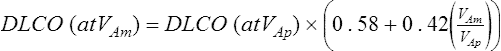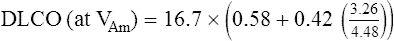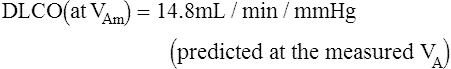Chapter 3
Diffusing Capacity Tests
1. Identify the steps for performing the single-breath Dlco.
2. List at least two criteria for an acceptable single-breath Dlco test.
3. Describe why Dlco is often reduced in emphysema.
1. Describe at least two nonpulmonary causes for reduced Dlco.
2. Explain the significance of a reduced Dl/VA.
3. Compare diffusion limitation caused by membranes and pulmonary capillary blood volume.
Carbon monoxide diffusing capacity
Description
Techniques
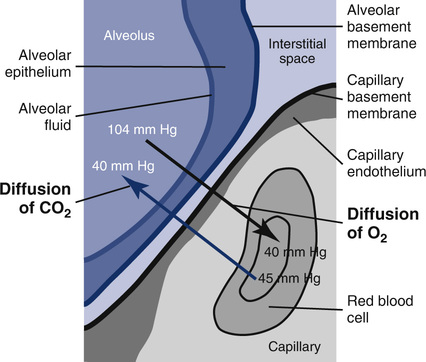
CO combines with hemoglobin (Hb) approximately 210 times more readily than oxygen (O2). In the presence of normal amounts of Hb and normal ventilatory function, the primary limiting factor to diffusion of CO is the status of the alveolocapillary membranes. This process of conductance across the membranes can be divided into two components: membrane conductance (Dm) and the chemical reaction between CO and Hb (Figure 3-1). Dm reflects the process of diffusion across the alveolocapillary membrane. Uptake of CO by Hb depends on the reaction rate (θ) and the pulmonary capillary blood volume (Vc). These two components occur in series, so the diffusion conductance can be expressed as:
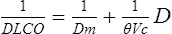
A small amount of CO in inspired gas produces measurable changes in the concentration of inspired versus expired gas. Because little or no CO is normally present in pulmonary capillary blood, the pressure gradient causing diffusion is basically the alveolar pressure (PAco). If the partial pressure of CO in the alveoli and the rate of uptake of the gas can be measured, the Dlco of the lung can be determined. There are several methods for determining Dlco (Table 3-1). All methods are based on the following equation:
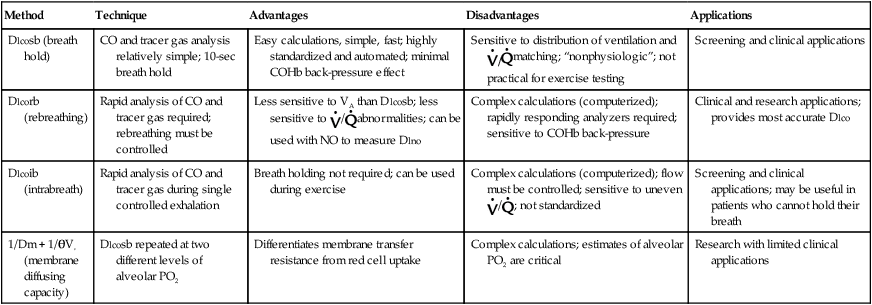
Table 3-1
Advantages and Disadvantages of Dlco Testing Methods
| Method | Technique | Advantages | Disadvantages | Applications |
| Dlcosb (breath hold) | CO and tracer gas analysis relatively simple; 10-sec breath hold | Easy calculations, simple, fast; highly standardized and automated; minimal COHb back-pressure effect | Sensitive to distribution of ventilation and |
Screening and clinical applications |
| Dlcorb (rebreathing) | Rapid analysis of CO and tracer gas required; rebreathing must be controlled | Less sensitive to VA than Dlcosb; less sensitive to |
Complex calculations (computerized); rapidly responding analyzers required; sensitive to COHb back-pressure | Clinical and research applications; provides most accurate Dlco |
| Dlcoib (intrabreath) | Rapid analysis of CO and tracer gas during single controlled exhalation | Breath holding not required; can be used during exercise | Complex calculations (computerized); flow must be controlled; sensitive to uneven |
Screening and clinical applications; may be useful in patients who cannot hold their breath |
| 1/Dm + 1/θVc (membrane diffusing capacity) | Dlcosb repeated at two different levels of alveolar PO2 | Differentiates membrane transfer resistance from red cell uptake | Complex calculations; estimates of alveolar PO2 are critical | Research with limited clinical applications |
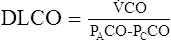
![]() co milliliters of CO transferred/minute (STPD)
co milliliters of CO transferred/minute (STPD)
PAco = mean alveolar partial pressure of CO
PCco = mean capillary partial pressure of CO, assumed to be 0
Dlco is expressed in milliliters of gas/minute/unit of driving pressure at STPD conditions.
Single Breath-Hold Technique (Modified Krogh’s Technique)
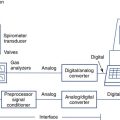
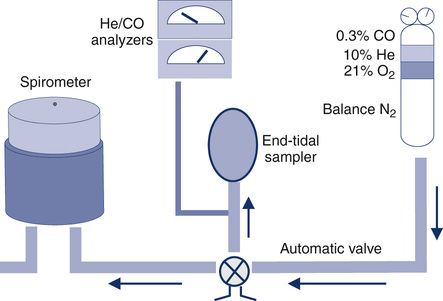
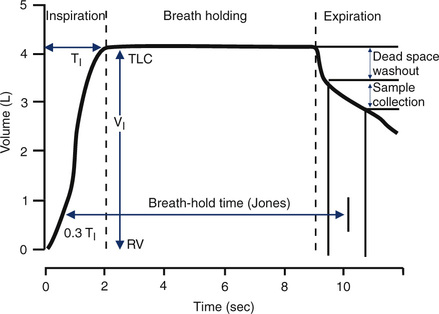
The patient exhales to RV and then inspires a vital capacity breath (referred to as the IVC or VI) from a system such as that in Figure 3-2. A special diffusion gas mixture is delivered either from a spirometer, a reservoir bag, or by means of a demand valve. The diffusion mixture usually contains 0.3% CO, a “tracer” gas, 21% O2, and the balance is N2. The tracer gas is usually an insoluble, inert gas such as helium (He), methane (CH4), or neon (Ne). The tracer used depends on the type of analyzer implemented to analyze the exhaled gas. The traditional method used He (usually about 10%) as the tracer gas. Rapidly responding infrared analyzers (see Chapter 11) have been used for continuous analysis of the small changes in CO. The same type of infrared analyzer can use CH4 as the tracer gas, so that a single analyzer can rapidly detect changes in both CO and the tracer. Another method uses gas chromatography (see Chapter 11) to detect changes in CO with neon used as the tracer gas.
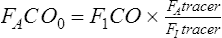
FAco0 = fraction of CO in alveolar gas at beginning of breath hold (time = 0)
FIco = fraction of CO in reservoir (usually 0.003)
FAtracer = fraction of tracer in alveolar gas sample
FItracer = fraction of tracer in inspired gas (varies with tracer gas used)
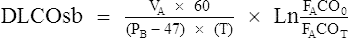
VA = alveolar volume, mL (STPD)
60 = correction from seconds to minutes
PB = barometric pressure, mm Hg
47 = water vapor pressure at 37 °C, mm Hg
T = breath-hold interval, seconds
FAco0 = fraction of CO in alveolar gas at beginning of breath hold
FAcoT = fraction of CO in alveolar gas at end of breath hold
VA may be calculated from the single-breath dilution of the tracer gas:
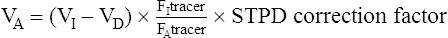
VI = volume of test gas inspired, mL (see Figure 3-3)
VD = dead space volume (anatomic and instrumental), mL
FAtracer = fraction of tracer in alveolar gas sample
FItracer = fraction of tracer in inspired gas (depends on tracer used)

FAtracer = fraction of tracer in alveolar gas, equal to FAco0
FAcoT = fraction of CO in alveolar gas at end of breath hold
Ln = natural logarithm of the ratio
This technique avoids the necessity of analyzing the absolute concentrations of the two gases. However, it requires that the analyzers be linear with respect to each other. Analysis of CO is often done using infrared analyzers (see Chapter 11), and their output is nonlinear. Care must be taken to ensure that corrected CO readings are used in the computation. These corrections are easily accomplished either electronically or via software in computerized systems. Systems that use the same detector for both CO and the tracer gas (e.g., infrared, gas chromatography) also need to provide linear output. The linearity of the system should be within 0.5% of full scale. This means that any drift or nonlinearity should cause no more than a 0.5% error when analyzing a known gas concentration (See Chapter 12).
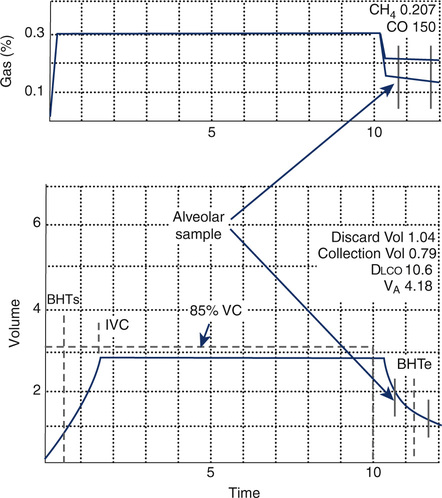
Dlco gas analysis is commonly performed with either a rapidly responding multigas analyzer or gas chromatography. Multigas analysis uses specialized infrared analyzers capable of detecting several gases simultaneously. These systems use methane (CH4) as a tracer gas. One advantage of multigas analysis is that CO and CH4 are measured rapidly and continuously (Figure 3-4) so that the calculated Dlco is available as soon as the exhalation has been completed. Gas chromatography (see Chapter 10) can also be used for Dlco gas analysis. Neon (Ne) is used as the tracer gas (Figure 3-5). Helium is used as a “carrier” gas for the chromatograph. Although gas analysis using chromatography is slow (60–90 seconds), it is extremely accurate.
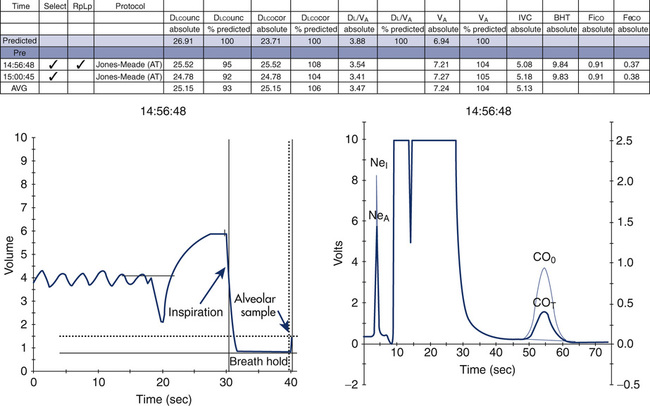
The timing device for the maneuver should be accurate to within 100 msec over a 10-second interval (1%). Most computerized systems time the maneuver automatically. However, a means of verifying the accuracy of the breath-hold time should be available. The Jones and Meade method of timing the breath hold should be used (see Figure 3-5). The Jones and Meade method measures breath-hold time from 0.3 of the inspiratory time to the midpoint of the alveolar sample collection.
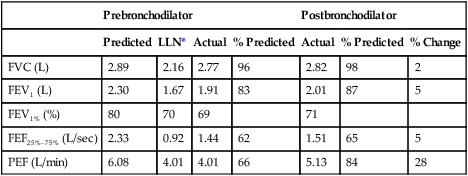
Dlcosb maneuvers should be performed after the patient has been seated for at least 5 minutes. The patient should refrain from exertion immediately before the test; exercise increases cardiac output, which increases Dlco. The patient should be instructed about the requirements of the maneuver and the technique demonstrated. Expiration to RV should be of a reasonable duration, usually 6 seconds or less. Patients who have airway obstruction may have difficulty exhaling completely in this interval. Inspiration to TLC should be rapid but not forced. Healthy subjects and patients with airway obstruction should be able to inspire at least 85% of their VC within 4 seconds (Criteria for Acceptability 3-1). The single-breath calculation assumes instantaneous filling of the lung. Prolonged inspiratory times will decrease the actual time of breath holding at TLC, typically resulting in a lower Dlco. The breath hold should be relaxed, against either the closed glottis or a closed valve. The patient should avoid excessive positive intrathoracic pressure (Valsalva maneuver) or excessive negative intrathoracic pressure (Müller maneuver). A Valsalva maneuver reduces pulmonary capillary blood volume and may produce a falsely low Dlco. A Müller maneuver increases pulmonary capillary blood volume and may falsely increase Dlco. Expiration after the breath hold should be smooth and uninterrupted. Exhalation should take less than 4 seconds, and alveolar gas sampling should occur in less than 3 seconds (again because the calculations assume instantaneous emptying of the lung). Patients who have moderate or severe airway obstruction may have difficulty achieving these criteria. The breath-hold time, measured using the Jones and Meade method (see Figure 3-3), should be 10 seconds ± 2 seconds. Prolonged inspiratory or expiratory times may result in breath-hold times greater than 12 seconds and should be noted on the report.
To obtain an alveolar sample, dead space gas needs to be washed out (i.e., discarded). A washout of 0.75–1.0 L is usually sufficient to clear the patient and sampling device dead space. For patients with small vital capacities (>2.0 L), the washout volume may be reduced to 0.5 L. A sample volume of 0.5–1.0 L should be collected, but a smaller sample may be necessary in patients whose VC is less than 1 L. In Dlcosb systems that analyze expired gas continuously (see Figure 3-4), inspection of the washout of the tracer gas may be used to select an appropriate alveolar sample. Rapidly responding gas analyzers that measure the CO and tracer gas simultaneously allow adjustment of washout volume (i.e., dead space) and sample volume after completion of the maneuver. These adjustments may be particularly useful in subjects who have very small vital capacities (e.g., pediatric patients or adults with severe restrictive disease). Such adjustments assume that both the CO and tracer gas concentrations reflect changes occurring at the mouth. Alveolar sampling may be adjusted to begin at the point where the tracer gas and CO indicate an “alveolar plateau” (see Figure 3-4). In patients who have uneven mixing or emptying of the lungs, there may not be a clear demarcation between dead space and alveolar gas. Adjustments to either washout (dead space) volume or sample volume should be noted on the report.
Two or more acceptable Dlcosb maneuvers (see Criteria for Acceptability 3-1) should be averaged. Duplicate determinations should be within 3 mL CO/min/mm Hg of each other, or within 10% of the largest value obtained from an acceptable effort. No more than five repeated maneuvers should be performed because of the effect of increasing carboxyhemoglobin (COHb) from inhalation of the test gas. There should be a 4-minute delay between repeated maneuvers to allow for washout of the tracer gas from the lungs.
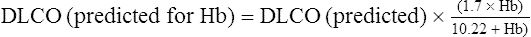
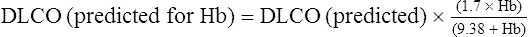
| Actual | Predicted | % | |
| Dlco mL/min/mm Hg | 19.0 | 25.0 | 76 |
| Dlco (Hb corrected) mL/min/mm Hg | 19.0 | 19.9 | 95 |

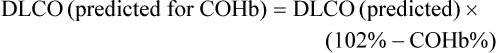
Dlco predicted for altitude = Dlco predicted/(1.0+0.0031[PIO2-150])
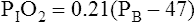
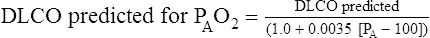
PAO2 = measured or estimated alveolar oxygen partial pressure.
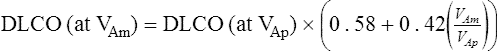
VAm = measured alveolar volume
VAp = predicted alveolar volume (i.e., TLC-VD)
A similar correction can be applied to the Dl/VA:
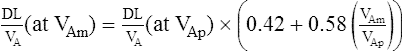
Rebreathing Technique
The patient rebreathes from a reservoir containing a mixture of 0.3% CO, tracer gas, and air (or an O2 mixture) for 30–60 seconds at a rate of approximately 30 breaths/min. The final CO, tracer, and O2 concentrations in the reservoir are measured after this interval. An equation similar to that used for the single-breath technique is used (see Criteria for Acceptability 3-1):
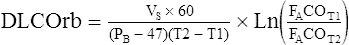
VS = volume of lung reservoir system (initial volume × FItracer/FAtracer)
60 = correction from seconds to minutes
PB = barometric pressure, mm Hg
47 = water vapor pressure, mm Hg
T2 -T1 = rebreathing interval, seconds
FAcoT1 = fraction of CO in alveolar gas at beginning of the rebreathing
FAcoT2 = fraction of CO in alveolar gas at the end of the rebreathing
Membrane Diffusion Coefficient and Capillary Blood Volume
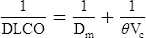
1/Dlco = reciprocal of diffusing capacity, or resistance
1/Dm = alveolocapillary membrane resistance
1/θVC = resistance of red blood cell membrane and rate of reaction with Hb
θ = transfer rate of CO/milliliter of capillary blood
The membrane component of resistance to gas transfer can also be estimated by measuring the rate of uptake of nitric oxide (NO). Dlno has been suggested as a direct measure of the conductance of the alveolocapillary membrane. Because NO combines with Hb approximately 280 times faster than CO, the rate of NO uptake by the blood (θNO) is very large and 1/θNO VC is negligible compared to 1/Dm for NO. Therefore, Dlno reflects the membrane resistance to gas diffusion in the lungs. Dlno can be measured with either a single-breath or rebreathing technique. A small amount of NO is added to the diffusion mixture and the uptake measured using a chemoluminescence analyzer (see Chapter 11).
Significance and Pathophysiology
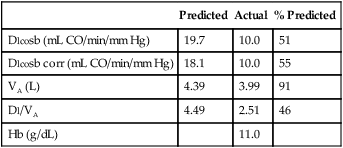
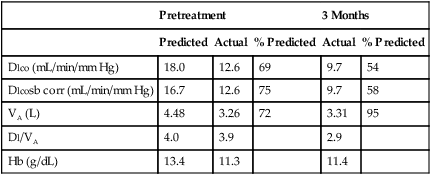
See Interpretive Strategies 3-1. The average Dlco value for resting adult patients by the single-breath method is approximately 25 mL CO/min/mm Hg (STPD) with significant variability. Women have slightly lower normal values, presumably because of smaller normal lung volumes. The expected Dlco value in a healthy patient varies directly with the patient’s lung volume. In some instances, it may be appropriate to adjust the predicted Dlco to account for decreased lung volume (see the section about techniques in the chapter). Dlco values can increase two to three times in healthy individuals during exercise in response to increased pulmonary capillary blood flow.
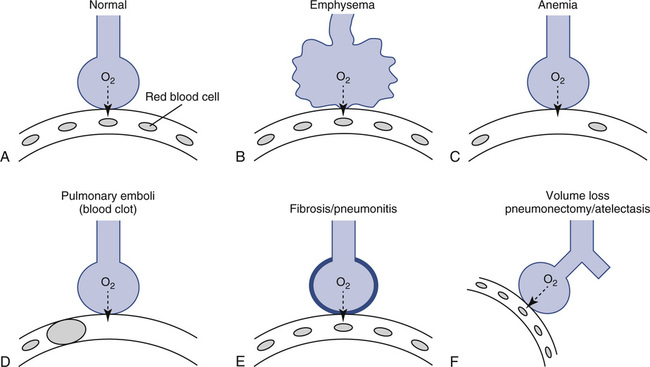
Dlco may be decreased in both acute and chronic obstructive lung disease. Dlco is decreased in emphysema for several reasons. Emphysematous lungs have a reduced surface area for gas exchange, with the loss of both alveolar walls and their associated capillary beds. As a result of the decreased surface area, less gas can be transferred per minute even if the remaining gas exchange units are structurally normal. In addition to loss of surface area for gas exchange, the distance from the terminal bronchiole to the alveolocapillary membrane increases in emphysema. As alveoli break down, terminal lung units become larger. Gas must diffuse farther just to reach the alveolocapillary surface. There is also a mismatching of ventilation and pulmonary capillary blood flow in emphysema. Disruption of alveolar structures causes loss of support for terminal airways. Airway collapse and gas trapping result in ventilation-perfusion (![]() /
/![]() ) abnormalities (Figure 3-6).
) abnormalities (Figure 3-6).
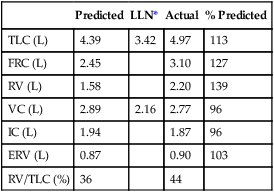
Dlco is directly related to lung volume (VA) in healthy individuals. The Dl/VA (also termed KCO) may be multiplied by the lung volume at which the measurement was obtained to express Dlco. This calculation is simple because VA must be measured to derive Dlco (see Figure 3-5). In healthy subjects, and even in those with mild to moderate restriction, VA approximates the TLC minus the assumed dead space (VD). Analysis of this relationship can be useful to differentiate whether decreased Dlco is the result of loss of lung volume (as in restriction) or from some other cause. In healthy individuals, alveolar volume and Dlco are proportional to body size (height). Two patients of different heights will have different Dlco and VA values, but their Dl/VA ratios will be similar. In healthy adults, Dl/VA is approximately 4–5 mL CO transferred/minute/liter of lung volume.
The Dlcosb is the most widely used method because it is relatively simple to perform, is noninvasive, and is more standardized than other methods. The rapidity with which repeated maneuvers can be performed also lends to its popularity. Most automated systems use the Dlcosb, contributing a certain degree of standardization to the methodology. Large differences in reported Dlco values exist between laboratories. This variability has been attributed to different testing techniques, problems in the gas analysis involved in the test, and differences in computations. Breath holding at TLC is not a physiologic maneuver. This and the fact that Dlco varies with lung volume cause some concerns about Dlcosb as an accurate description of diffusing capacity. Dlcosb is not practical for use during exercise. Some patients have difficulty expiring fully, inspiring fully, or holding their breath. The American Thoracic Society-European Respiratory Society (ATS-ERS) have provided guidelines to improve the standardization of the single-breath maneuver (Box 3-1).
Measurement of Dlco by the intrabreath method (Dlcoib) offers the advantage of not requiring a breath hold at TLC. However, the patient must inspire a large enough volume of test gas so that the subsequent exhalation will clear the instrument and anatomic VD. In addition, the single-breath exhalation must be slow and even. In some systems, a flow restrictor may be necessary to limit expiratory flow. The single-breath, slow-exhalation method produces values similar to those obtained by the breath-hold method in healthy patients when flow is maintained at 0.5 L/sec. Uneven distribution of ventilation may produce intrabreath Dlco values that are artificially elevated. Because the evenness of ventilation distribution can be assessed from the washout of CH4, unacceptable Dlco values can be detected. Table 3-1 compares some advantages and disadvantages of Dlco testing methods.
Numerous other physiologic factors can influence the observed Dlco:
1. Hemoglobin and hematocrit (Hct). Decreased Hb or Hct reduces Dlco, whereas increased Hb and Hct elevate Dlco. Dlco may be corrected if the patient’s Hb is known. CO uptake varies approximately 7% for each gram of Hb. The predicted Dlco may be corrected so that the value reported is compared to a standardized Hb level of 14.6 g% for men and 13.4 g% for women and children younger than 15 years. When this correction is applied, the predicted Dlco will be reduced (and the percentage of predicted increased) if the patient’s Hb is less than the standard value (14.6 g% or 13.4 g%, respectively). Conversely, the predicted Dlco increases (and the percentage of predicted decreases) if the Hb is greater than the standard value. Both corrected and uncorrected Dlco predicted values should be reported, along with the Hb value used for adjustment. Care should be taken to use Hb values that are representative of the patient’s actual Hb level at the time of the Dlco test.
2. COHB. Increased COHb levels, often found in smokers, reduce Dlco. Smokers may have COHb levels of 10% or greater, causing significant CO back-pressure. The diffusion gradient for CO across alveolocapillary membranes is assumed to equal the alveolar pressure of CO. In healthy nonsmoking patients, very little CO (usually less than 2% COHb) is present in pulmonary capillary blood. When there is carboxyhemoglobinemia, diffusion of CO is reduced because the gradient across the membrane is reduced. COHb also shifts the oxyhemoglobin dissociation curve, further altering gas transfer. Each 1% increase in COHb causes an approximate 1% decrease in the measured Dlco. CO back-pressure corrections can also be made by estimating the partial pressure of CO in the pulmonary capillaries. This pressure can be used to correct the FAco0 and the FAcoT. More commonly, however, the predicted Dlco is corrected for the presence of COHb in excess of 2% (see the section, Techniques).
3. Alveolar Pco2. Increased Pco2 elevates Dlco because the alveolar Po2 is necessarily decreased. Significant increases in alveolar Pco2 (i.e., moderate to severe hypoventilation) reduce the alveolar Po2.
4. Pulmonary capillary blood volume. Increased blood volume in the lungs (VC) causes increased Dlco. Increases in pulmonary capillary blood volume may result from increased cardiac output as occurs during exercise. Patients should be seated and resting for several minutes before Dlco testing is performed. Pulmonary hemorrhage and left-to-right shunts may also cause an increase in blood volume in the lungs. In each of these cases, the increase in Dlco is related to the increased volume of Hb available for gas transfer. Excessive negative intrathoracic pressure during breath holding can increase pulmonary capillary volume and elevate the Dlco. Conversely, excessive positive intrathoracic pressure (Valsalva maneuver) can reduce pulmonary blood flow and decrease Dlco.
5. Body position. The supine position increases Dlco. Changes in body position affect the distribution of capillary blood flow.
6. Altitude above sea level. Dlco varies inversely with changes in alveolar oxygen pressure (PAO2). At altitudes significantly greater than sea level, Dlco increases unless corrections are made (see the section, Techniques).
7. Asthma and obesity. Asthma and obesity have been associated with an elevated Dlco in some studies. Increased pulmonary capillary blood volume may explain these observations, but the exact physiology is unclear.
A simple method of evaluating the quality of the test is to compare the TLC–VA relationship, whereas the VA should never be larger than the measured TLC regardless of the TLC testing methodology (see Chapter 12).
The method of timing of breath hold also influences the calculation of Dlco (see Figure 3-3). Most systems measure breath-hold time by one of three methods:
1. Jones and Meade method: includes 0.7 of the inspiratory time to the midpoint of the alveolar sample
2. Epidemiology Standardization Project (ESP) method: from the midpoint of inspiration (half of the VI) to the beginning of alveolar sampling
3. Ogilvie method: from the beginning of inspiration (VI ) to the beginning of alveolar sampling
Theoretically, breath-hold time is considered the time during which diffusion occurs. That is, the Dlco equation assumes that the entire breath hold is at TLC. However, because some gas transfer takes place during inspiration and expiration, Dlco will be influenced by the proportion of time spent in these two phases. A three-equation method has been proposed that uses separate equations for the three phases of the maneuver (i.e., inspiration, breath hold, expiration). This method has not been widely used, however. The timing method may become significant if the reference values used for comparison were generated by one of the other methods. The Jones and Meade method is the recommended method (see Box 3-1). Rapid inspiration from RV to TLC and rapid expiration to the alveolar sampling phase reduces differences resulting from the timing methods (see Box 3-1). Conversely, patients who display prolonged inspiratory or expiratory times may have reduced Dlco values.
Alveolar sampling technique also affects Dlco measurement. Alveolar samples should be collected within 4 seconds, including washout and alveolar sampling. A sample volume of 0.5–1.0 L is recommended. Patients with a small VC (i.e., less than 2.0 L) may require a smaller volume, just as with the washout. When only a small sample is obtained, the gas may not accurately reflect alveolar concentrations of CO and tracer gas, particularly in the presence of ![]() /
/![]() abnormalities. Continuous analysis of the expirate using rapidly responding analyzers allows identification of alveolar gas. Infrared analyzers that can simultaneously analyze the tracer gas and CO will allow the entire breath to be analyzed. These instruments permit adjustment of the alveolar sampling window so that a representative gas sample can be obtained (see Figure 3-4).
abnormalities. Continuous analysis of the expirate using rapidly responding analyzers allows identification of alveolar gas. Infrared analyzers that can simultaneously analyze the tracer gas and CO will allow the entire breath to be analyzed. These instruments permit adjustment of the alveolar sampling window so that a representative gas sample can be obtained (see Figure 3-4).
Summary
• Dlco can be measured by various techniques, including the single-breath, rebreathing, and intrabreath methods.
• The single-breath method, or Dlcosb, is the most commonly used. Dlcosb is noninvasive and can be repeated easily to obtain multiple measurements. Many automated Dlcosb systems are available.
• The ATS-ERS and others have published standardization guidelines for Dlcosb. Careful attention to standards and clinical practice guidelines can reduce the variability in Dlcosb measurements in different laboratories.
• Dlco measurements are used diagnostically for a variety of diseases. Because Dlco assesses gas exchange, it is useful in both obstructive and restrictive disease patterns.
• Dlco measurements may be affected by a variety of factors, such as Hb level, COHb, or altitude. Techniques for correcting Dlco for these factors include adjusting the predicted values used for interpretation.