasionally atrial fibrillation spontaneously resolves during initial therapy.5
 Mechanism of Action
Mechanism of Action
Digoxin is a cardiac glycoside with specific effects on the myocardium. Inhibition of the sodium/potassium–adenosine triphosphatase (Na+/K+-ATPase) pump increases intracellular sodium concentration and subsequently increases intracellular calcium concentration by stimulation of sodium-calcium exchange.1,11 The pharmacologic effects of digoxin include increased force of systolic contraction (i.e., positive inotropic activity); decreased activation of the sympathetic nervous system and renin-angiotensin system (neurohormonal deactivating effect); sensitization of arterial baroreceptor nerve endings, which then normalizes the reflex vasodilation response to cardiac unloading; and decreased heart rate and conduction velocity within the atrioventricular (AV) node (vagomimetic effect). Neurohormonal effects occur at low dosages, independent of inotropic effects. Hemodynamic improvement is observed in CHF related to both the inotropic and neurohormonal effects of digoxin. The vagal effects of digoxin result in slowed conduction and prolongation of AV node refractoriness, which slows the ventricular response in patients with atrial fibrillation. The overall response to digoxin is an increase in cardiac output and reduction in pulmonary artery pressure, systemic vascular resistance, plasma norepinephrine level, and pulmonary capillary wedge pressure. Minimal changes in blood pressure occur with initiation of therapy.1,12–13
 Pharmacokinetics
Pharmacokinetics
Intravenous (IV) preparations are 100% bioavailable, whereas most oral formulations provide only 60% to 80% bioavailability.1 Capsules containing liquid have increased bioavailability, being about 90% to 100% of the IV formulation. Therefore, dosing considerations are important when switching between oral and IV preparations. Digoxin absorption occurs primarily in the small intestine. When some digoxin oral preparations are taken after meals, the rate of absorption is slowed, but the total amount of digoxin absorbed remains unchanged.1 Impaired absorption after oral administration can occur if intestinal function is impaired, although partial gastrectomy or jejunoileal bypass does not affect absorption to an appreciable extent.1,14–15
The distribution phase of digoxin metabolism is prolonged after oral or IV administration. For patients started on oral therapy, the onset of action occurs within 0.5 to 2 hours, and peak effects are seen within 6 to 8 hours.1 After IV administration, onset occurs in 5 to 30 minutes, and peak effect is observed within 1 to 5 hours.16 This delay in pharmacologic effect may be undesirable in the setting of acute atrial fibrillation. Pharmacologic effects typically persist for 3 to 4 days after withdrawal of digoxin therapy.
Approximately 20% to 30% of digoxin is protein bound in patients with normal renal function or uremia.1 Digoxin is extensively bound to multiple tissues, particularly to Na+/K+-ATPase in cardiac and skeletal muscle, and demonstrates a large volume of distribution, which averages 6 to 7 L/kg of total body weight in patients with normal renal function. A decrease in the volume of distribution occurs in patients with renal dysfunction or dialysis.
With normal renal function, the elimination half-life is 36 to 48 hours. Elimination is prolonged in patients with renal dysfunction, being about 3.5 to 5 days in anuric patients.11 Metabolism occurs primarily in the liver, but the drug also is metabolized by bacteria within the large intestine after oral administration.1 Excretion of digoxin is predominantly in the urine as unchanged drug. The drug is cleared by glomerular filtration and active tubular secretion. Small amounts are excreted in bile and feces. Approximately 30% of the total digoxin load in the body is eliminated daily in patients with normal renal function. The metabolism and excretion of digoxin is not appreciably altered in patients with liver disease if normal renal function is present. Importantly, increased urinary output does not result in enhanced elimination of digoxin, because elimination is dependent on age, gender, and serum creatinine. Estimations of creatinine clearance (CrCl) in milliliters per minute can be calculated from the patient’s age (in years) and the serum creatinine concentration (in mg/dL) by the modified Cockcroft and Gault equation:
 Dosing Recommendations
Dosing Recommendations
General Considerations
Lean body mass should be used to calculate the appropriate digoxin dosage for adult patients in intensive care units (ICUs), because no appreciable amount of digoxin is distributed to body fat.17 Age, renal function, and weight all have to be considered when calculating both loading and maintenance doses for initiation of digoxin therapy.18 Digoxin dosages in the pediatric population must be carefully titrated, especially in neonates. For children from infancy to age 10 years, substantially higher dosing is necessary in comparison with adult patients (see later discussion). In addition, concomitant medications (discussed later) may influence serum digoxin levels and should be considered when initiating therapy.
Initial Loading Dose
Recent literature does not support initial bolus dosing for patients with CHF.18 If deemed appropriate, a daily dose of 8 to 12 µg/kg is suggested for adult patients in heart failure who are in normal sinus rhythm. Adult patients with CHF may receive initial dosing (62.5 to 250 mg/day), based on ideal body weight and kidney function.19 In the acute setting, administering an initial loading dose is recommended for management of supraventricular tachyarrhythmias. Determining lean body weight (LBW in kilograms) is necessary for calculating digoxin loading and maintenance dosing. Appropriate dosing weight can be calculated from the following equations:
or
or
An initial IV loading dose for adults of 10 to 15 µg/kg based on LBW is necessary for adequate ventricular rate control in the setting of atrial fibrillation or atrial flutter. Patients with impaired renal function and those older than 70 years of age require lower initial loading doses; a 50% dose reduction is recommended. Typically, the loading dose is administered as approximately half of the total dose immediately (maximum of 500 µg administration at one time), followed in 6 to 8 hours by 25% of the total dose, with the remaining 25% given after another 6 to 8 hours.18 For example, a loading dose of 1000 µg should be administered as a 500-µg IV bolus, followed by 250 µg IV every 6 hours for 2 doses. To prevent toxicity, a thorough clinical evaluation of the ICU patient should be completed before additional bolus doses are given during the loading dose phase of therapy.
Maintenance Dosing
If the initial loading dose of digoxin successfully controls the ventricular response of a supraventricular arrhythmia, a maintenance dose should be initiated.18 The maintenance dose is also determined by renal function and the patient’s LBW. The maintenance dose needed by patients not previously receiving digoxin therapy can be estimated from the loading dose and the percentage of drug eliminated each day as follows:
Patients who are switched from IV to oral therapy must have dosage adjustments made as necessary.18 If changing from IV therapy to oral tablets or elixir, the digoxin dosage should be increased by approximately 20% to 25%. However, no dosage adjustment is needed if the oral therapy uses liquid-filled capsules. For example, 100 µg of the IV product is approximately equivalent to 100 µg of the liquid-filled capsules (Lanoxicaps) or 125 µg of the tablet (Digitek, Lanoxin) or the elixir formulation.
 Special Populations
Special Populations
Thyroid Dysfunction
Thyroid dysfunction results in an altered pharmacodynamic profile. Hypothyroid patients require decreased digoxin dosages compared to euthyroid ICU patients.14,15,18 Hyperthyroid patients commonly need increased digoxin dosages, potentially secondary to increased resistance to digoxin therapy. Alterations in absorption, tissue distribution, renal excretion, and sensitivity of digitalis receptors in patients with thyroid disease have been proposed as mechanisms to explain altered serum digoxin concentrations.14,15
Electrolyte Disturbances
Hypokalemia enhances the effects of digoxin by increasing the cardiac effects due to depletion of intracellular potassium.18 Hypomagnesemia requires larger digoxin doses for rate control in the setting of atrial fibrillation.18 Repletion of potassium and magnesium to adequate levels should be completed before initiation of digoxin therapy to prevent potential proarrhythmic effects. Significant hypercalcemia may enhance digoxin toxicity.18
Heart Disease
For patients with coronary artery disease, cor pulmonale, or extensive myocardial damage including previous myocardial infarction, a reduction of digoxin dosage may be necessary.18 Digoxin has been reported to increase mortality in patients with acute ischemic syndromes,20,21 although more recent data do not support this idea.16 The increase in sensitivity to digoxin based on underlying cardiac disease mandates caution and careful patient monitoring.
Gender
When used to treat heart failure and decreased left ventricular function, digoxin was found to have different effects on all-cause mortality in men compared to women.22 Specifically, digoxin was associated with increased all-cause mortality among women in a population with heart failure and depressed left ventricular systolic function.22 The impact of gender on the pharmacologic effects of digoxin used to treat supraventricular arrhythmias is currently unknown, and dosage adjustments are not recommended on the basis of gender at this time.
Pregnancy
Digoxin is a category C medication and should be considered for pregnant patients only if the benefits clearly outweigh the risks, and no alternative is available. The impact in terms of fetal harm or reproductive capacity is unknown.1
Pediatrics
Individualized dosing is extremely important in pediatric patients. In newborns, a reduction in renal clearance of digoxin is observed, necessitating dosage adjustments, especially in premature infants.18 Divided daily dosing is often necessary in infants and those younger than 10 years of age. The elixir formulation is especially suitable for the pediatric population. Loading dosages of the pediatric elixir differ based on age: 20 to 30 µg/kg for premature infants, 25 to 35 µg/kg for full-term newborns, and 35 to 60 µg/kg for children younger than 2 years of age. For children aged 2 to 5 years, oral loading doses of 30 to 40 µg/kg are appropriate, and for those aged 5 to 10 years, the oral loading dose is 20 to 35 µg/kg. Children older than 10 years of age require 10 to 15 µg/kg initially. Maintenance doses for pediatric patients are approximately 25% of the oral loading dose necessary to achieve the optimal therapeutic effect. If IV therapy is necessary, the dose is approximately 80% of the total oral elixir requirement.
 Therapeutic Monitoring
Therapeutic Monitoring
Measurements of digoxin concentration are useful in certain situations to assist in evaluating the effects of the drug on the disease state being treated and to avoid toxicity.18 For treatment of supraventricular tachyarrhythmias, the usual therapeutic range for serum digoxin concentration is 1 to 2 ng/mL. However, patients can require serum concentrations as great as 3 ng/mL. The concentration is correlated with effectiveness or toxicity in a particular patient. The same level that is toxic in one patient may be therapeutic in another. Therefore, dose titration should be based on the heart rate and signs or symptoms of toxicity rather than the absolute digoxin concentration.
Evidence to support the use of serum concentrations to ensure efficacy in the treatment of heart failure is lacking. Lower digoxin concentrations (0.5-0.8 ng/mL) appear to provide equal or superior efficacy and avoid toxicity. Gheorghiade et al.23 found that exercise time, heart failure scores, heart rate, and neurohormonal findings were similar among patients with serum digoxin concentrations of 0.67 ± 0.22 ng/mL compared to those at 1.22 ± 0.35 ng/mL. Mean concentrations of 0.8 ng/mL provided a reduction in rate of hospitalizations and worsening heart failure.4,24 Rathore et al.25 demonstrated that patients with digoxin concentrations of 0.5 to 0.8 ng/mL had a reduction in absolute mortality rate of 6.3% compared with patients who received placebo. However, no reduction in mortality was observed for patients with concentrations of 0.9 to 1.1 ng/mL compared to the placebo group, and an increase in mortality was found for patients with levels of 1.2 ng/mL or greater.
Measurements of serum digoxin concentrations may be particularly useful when kinetic parameters are changing.18 For example, in patients with improving or declining renal function or in situations in which a drug interaction could decrease absorption or digoxin clearance, monitoring levels is helpful. Digoxin concentrations can be obtained periodically to detect excessive drug levels and prevent toxicity.
Proper timing of digoxin measurements is critical. Although digoxin is found in the plasma compartment within a brief period after administration, the medication distributes slowly into the heart and other tissues.26 Because the heart is the site of action, digoxin concentrations measured less than 4 hours after IV administration or 6 hours after oral administration are misleading. The optimal time to measure digoxin levels is 12 to 24 hours after administration. For patients with normal renal function, digoxin concentrations do not reach steady state for 7 to 10 days in the absence of a loading dose. As renal function declines, clearance of digoxin is impaired, and the time to reach steady state is prolonged. In patients with end-stage renal failure, this duration is extended to 15 to 20 days. Levels obtained before the drug has reached steady state can be useful to prevent toxicity or assess a trend. However, these concentrations do not reflect the maximum concentration at steady state.
 Contraindications
Contraindications
Contraindications to the use of digoxin include ventricular fibrillation and hypersensitivity to digoxin or digitalis compounds.1 The risk of digoxin toxicity is higher in patients with preexisting sinus node disease or incomplete AV block, in those with an accessory AV pathway (Wolff-Parkinson-White syndrome), and in those who have heart failure with preserved left ventricular systolic function (isolated diastolic dysfunction). Patients with sinus node disease can develop severe sinus bradycardia or sinoatrial block. An advanced or complete AV block may develop in individuals with a previously incomplete block. The use of digoxin in patients with an accessory AV pathway may result in increased frequency of anterograde conduction via the accessory pathway, with a rapid ventricular response or atrial fibrillation. Individuals with restrictive cardiomyopathy, constrictive pericarditis, amyloid heart disease, or acute cor pulmonale are particularly susceptible to digoxin toxicity.1 Digoxin therapy can adversely affect patients with idiopathic hypertrophic subaortic stenosis by causing further obstruction to outflow.
 Drug-Drug and Drug-Assay Interactions
Drug-Drug and Drug-Assay Interactions
Digoxin is a substrate of P-glycoprotein,27–33 while amiodarone,27 verapamil,28 quinidine,29,30 clarithromycin,31 itraconazole,32 and cyclosporin A33 are potent inhibitors of P-glycoprotein. P-glycoprotein is encoded by the multidrug-resistance (MDR1) gene and is found in kidney, liver, colon, jejunum, adrenal glands, blood-brain barrier, placenta, and testis.28 The role of P-glycoprotein in the body appears to be to act as an ATP-dependent efflux pump.
Within 5 to 7 days after institution of amiodarone therapy in patients receiving digoxin, amiodarone inhibits P-glycoprotein function in kidneys and liver, resulting in a decrease in both renal and nonrenal clearance of digoxin.7,18,34 Renal and nonrenal clearance of digoxin also decreases with concurrent administration of verapamil, resulting in a 70% to 100% increase in serum digoxin concentration.7,18 Although not as extensively studied, a decrease in digoxin clearance also may occur with concomitant administration of diltiazem.35 Administration of digoxin plus verapamil or digoxin plus diltiazem should be avoided by selecting an alternative agent to the aforementioned calcium channel blockers. Quinidine decreases renal and nonrenal clearance of digoxin and increases the rate and extent of digoxin absorption. If amiodarone, verapamil, or quinidine is administered to a patient taking digoxin, the digoxin dose should be decreased by 50%, and serum digoxin concentrations should be monitored closely.
With the administration of clarithromycin, the oral bioavailability of digoxin increases and nonglomerular renal clearance of digoxin decreases.31 This results in a 1.8-fold increase in digoxin concentration. By inhibiting P-glycoprotein, itraconazole decreases the renal clearance of digoxin by approximately 20%, increases oral bioavailability by 30%, and results in a twofold increase in digoxin serum concentrations.32 Renal excretion of digoxin is also inhibited by administration of cyclosporine.33 Serum digoxin concentrations should be monitored closely when clarithromycin, itraconazole, or cyclosporine therapy is started in a patient receiving digoxin.
In patients with severe heart failure, captopril causes a 1.6-fold increase in peak digoxin concentrations.36 This effect may not occur in patients with New York Heart Association class II or III heart failure. The mechanism of the interaction is unknown; however, it may be caused by a decrease in glomerular filtration and tubular secretion of digoxin.
Spironolactone decreases renal37 and nonrenal7,18 clearance of digoxin. In addition, spironolactone and canrenone, a metabolite of spironolactone, cross-react with several of the assays used to monitor digoxin concentrations.38,39 An increase in the apparent digoxin concentration was observed when the drug was assayed by fluorescence polarization immunoassay (FPIA), aca,38 or Elecsys 2020.39 In contrast, a decrease in the apparent concentration occurred when the microparticle enzyme immunoassay (MEIA),38 AxSYM MEIA II,39 IMx MEIA II,39 or Dimension Systems39 were used to measure digoxin levels. Spironolactone did not appear to interact with the chemiluminescent assay (CLIA),38 EMIT 2000,39 Tina Quant,39 or Vitros slides.39 Interference with the MEIA and FPIA assays was eliminated when free concentrations were measured.38 Digoxin concentrations should be monitored more frequently after starting spironolactone to avoid accumulation of the medication; also, CLIA, EMIT 2000, Tina Quant, Vitros slides, or free levels should be used to accurately measure digoxin concentrations.
There are multiple medications that decrease the bioavailability of digoxin and result in lower serum concentrations.7,18 Cholestyramine, colestipol, kaolin-pectin, and oral antacids decrease the absorption of oral digoxin by binding digoxin in the gastrointestinal tract. These medications should be administered at least 2 hours apart to prevent this effect. Metoclopramide decreases the absorption of digoxin tablets by increasing gastrointestinal motility The administration of digoxin capsules instead of tablets in patients receiving metoclopramide is suggested to avoid this reaction. Absorption of digoxin is lowered by the concurrent administration of neomycin or sulfasalazine. This interaction should be avoided; however, if a patient needs to receive both medications, the doses should be spaced by approximately 2 hours.
Patients receiving levothyroxine and digoxin should have close monitoring of thyroid hormone levels and digoxin concentrations. Hyperthyroidism was shown to decrease digoxin levels by increasing the volume of the central compartment.40 In contrast, hypothyroidism may have no effect or may cause an increase in the digoxin concentration.40,41
Rifampin administration induces intestinal P-glycoprotein activity.42 This results in a decrease in digoxin oral bioavailability by approximately 30%, with no apparent change in digoxin renal clearance. Because of the decrease in bioavailability, maximum plasma digoxin concentrations are reduced by 58%.
 Adverse Effects
Adverse Effects
Numerous cardiac arrhythmias may result from digoxin toxicity.7,34 Some predisposing factors for digoxin toxicity include hypokalemia along with hypercalcemia, renal insufficiency, and hypothyroidism. Cardiac effects can manifest as an increase in vagal tone, causing sinus bradycardia. In the early phase of an overdose or in acute toxicity, bradycardia is likely to respond to atropine administration. However, atropine may be ineffective in later phases of acute poisoning. Frequent premature ventricular complexes are another electrocardiographic manifestation of early-phase digoxin toxicity. These phenomena are believed to be due to spontaneous calcium release from calcium-overloaded sarcoplasmic reticulum (SR),43 which is brought upon by cytosolic calcium accumulation secondary to channeling of sodium efflux away from Na+/K+-ATPase and toward the sodium-calcium exchanger. These frequent premature ventricular complexes can degenerate to bigeminal activity and subsequent bidirectional ventricular tachycardia. Such ventricular arrhythmias, along with complete heart block and ventricular fibrillation, are indicative of the late stages of digoxin toxicity.
Noncardiac digoxin toxicities include gastrointestinal effects (anorexia, nausea, vomiting, diarrhea, abdominal pain), central nervous system abnormalities, and hyperkalemia.1,7 Possible central nervous system effects include lethargy, confusion, weakness, headache, delirium, psychosis, transient amblyopia, photophobia, blurred vision, scotomata, photopsia, decreased visual activity, and color irregularities such as yellow-green or red-green halos around lights. Hyperkalemia results from excessive blockade of the Na+/K+-ATPase pump and is an index for outcome. Acute manifestations of digoxin toxicity are often more severe than chronic adverse effects.
 Treatment of Digoxin Toxicity
Treatment of Digoxin Toxicity
The treatment of digoxin toxicity includes several steps which vary based on the acuteness of the situation. In acute overdoses, prevention of further absorption using activated charcoal should be instituted.44 Syrup of ipecac, insertion of a gastric tube, and gastric lavage should be avoided, because vomiting induced by these methods intensifies vagal tone.
Supportive care is required to manage electrolyte disturbances and dysrhythmias.34,44,45 Hyperkalemia should be treated by standard approaches. Sodium polystyrene sulfonate (Kayexalate) may remove potassium, and if hyperkalemia is severe, digoxin immune Fab should be administered (see next section). Caution should be used in administering both digoxin immune Fab and sodium polystyrene sulfonate, because hypokalemia may occur.
In the case of life-threatening arrhythmias, digoxin immune Fab should be administered.34,44 If administration of digoxin immune Fab is delayed or treatment is needed until the onset of the effect of this agent, advanced cardiac life support (ACLS) protocols should be followed.
Digoxin Immune Fab
The digoxin immune Fab (ovine) products available in the United States are Digibind and DigiFab. The products are developed by immunizing sheep with a digoxin analog and then isolating the digoxin-specific Fab fragments from ovine blood.46,47 Digoxin immune Fab is used for the treatment of acute and chronic life-threatening digoxin toxicity or overdose. In addition, digoxin immune Fab is used to bind other digitalis glycosides.44 Digoxin binds to digoxin immune Fab with a higher affinity compared to its sodium pump receptors. Once formed, the Fab-digoxin complex is eliminated by the kidneys and the reticuloendothelial system.
Based on an in vivo kinetic study of healthy volunteers, Digibind and DigiFab result in similar reductions in free serum digoxin concentrations.48 Resolution of gastrointestinal symptoms occurs within minutes after beginning a bolus infusion.49,50 Within 30 to 60 minutes, hyperkalemia secondary to skeletal muscle Na+/K+-ATPase inhibition starts to resolve, and electrocardiogram abnormalities cease. This effect can last for several days, requiring the complex and the drug to be cleared renally,46,47 particularly since digoxin immune Fab is not removed by hemodialysis.
Each vial contains 38 mg (Digibind) or 40 mg (DigiFab) of digoxin immune Fab and binds approximately 0.5 mg of digoxin.46,47 Adult and pediatric patients who acutely ingest an unknown amount of digoxin or other digitalis glycoside should receive 20 vials of either product. Pediatric patients should be closely monitored for volume overload. Administration of all 20 vials at once is likely to result in a faster onset of action but may increase the risk of an allergic reaction. Alternatively, 10 vials may be administered with careful observation of the patient, after which 10 additional vials may be given if clinically indicated.
Adults exhibiting toxicity due to chronic dosing of digoxin and for whom a digoxin level is unavailable should be given 6 vials of either product.46,47 One vial should be sufficient for infants and children weighing 20 kg or less.
If tablets instead of capsules were ingested, the number of vials needed can then be calculated:
Calculation of the digoxin immune Fab dose can also be based on the steady-state digoxin concentration.46,47 Concentrations obtained in an acute overdose may be misleading and may result in underdosing, because digoxin can continue to be absorbed via the gastrointestinal tract. Calculation of the number of vials of Fab product required for an adult patient who is experiencing digoxin toxicity is based on the serum digoxin level (in ng/mL) and the patient’s weight in kilograms as follows:
For digitoxin, the calculation is as follows:
The rate of administration has varied in clinical trials and case reports. Doses are typically given as a bolus over 15 to 30 minutes.50–52 Schaumann and colleagues53 evaluated the kinetics of digoxin immune Fab in 17 patients with acute overdose. They concluded that a bolus dose of 160 mg (4 vials) over 30 minutes, followed by an infusion of 0.5 mg/minute over 8 hours, optimally binds digoxin as it rediffuses into the blood from the tissues. Patients experiencing rebound toxicity 8 to 12 hours after the initiation of treatment could be given 0.1 mg/min.
There are no known contraindications to the use of digoxin immune Fab.46,47 However, allergic reactions and anaphylactic reactions have occurred. Patients at a higher risk for experiencing allergic reactions are those who are allergic to papain, chymopapain, other papaya extracts, pineapple enzyme bromelain, dust mites, or latex. Because the drug is an animal product, individuals who are allergic to sheep or wool are at higher risk. In addition, patients who have previously received digoxin immune Fab are at an increased risk. However, skin testing has not been shown to be useful and results in delay of therapy.
Patients must be closely monitored for significant decreases in potassium concentrations, as well as for deterioration secondary to the withdrawal of an inotropic agent in patients with low cardiac output states.46,47 There is a theoretical risk of development of antibodies to the drug; however, this occurrence has not been reported.
After acute digoxin administration, rebound of free digoxin concentrations was observed 8 to 24 hours after initiation of Fab therapy.48,53 The cause of this phenomenon is not entirely clear. Proposed mechanisms include a release of free digoxin by metabolic degradation of the Fab-digoxin complex46 and rediffusion of free digoxin from the tissues into the serum.53 Patients should be observed closely for indications of a rebound effect. However, monitoring of total serum concentrations is of little utility , because immune Fab interacts with most assay methods. Free digoxin concentrations in ultrafiltration samples provide the most accurate results.54
Key Points
The Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336:525-533.
Packer M, Gheorghiade M, Young JB, et al. Withdrawal of digoxin from patients with chronic heart failure treated with angiotensin-converting-enzyme inhibitors. RADIANCE Study. N Engl J Med. 1993;329:1-7.
Rathore SS, Curtis JP, Wang Y, et al. Association of serum digoxin concentration and outcomes in patients with heart failure. JAMA. 2003;289:871-878.
Rathore SS, Wang Y, Krumholz HM. Sex-based differences in the effect of digoxin for the treatment of heart failure. N Engl J Med. 2002;347:1403-1411.
Rich MW, McSherry F, Williford WO, et al. Effect of age on mortality, hospitalizations and response to digoxin in patients with heart failure: the DIG study. J Am Coll Cardiol. 2001;38:806-813.
1 GlaxoSmithKline. Lanoxin (digoxin) Tablets, USP [product information]. Research Triangle Park, NC: GlaxoSmithKline; 2009.
2 Uretsky BF, Young JB, Shahidi FE, et al. on behalf of the PROVED Investigative Group. Randomized study assessing the effect of digoxin withdrawal in patients with mild to moderate chronic congestive heart failure. J Am Coll Cardiol. 1993;22:955-962.
3 Packer M, Gheorghiade M, Young JB, et al. Withdrawal of digoxin from patients with chronic heart failure treated with angiotensin-converting-enzyme inhibitors. RADIANCE Study. N Engl J Med. 1993;329:1-7.
4 The Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336:525-533.
5 Dec GW. Digoxin remains useful in the management of chronic heart failure. Med Clin North Am. 2003;82:317-337.
6 Rawles JM, Metcalfe MJ, Jennings K. Time of occurrence, duration, and ventricular rate of paroxysmal atrial fibrillation: the effect of digoxin. Br Heart J. 1990;63:225-227.
7 Falk RH, Knowlton AA, Bernard SA, Gotlieb NE, Battinelli NJ. Digoxin for converting recent onset atrial fibrillation—a randomized double-blind trial. Ann Intern Med. 1987;106:503-506.
8 Beasley R, Smith DA, McHaffie DJ. Exercise heart rates at different serum digoxin concentrations in patients with atrial fibrillation. Br Med J. 1985;290:9-11.
9 Furshi R, Kistner N, Sarma JS, Longmate JA, Singh BN. Ventricular rate control in chronic atrial fibrillation during daily activity and programmed exercise: a crossover open-label study of five-drug regimens. J Am Coll Cardiol. 1999;33:30410.
10 Tisdale JE, Padhi ID, Goldberg AD, et al. A randomized, double-blind comparison of intravenous diltiazem and digoxin for atrial fibrillation after coronary artery bypass surgery. Am Heart J. 1998;135:739-747.
11 Schreck DM, Rivera AR, Tricarico VJ. Emergency management of atrial fibrillation and flutter: Intravenous diltiazem versus intravenous digoxin. Ann Emerg Med. 1997;29:135-140.
12 Ferrari A, Gregorini L, Ferrari MC, Preti L, Mancia G. Digitalis and baroreceptor reflexes in man. Circulation. 1981;63:279-285.
13 Ferguson DW, Abboud FM, Mark AL. Selective impairment of baroreflex-mediated vasoconstrictor responses in patients with ventricular dysfunction. Circulation. 1984;69:451-460.
14 Ochs HR, Greenblatt DJ, Bodem G, et al. Disease-related alterations in cardiac glycoside disposition. Clin Pharmacokinet. 1982;7:434-451.
15 Aronson JK. Clinical pharmacokinetics of digoxin. Clin Pharmacokinet. 1980;5:137-149.
16 Eichhorn EJ, Gheorghiade M. Digoxin. Prog Cardiovasc Dis. 2002;44:251-266.
17 Ewy GA, Groves BM, Ball MF, et al. Digoxin metabolism in obesity. Circulation. 1971;44:810-814.
18 Reuning RH, Geraets DR, Rocci ML, et al. Digoxin. In Evans WE, Schentag JJ, Jusko WJ, Relling MV, editors: Applied Pharmacokinetics: Principles of Therapeutic Drug Monitoring, 3rd ed, Vancouver, WA: Applied Therapeutics, 1992. Chapter 20
19 Bauman JL, DiDemnico RJ, Viana M, Fitch M. A method of determining the dose of digoxin for heart failure in the modern era. Arch Intern Med. 2006;166:2539-2545.
20 Yusuf S, Garg H, Held P, et al. Need for a large randomized trial to evaluate the effects of digitalis on morbidity and mortality in congestive heart failure. Am J Cardiol. 1992;69:64G-70G.
21 Leor J, Goldbourt U, Behar S, et al. Digoxin and mortality in survivors of acute myocardial infarction: Observation in patients at low and intermediate risk. Cardiovasc Drug Ther. 1995;9:609-617.
22 Rathore SS, Wang Y, Krumholz HM. Sex-based differences in the effect of digoxin for the treatment of heart failure. N Engl J Med. 2002;347:1403-1411.
23 Gheorghiade M, Hall VB, Jacobsen G, et al. Effects of increasing maintenance dose of digoxin on left ventricular function and neurohormones in patients with chronic heart failure treated with diuretics and angiotensin-converting enzyme inhibitors. Circulation. 1995;92:1801-1807.
24 Rich MW, McSherry F, Williford WO, et al. Effect of age on mortality, hospitalizations and response to digoxin in patients with heart failure: The DIG study. J Am Coll Cardiol. 2001;38:806-813.
25 Rathore SS, Curtis JP, Wang Y, et al. Association of serum digoxin concentration and outcomes in patients with heart failure. JAMA. 2003;289:871-878.
26 Winter ME. Digoxin. In: Koda-Kimble MA, editor. Basic Clinical Pharmacokinetics. 3rd ed. Vancouver, WA: Applied Therapeutics; 1994:198-235.
27 Kakumoto M, Takara K, Sakaeda T, et al. MDR1-mediated interaction of digoxin with antiarrhythmic or antianginal drugs. Biol Pharm Bull. 2002;25:1604-1607.
28 Verschraagen M, Koks CHW, Schellens JHM, et al. P-glycoprotein system as a determinant of drug interactions: The case of digoxin-verapamil. Pharmacol Res. 1999;40:301-306.
29 Fromm MF, Kim RB, Stein CM, et al. Inhibition of P-glycoprotein-mediated drug transport: A unifying mechanism to explain the interaction between digoxin and quinidine. Circulation. 1999;99:552-557.
30 Bauer LA, Horn JR, Pettit H. Mixed-effect modeling for detection and evaluation of drug interactions: Digoxin-quinidine and digoxin-verapamil combinations. Ther Drug Monit. 1996;18:46-52.
31 Rengelshausen J, Goggelmann C, Burhenne J, et al. Contribution of increased oral bioavailability and reduced nonglomerular renal clearance of digoxin to the digoxin-clarithromycin interaction. J Clin Pharmacol. 2003;56:32-38.
32 Jalava K-M, Partanen J, Neuvonen PJ. Itraconazole decreases renal clearance of digoxin. Ther Drug Monit. 1997;19:609-613.
33 Okamura N, Hirai M, Tanigawara Y, et al. Digoxin-cyclosporin A interaction: Modulation of the multidrug transporter P-glycoprotein in the kidney. J Pharmacol Exp Ther. 1993;266:1614-1619.
34 Hack JB, Lewin NA. Cardiac glycosides. In: Goldfrank LR, Flomenbaum NE, Lewin NA, et al, editors. Goldfrank’s Toxicologic Emergencies. 7th ed. New York: McGraw-Hill, Medical Publishing Division; 2002:724-740.
35 Suematsu F, Yukawa E, Yukawa M, et al. Pharmacoepidemiologic detection of calcium channel blocker-induced change on digoxin clearance using multiple trough screen analysis. Biopharm Drug Dispos. 2002;23:173-181.
36 Kirimli O, Kalkan S, Guneri S, et al. The effects of captopril on serum digoxin levels in patients with severe congestive heart failure. Int J Clin Pharmacol Ther. 2001;39:311-314.
37 Hedman A, Angelin B, Arvidsson A, et al. Digoxin-interactions in man: spironolactone reduces renal but not biliary digoxin clearance. Eur J Clin Pharmacol. 1992;42:481-485.
38 Dasgupta A, Saffer H, Wells A, et al. Bidirectional (positive/negative) interference of spironolactone, canrenone, and potassium canrenoate on serum digoxin measurement: Elimination of interference by measuring free digoxin or using a chemiluminescent assay for digoxin. J Clin Lab Anal. 2002;16:172-177.
39 Steimer W, Muller C, Eber B. Digoxin assays: Frequent, substantial, and potentially dangerous interference by spironolactone, canrenone, and other steroids. Clin Chem. 2002;48:507-516.
40 Shenfield GM, Tompson J, Horn DB. Plasma and urinary digoxin in thyroid dysfunction. Eur J Clin Pharmacol. 1977;12:437-443.
41 Croxson MS, Ibbertson HK. Serum digoxin in patients with thyroid disease. BMJ. 1975;3:566-568.
42 Greiner B, Eichelbaum M, Fritz P, et al. The role of intestinal P-glycoprotein in the interaction of digoxin and rifampin. J Clin Invest. 1999;104:147-153.
43 Diaz ME, Trafford AW, O’Neill SC, et al. Measurement of sarcoplasmic reticulum calcium content and sarcolemmal calcium fluxes in isolated rat ventricular myocytes during spontaneous Ca2+ release. J Physiol. 1997;501:3-16.
44 Linden CH. Digitalis glycosides. In: Ford MD, Delaney KA, Ling LJ, Erickson T, editors. Clinical Toxicology. Philadelphia: WB Saunders; 2001:379-390.
45 Ahee P, Crowe AV. The management of hyperkalemia in the emergency department. J Accid Emerg Med. 2000;17:188-191.
46 Digibind Product Information. Research Triangle Park, NC: GlaxoSmithKline; August 2003.
47 DigiFab Product Information. Nashville, TN: Protherics Inc.; August 2001.
48 Ward SB, Sjostrom L, Ujhelyi MR. Comparison of the pharmacokinetics and in vivo bioaffinity of DigiTAb versus Digibind. Ther Drug Monit. 2000;22:599-607.
49 Spiegel A, Marchlinski FE. Time course for reversal of digoxin toxicity with digoxin-specific antibody fragments. Am Heart J. 1985;6:1397-1399.
50 Wegner TL, Butler VP, Haber E, et al. Treatment of 63 severely digitalis-toxic patients with digoxin-specific antibody fragments. J Am Coll Cardiol. 1985;5:118A-123A.
51 Smith TW, Butler VP, Haber E, et al. Treatment of life-threatening digitalis intoxication with digoxin-specific Fab antibody fragments. N Engl J Med. 1982;307:1357-1362.
52 Rabetoy GM, Price CA, Findlay JWA, et al. Treatment of digoxin intoxication in a renal failure patient with digoxin-specific antibody fragments and plasmapheresis. Am J Nephrol. 1990;10:518-521.
53 Schaumann W, Kaufmann B, Neubert P, et al. Kinetics of the Fab fragments of digoxin antibodies and of bound digoxin in patients with severe digoxin intoxication. Eur J Clin Pharmacol. 1986;30:527-533.
54 McMillin GA, Owen WE, Lambert TL, et al. Comparable effects of Digibind and DigiFab in thirteen digoxin immunoassays. Clin Chem. 2002;48:1580-1584.