Problem 1 Difficulties with postoperative fluid balance in a 58-year-old man
Investigation 1.1 Blood results
| Haemoglobin | 165 g/L |
| White cell count | 9.6 × 109/L |
| Platelets | 350 × 109/L |
| Sodium | 149 mmol/L |
| Potassium | 3.4 mmol/L |
| Urea | 10.0 mmol/L |
| Creatinine | 0.12 mmol/L |
| Chloride | 112 mmol/L |
| Bicarbonate | 29 mmol/L |
| Glucose | 4.4 mmol/L |
| Bilirubin | 19 µmol/L |
| Total protein | 65 g/L |
| Globulins | 27 g/L |
| Albumin | 38 g/L |
| ALT | 25 U/L |
| AST | 39 U/L |
| ALP | 74 U/L |
| GGT | 17 U/L |
| LDH | 110 U/L |
| Amylase | 65 U/L |
| Calcium | 2.16 mmol/L |
| Phosphate | 1.15 mmol/L |
| Uric acid | 0.21 mmol/L |
| Cholesterol | 3.6 mmol/L |
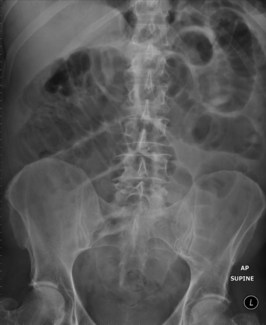
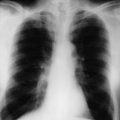
His abdominal X-ray is shown in Figure 1.1.
Conservative management has not been successful and an emergency operation is arranged.
Investigation 1.2 Fluid balance chart on admission
| Fluid Input | |
| IV fluids | 4200 mL |
| Fluid Output | |
| Urine total | 400 mL |
| Urine last 4 hours | 22/16/12/0 mL |
| Nasogastric tube | 700 mL |
| Wound drain | 200 mL |
Investigation 1.3 Fluid balance 24 hours later
| Fluid Input | |
| IV fluids | 3700 mL |
| Fluid Output | |
| Urine total | 800 mL |
| Urine last 4 hours | 15/13/9/8 mL |
| Nasogastric tube | 2800 mL |
| Wound drain | 400 mL |
The early morning electrolytes are as follows:
Investigation 1.4 Electrolytes
| Sodium | 138 mmol/L |
| Potassium | 2.7 mmol/L |
| Chloride | 102 mmol/L |
| Bicarbonate | 29 mmol/L |
| Urea | 7.0 mmol/L |
| Creatinine | 0.08 mmol/L |
Answers
The blood results show the following:
A.4 The key requirement is to correct any pre-existing fluid deficit prior to surgery.
Revision Points
Fluid Balance
Approximately 60% of body weight is made up of water.
In a 70 kg man, this is 42 litres (L):
Fluid management requires understanding and calculation of three principal factors:
Issues to Consider
, http://archive.student.bmj.com/issues/04/04/education/144.php. A didactic page on fluid balance
Shields C.J. Towards a new standard of perioperative fluid management. Therapeutics and Clinical Risk Management. 2008;4(2):569-571. A useful review of current thinking
Grocott M.P., Mythen M.G., Gan T.J. Perioperative fluid management and clinical outcomes in adults. Anesthesia and Analgesia. 2005;100(4):1093-1106. Another review