|
1. Oxford classification: split system
|
|
M
|
Mesangial hypercellularity
|
≤0.5 (M0) or ≥0.5 (M1)
|
|
E
|
Endocapillary hypercellularity
|
Absent (E0) or present (E1)
|
|
S
|
Segmental sclerosis
|
Absent (S0) or present (S1)
|
|
T
|
Tubular atrophy/interstitial fibrosis
|
<25 % (T0), 26-50 % (T1), or >50 % (T2)
|
|
2. Japanese histological grading classification: lumped system
|
|
Histological grade
|
No of lesions*/total no. of glomeruli
|
Active lesions only
|
Active lesion + chronic lesion
|
Chronic lesion only
|
|
H-Grade I
|
0–24.9 %
|
A
|
A/C
|
C
|
|
H-Grade II
|
25–49.9 %
|
A
|
A/C
|
C
|
|
H-Grade III
|
50–74.9 %
|
A
|
A/C
|
C
|
|
H-Grade IV
|
75 %
|
A
|
A/C
|
C
|
Table 5.2
Pathological parameters of Oxford and JHGC
|
Active glomerular lesions
|
Mesangial hypercellularity
|
○
|
×
|
|
Endocapillary hypercellularity
|
○
|
×
|
|
Cellular or fibrocellular crescent
|
×
|
○ (late progressor)
|
|
Chronic glomerular lesions
|
Global sclerosis
|
×
|
○ (early and late progressor)
|
|
Segmental sclerosis
|
×
|
○ (early progressor)
|
|
Segmental sclerosis or adhesion
|
○
|
×
|
|
Fibrous crescent
|
×
|
○ (early progressor)
|
| |
Adhesion
|
×
|
×
|
|
Tubulointerstitium
|
Tubular atrophy/interstitial fibrosis
|
○
|
×
|
|
Vascular lesions
|
Interlobular artery
|
×
|
×
|
|
Afferent artery
|
×
|
×
|
5.2.1 Oxford Classification
5.2.1.1 Development
An international committee of pathologists and nephrologists from four continents were first convened in 2004 for the purpose of developing a new, evidence-based, international consensus histologic classification for IgAN. The 265 cases were assembled including biopsies from 206 adults and 59 children and were from 17 different centers based in eight countries and four continents.
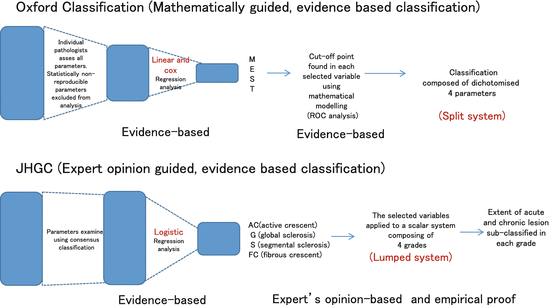
The following rigorous procedure was taken to develop the evidence-based classification (Fig.
5.1). First, the histological parameters which are necessary to take in the lesions of IgAN were discussed and determined. Using these selected lesions, a score sheet was produced to quantify these selected histological parameters consisting of glomerular, tubulointerstitial, and vascular lesions. Second, on the basis of data-using score sheets, reproducibility was tested by the Intraclass Correlation Coefficient (ICC), and the parameters with poor reproducibility were excluded for further analysis. Third, the histological parameters with reasonable reproducibility were assessed by linear regression analysis and Cox regression analysis to determine if they were independent predictors for the renal functional outcome for a minimum of 3 years and/or until an end point of end-stage renal disease (ESRD) or ≥50 % decline in estimated glomerular filtration rate (eGFR).
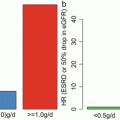
As a consequence, three histologic parameters were selected as independent predictors within this classification (M: mesangial hypercellularity, S: segmental sclerosis, and T: tubular atrophy/interstitial fibrosis (TA/IF)). One additional histologic parameter—endocapillary hypercellularity (E) in >= 1 glomerulus—was not significantly correlated with the above outcomes by multivariate analysis but was strongly correlated with response to immunosuppressive therapy and hence this additional parameter was included in the classification. Finally, in order to dichotomize each of the selected parameters to produce a split classification system, cutoff points were determined by ROC (Receiver Operating Characteristic) analysis. Thus, the recommendation of the classification with IgAN includes four independently reported histologic parameters (M, E, S, and T): M0 or M1, indicating mesangial hypercellularity in ≤50 % versus >50 % of glomeruli; E0 or E1, indicating endocapillary hypercellularity in 0 versus ≥1 glomeruli; S0 or S1, indicating segmental sclerosis in 0 versus ≥1 glomeruli; and T0, T1, or T2, indicating TA/IF in ≤25 %, 26–50 %, or >50 % of renal cortex, respectively. They produced a four-parameter dichotomous scoring system to describe IgAN lesions.
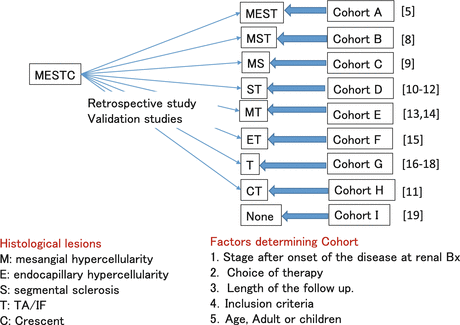
5.2.1.2 Validation Studies of Oxford Classification
The Oxford Histologic Classification has gained a significant level of worldwide acceptance and has been the subject of several single-center or multicenter validation studies. Validating the classification in a diverse range of cohorts treated in different ways is important to confirm that the classification can be widely applied. Among validation studies, the selected independent predictors for renal functional outcome by multivariate analysis were M, S, and T [
8] in one, M and S in one, 2 [
9], S and T in two [
10–
12], M and T in two [
13,
14], E and T in one, [
15], only T in three, [
16–
18], only CT in one [
11], and none in one study [
19], respectively (Fig.
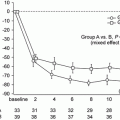
5.2).
Confirmatory Studies
Coppo et al. compared the applicability of the Oxford classification to predict renal outcomes in 59 children as compared with the 206 adults included in the original Oxford study cohort. M1 vs. M0 and S1 vs. S0 were associated with an increased rate of renal functional decline. In contrast, E1 vs. E0 was not. These findings are similar to those reported in the entire Oxford cohort and suggested that selected parameters were not influenced by the patient age. However, the effects of the T variable were not directly examined because there were too few children with tubulointerstitial lesions to draw conclusions [
9]. European validation study of the Oxford classification of IgA (VALIGA) also confirmed the results from the original Oxford classification study using 1,147 patients from 13 European countries. Over a median follow-up of 4.7 years, M, S, and T lesions independently predicted the loss of eGFR and a lower renal survival. In individuals with eGFR less than 30 ml/min per 1.73 m
2, the M and T lesions independently predicted a poor survival [
8].
Using a cohort of 187 adults and children with IgAN from 4 North American centers, S and T scores were confirmed as independent predictors of renal functional decline, and this study also confirmed that endocapillary proliferation (E1) was associated with an increased rate of renal functional decline in patients not treated with immunosuppression but not in patients receiving immunosuppression, similar to findings in the original Oxford cohort. M score was not an independent predictor of renal functional decline in the study [
10]. Katafuchi et al. studied 702 Japanese adults and children by multivariate analysis and found that S and T lesions were associated with ESRD. M1 showed a trend toward high risk of ESRD compared with M0. E score showed no significant association with the development of ESRD. When crescent (C) was added to the multivariate analysis, C and T, but not S, were significantly associated with the development of ESRD. They documented also that an association of crescent with ESRD was not observed in patients who met the inclusion criteria of Oxford cohort but was evident in those who did not [
11].
Shi et al. evaluated 410 Chinese patients and found that S and T scores were independent predictors of ESRD. Furthermore, patients having >25 % of glomeruli with endocapillary proliferation (termed E25), crescents, and M1 were more likely to be treated with immunosuppressive agents, but none of these parameters were independent predictors of prognosis [
12]. Zeng et al. found that only M1 and T scores were associated with an increased rate of eGFR decline or reduced renal survival from a combined event by multivariate analysis using 1,026 adult IgAN patients from 18 centers in China [
13]. Shima et al. defined as a decline in eGFR to <60 ml/min per 1.73 m
2 in 161 consecutive Japanese IgAN children (<20 years old) and found that M and T scores (but not S) and crescents in >30 % of glomeruli (but not in ≥1 glomerulus) were significant predictors of renal outcome in multivariate analyses [
14]. Lee et al. reported on 69 adult patients followed for >36 months and found that E1 and T1/T2 lesions independently predicted a ≥50 % decline in eGFR or ESRD, even though patients with E1 lesions received more immunosuppression than those with E0 [
15].
Divergent Studies (One or Less Parameters)
Four studies found only one of the Oxford M, S, and T scores, most often the T score, to be independently predictive of clinical outcome. El Karoui et al. studied 128 adult patients and found only T score predicted rate of eGFR decline, and only M score was associated with doubling of serum creatinine or ESRD by multivariate analysis [
16]. In the study by Kang et al. using 197 Korean adult patients, they showed that only T lesions predicted a 50 % reduction in eGFR or ESRD. Lee et al. reported on 69 adult patients followed for >36 months. E1 and T1/T2 lesions independently predicted a ≥50 % decline in eGFR or ESRD, even though patients with E1 lesions received more immunosuppression than those with E0 [
17]. Le et al., in a study of 218 Chinese children, found that only T1/2 was an independent predictor of poor outcome in multivariate analysis [
18]. Finally, Alamartine et al. studied 183 French adults including very mild and very severe forms of the disease for an average of 77 months; only baseline eGFR was predictive of reaching this end point by multivariate analysis [
19].
As shown above, the Oxford classification offers a simple approach for predicting renal outcome by describing the presence or absence of active (M, E) and chronic lesions (S, T). It is also the only current glomerular disease classification that was developed in a truly evidenced-based manner.
5.2.2 Japanese Histological Grade Classification (JHGC)
5.2.2.1 Development
As mentioned in the introduction, within Japan, an alternate classification system has been developed. This came from the work of the IgAN Study Group of the Progressive Renal Diseases Study Committee under the auspices of the Ministry of Health, Labor and Welfare. A multicenter case-control study on IgAN was conducted to develop an evidence-based clinicopathological classification for predicting long-term renal outcome.
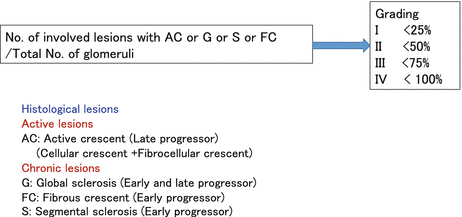
This working group consisted of 16 centers. The cohort was drawn from patients seen in Japanese hospitals. Lesions were assessed independently by two pathologists, and if they did not agree, lesions were reviewed by both pathologists until a consensus was reached. The initial panel of histological parameters examined was very similar to that of the Oxford classification. During a median follow-up of 9.3 years after biopsy, 49 out of 287 patients (19 %) progressed to ESRD. The associations between pathological variables and the need for chronic dialysis were examined by multivariate logistic regression analysis separately in patients who required dialysis earlier than 5 years (early progressors) and those who required dialysis within 5–10 years (late progressors) after biopsy. Independent pathological variables predicting progression to ESRD were global sclerosis, segmental sclerosis, and fibrous crescents for early progressors and global sclerosis and cellular/fibrocellular crescents for late progressors (Fig.
5.3). Four histological grades (HGs), such as HG 1, HG 2, HG 3, and HG 4, were established corresponding to <25 %, 25–49 %, 50–74 %, and ≥75 % of glomeruli, exhibiting cellular or fibrocellular crescents, global sclerosis, segmental sclerosis, or fibrous crescents. Eleven (7 %) patients in HG 1, 12 (16 %) in HG 2, 13 (31 %) in HG 3, and 13 (68 %) in HG 4 progressed to ESRD. Multivariate logistic analysis revealed that the risk of progression to ESRD was significantly higher in HG 2, 3, and 4 than in HG 1 (odds ratio, 2.4, 5.7, and 27.6 vs. 1.0). Age, mean arterial pressure, and urinary protein excretion were higher, while eGFR was lower in higher grades, suggesting that our histological grading agrees with the prognostic clinical features of IgAN at the time of diagnosis. The risk of progression to ESRD and the rate of GFR decline were significantly greater in higher grades, indicating that the grading can identify the magnitude of the risk of progression to ESRD as well as the deterioration rate of renal function [
7]. In addition to the grading system, this classification allowed for active (A) chronic lesions (C) and mixed (A/C) to be produced as a subgroup of each grade which allows identification of a balance between activity and chronicity.
5.2.2.2 Validation Studies
Compared to the Oxford classification scheme, a limited number of validation studies have been conducted, and these have been confined to Japanese cohorts. Sato et al. validated the JHGC in a Japanese single-center cohort [
20]. This was a retrospective study in 198 Japanese adult patients with IgAN. Clinical parameters including blood pressure, urinary protein, eGFR, and outcomes were evaluated in these patients. The glomerular lesion percentage score (GLPS) [number of glomeruli with cellular crescents, fibrocellular crescents, global sclerosis, segmental sclerosis, or fibrous crescents per number of total obtained glomeruli] was assessed in each patient and categorized into histologic grades of HG1 (<25 %), HG2 (25–49 %), and HG3/4 (≥50 %). Associations of GLPS (or HG) with disease progression (50 % eGFR decline or ESRD-requiring dialysis) within 10 years after biopsy and the rate of annual eGFR decline were examined. During a median follow-up period of 12.0 years after biopsy, disease progression occurred in 12.8 % (12/94) of HG1 patients, 32.3 % (21/65) of HG2 patients, and 46.2 % (18/39) of HG3/4 patients. The risk of disease progression was significantly higher in the HG2 and HG3/4 groups than in the HG1 group (odds ratios: 3.3 and 5.9 vs.1). A higher GLPS was significantly associated with a higher risk of disease progression and a greater annual eGFR decline. JHGC based on GLPS (or HG) was well correlated with long-term prognosis in the cohort of Japanese adult patients with IgAN [
20].
5.3 Comparison Between Oxford Classification and Japanese Histological Grade Classification
Despite similar objective (the communication of complex pathological information using a simplified scoring system), the Oxford and Japanese histological grade classification schemes differ on a number of points, both in the methodological approach to developing the classification schemes and in the predictive pathological parameters suggested by each classification scheme. As these two classification systems coexist in Japan, there has been a great deal of motivation to compare the two in order to try and determine which approach may produce the most informative information for the sake of clinicians and ultimately for the benefit of the patients. In order to provide background to our subsequent discussion of the two classification schemes, the differences in their development and methodology are detailed below.
5.3.1 Differences in Development of Classification Schemes
5.3.1.1 Inclusion Criteria
The cohorts used in the studies were different between JHGC study and the Oxford classification study. The differences were influenced mainly by the inclusion criteria. The Oxford classification excluded patients with proteinuria less than 0.5 g/day and those with an initial eGFR of <30 ml/min per 1.73 m2, whereas in the JHGC study, there was no limitation for the cohort. This is a relevant variation as a significant fraction of Japanese patients had asymptomatic proteinuria with microhematuria or isolated microhematuria in their initial clinical presentation and were detected owing to the health-screening programs of Japan, whereas these patients would have been excluded from analysis within the Oxford cohort. This means that despite the overall Japanese cohort presenting with less severe IgAN lesions, it also included those with severe lesions, providing a wider range of IgAN lesions from which to develop its classification.
5.3.1.2 Baseline Differences Between Cohorts Used in the Studies (Table 5.3)
Table 5.3
Clinical profile of the cohort
|
Cohort
|
265
|
287
|
|
Median age (yrs)
|
32 (4–73)
|
35 (18–70)
|
|
Female
|
28 %
|
49 %
|
|
Pediatric at time of biopsy (<18 yrs)
|
22 %
|
9.1 %
|
|
Ethnicity Caucasian/African/Asian/Other)
|
66, 3, 27, 4 %
|
Japanese
|
|
MAP (mmHg)
|
98 ± 18
|
94 ± 14
|
|
eGFR (ml/min/1.73 m2)
|
83 ± 36
|
78 ± 25
|
|
Proteinuria(g/day)
|
1.7 (0.5–18.5)
|
0.8 (0.0–7.6)
|
|
Period of follow-up (Months)
|
69(12–268)
|
110 (17–602)
|
|
Treated with RAS blockade (ACEi, ARB)
|
74 % (68 %, 22 %)
|
77 %
|
|
Steroid
|
29 %
|
37 %
|
|
eGFR slope (ml/min/1.73 m 2 /y)
|
−3.5 ± 8.4
|
−2.9 ± 3.8
|
When comparing baseline characteristic between the two cohorts, the majority of parameters were different, possibly due to a combination of the differences in inclusion criteria as mentioned above and other factors. These differences are summarized in Table 5.3. The difference between the cohorts included the proportion of pediatric and female patients, follow-up period, degree of proteinuria, and the eGFR slope. The mean rate of renal function decline was 3.5 ± 8.4 ml/min per 1.73 m2 per year (–3.7 ± 6.6 in adults and –2.7 ± 1.05 in children,) in Oxford, whereas eGFR decline was −2.9 ± 3.8 in JHGC. Considering the lower degree of proteinuria and lower eGFR slope in the JHGC study, overall this cohort consists of earlier stage of the disease process of IgAN. This speculation is supported by the evidence that correlation efficient between endocapillary hypercellularity and mesangial hypercellularity is 0.5 in JHGC and 0.3 in Oxford, whereas correlation efficient between endocapillary hypercellularity and crescent was 0.2 in JHGC and 0.4 in Oxford, respectively (data not shown) [6, 7] . Since crescent became an independent predictor between 5 and 10 years (late progressor) in JHGC study and mean follow-up period is 69 months (5.8 years) in Oxford study, crescent may not be selected in Oxford study due to the shorter follow-up time [5–7].
5.3.1.3 Statistical Methodology Involved
The mathematical and statistical approaches to parameter selection differed between the two approaches. Of the two, the Oxford classification can be said to be the most rigorously developed as it is the product of a blinded analysis by multiple pathologists, it tested the reproducibility of each parameter, and it excluded those showing poor reproducibility. It then subjected parameters that passed this initial criterion of good interobserver reproducibility to regression analysis in both univariate and multivariate linear and Cox regression models in order to find those parameters which modeled both renal functional decline (through eGFR slope) and outcomes (measured by survival from 50 % reduction in renal function or ESRD). In contrast, the JHGC used a consensus-based approach to parameters without a quantitative estimation of the reproducibility of each parameter. The parameters assessed in this manner were then tested by multivariate logistic regression analysis in order to determine which parameter most closely followed lesion for progression in early progressors (dialysis <5 years after biopsy) or late progressors (dialysis 5–10 years after biopsy) (Fig.
5.1).
Hence, despite similarities (retrospective cohorts, evidence-based modeling), the Oxford classification has the more rigorous mathematical approach to reproducibility by only including highly reproducible histological features as examined by multiple pathologists. In contrast, the JHGC still relies on a degree of expertise by the examining pathologist in classifying the lesions examined. When comparing the effectiveness of the models used, linear and Cox regression models are preferable to assess the renal functional outcome in middle observation time of 5.8 years in Oxford study. On the other hand, in logistic analysis used in the JHGC study, a number of events attaining the hard end point become more in the longer observation period. So the observation period in this study was divided into early phase (within 5 years) and late phase (between 5 and 10 years), allowing the parameters assessed to encompass a greater spectrum of the natural history of IgAN.
5.3.1.4 Differences in the Predictive Pathological Parameters Obtained from Both Classification Schemes
While having similar objectives and approaches, the Oxford classification and the JHGC obtained different pathological parameters as key components in their model of the best way to quantify IgAN lesions, despite both models starting with a similar panel of pathological characteristics. The only overlap between the two scoring systems was the adoption of segmental sclerosis as a component of the overall score in both the Oxford classification and the JHGC.
In the Oxford classification, the additional parameters selected on their basis of predicting renal function and outcome were tubular atrophy/interstitial fibrosis (T) and mesangial hypercellularity (M), while in the JHGC, the parameters were global sclerosis, cellular crescent, fibrocellular crescent, and fibrous crescents. Within these, there are probably parallels as global sclerosis and TA/IF showed a high degree of correlation in the JHGC study (R = 0.741 and p < 0.001 by Spearman’s rank correlation). While the reproducibility of TA/IF was high in the Oxford study (ICC, 0.78/0.79) and this reproducibility was one of the reasons for its selection, IF/TA was estimated by semiquantitative methods (eyeball estimation) in both studies and hence may only be reliable only in an expert’s estimation. From this point of view, global sclerosis showed outstanding reproducibility in the Oxford classification (ICC;0.90), as well as being a more quantitative variable so hence was adopted in preference to IF/TA as predictors in the JHGC. Despite its utility in traditional expert’s opinion classification, vascular lesion was not adopted in either classification because reproducibility of quantification for vascular lesions was considered to be insufficient in both studies. One additional histologic parameter—endocapillary hypercellularity was not significantly correlated with the above outcomes by multivariate analysis but was strongly correlated with response to immunosuppressive therapy—could be a predictor for poor prognosis in the patients without immunosuppressive therapy in the Oxford study, but a similar analysis was not performed in the JHGC.
Of note, despite its clinical utility, crescent (C) was not found to be significant predictors of clinical outcomes in the Oxford study but was included in the JHGC. A possible explanation behind this is that the rapidly progressing patients (progression to ESRD within 12 months of biopsy and/or those with an initial eGFR of <30 ml/min per 1.73 m
2) were excluded from the Oxford study, but included in the Japanese study. Given that crescent is a parameter associated with rapidly progressive disease, it is possible that the cohort selection predisposed the models to preselect different characteristics [
11]. Thus, this may explain the lack of selection of crescents in the Oxford classification.
5.3.1.5 Treatment and Outcome Differences Between Cohorts Used in the Studies
Immunosuppressive therapy is a common treatment approach used in IgAN. Twenty-nine percent of the patients enrolled (47 % of children and 23 % of adults) received immunosuppressive therapy in the Oxford study, whereas 37 % of the patients received variable dosages of corticosteroids with additional immunosuppressive agents in the JHGC. The difference in rates of therapy between these two groupings may be explained by their inclusion criteria and differences in the nature and natural history of IgAN lesions. While dealt with in more detail below, IgAN lesions can be subclassified into active and chronic lesions. The former responds to immunosuppressive therapy, while the latter does not. In traditional expert’s opinion-based pathology, active lesions can be characterized by the presence of various pathological parameters (mesangial hypercellularity, endocapillary hypercellularity, and cellular crescents) and thus in these retrospective cohort were most probably recognized and more likely to be treated by immunosuppressive therapy. As the Oxford cohort limited the inclusion of early stage lesions by its inclusion criteria while the cohort of JHGC consists of earlier stage cases, it is unsurprising that there may be differences in the rates of treatment. Additionally, this difference in cohort composition may have biased the models selection of significant outcomes, as if in the Japanese cohort, a higher proportion of early lesions was included and treated parameters that are reversible upon steroid treatment (such as those mentioned above) would not be independent predictors for renal outcome in contrast to the Oxford study. Interestingly, fibrous crescents, which are traditionally interpreted as a marker of lesions that will not respond to steroid therapy, were selected as early progressor in the JHGC [
7], which is in line with the idea that these lesions are non-responsive to treatment. Such an assessment was not made in the Oxford cohort as the quantification of fibrous crescents failed the initial selection step of interindividual reproducibility [
5,
6].
5.4 The Relative Advantages of a Lumped System of Classification Compared to a Split System of Classification
The purpose of the histological classification is principally to identify the risk of progression of renal disease, enabling us to improve individual patient prognostication, to identify the potential for response to immunosuppression or other specific treatments, and to refine recruitment to clinical trials. With these aims in mind, it is important to look at the function of both the Oxford and JHG classification schemes in meeting these needs. Before doing so, however, it is important to review the natural history of IgAN in order to better understand the clinical implications of various histological aspects of the disease, because renal biopsy to diagnose IgAN is undertaken before therapy and is one of the main parameters used in classifying disease course and subsequent treatment.
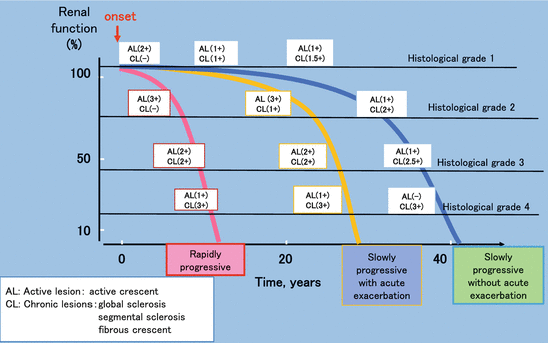
One important aspect of IgAN is that they can have very different disease courses. A diagrammatic overview of the natural history of IgAN is shown in Fig.
5.4. There are three types of disease courses expected by the clinician; rapidly progressive, slowly progressive with acute exacerbation, and slowly progressive type without acute exacerbation. These disease types are important as they have an impact on the patient’s progression and guide clinicians in regards to treatment and overall prognosis. In the early phase, active lesions are prominent and chronic lesions are rare. Active lesions, which affect renal function, are characterized by crescents. The frequency and/or severity of these lesions has traditionally guided pathologists and clinicians in choosing immunosuppressant therapy for an individual patient. In addition to crescents within the acute lesions, mesangial hypercellularity and endocapillary hypercellularity predict for lesions that will respond well to immunosuppressant therapy and hence will not develop into chronic lesions characterized by a greater degree of structural alteration. In contrast, glomerular tuft necrosis can develop into cellular crescents and finally into more permanent structural alterations such as fibrocellular and fibrous crescent together with adhesion and segmental sclerosis. Global sclerosis and TA/IF are terminal lesions of fibrous crescent and segmental sclerosis and thus indicate more chronic disease. In the majority of individual cases, the lesions of IgAN stand on the balance of acute and chronic lesions. As renal biopsy is performed before a diagnosis and treatment is made and is a static measure (i.e., may be performed at any point on the disease course depending on how quickly the initial diagnosis was made), assessment of a number of the factors above may be important in differentiating the three possible disease processes present and the most appropriate choice of therapy.
With this aim, we would argue that the lumped system as used by the JHGC is a more favorable procedure than that of split system used by the Oxford classification system. The reason for this is that the Oxford classification system reduced the complexity and diversity of the histology observed in the biopsy into a series of dichotomous values (present/absent), thus potentially losing valuable information on the pathology within the lesion. This is especially true when trying to assign an individual to one of the three disease courses mentioned above. In addition, the current Oxford system reports four independent parameters without any information of how varied combinations of these parameters will interact. This means that the utility that this classification scheme provides to clinicians, in terms of identifying the disease course in patients, still relies on interpretation of the various values from the pathology report.
In contrast, the JHGC utilizes a lumped system consisting of markers for both chronic and active lesions and utilizes a scalar system in order to grade each component (Fig.
5.3). In this system, four histological grades, HG 1, HG 2, HG 3, and HG 4, are assigned corresponding to <25 %, 25–49 %, 50–74 %, and ≥75 % of glomeruli, exhibiting cellular or fibrocellular crescents, global sclerosis, segmental sclerosis, or fibrous crescents. In addition, the JHCG then combines these parameters to produce a singular lumped value incorporating all elements of the classification. By this systemic scoring, the histology of the patients from early phase of the disease to advanced phase can be graded, which correlates well with grade of the renal functional deterioration (Fig.
5.4). The reproducibility of the JHGC is 0.85 in ICC in a multicenter case-control prospective study in the IgAN Study Group of the Progressive Renal Diseases Study Committee organized by the Ministry of Health, Labor and Welfare (preliminary data), suggesting recognition of glomerular lesion either cellular and fibrocellular crescent, global sclerosis, segmental sclerosis, or fibrous crescent, and quantitative scoring of the summation of the number of the constituting lesions can be a reproducible procedure. While this approach needs further validation studies across a greater range of cohorts, we believe that the greater overview of the lesion it provides makes it a better tool for communication between pathologists and physicians as to the full nature of the lesions observed in IgAN biopsies (Fig.
5.4). Indeed, the potential utility of a lumped rather than split classification system has been highlighted by the suggestion that the Oxford classification system could be improved by developing and validating it in this manner.
5.5 Is Evidence-Based Classification Superior to Expert’s Opinion-Based Classification?
As mentioned in the introduction, there is a continuing discussion of the merits of evidence-based classification as opposed to expert opinion-based classification. In this article, we have described two separate evidence-based classification systems for IgAN lesions. The Oxford Classification of IgAN is the only classification of glomerular disease that is rigorously evidence-based through the nature of its development. In choosing the parameters to be included in the system, priority was given to those that were statistically reproducible between individual pathologists, and only when histological characteristics were shown to be such were they then tested for their ability to describe the disease course. Values that then predicted outcome in the specific cohort selected for the development of this system were subsequently dichotomized in order to produce the Oxford MEST classification (Fig.
5.1). However, the limitation of such a purely evidence-based method may be its ability to be generalized to a wide range of cohorts whose clinical parameters do not match those used to develop the method. This could potentially be attributed to its complete reliance on mathematical development without combining mathematical testing with understanding of the natural history and contributing histopathology of the disease. This potential weakness is evident in the fact that none of the validation studies of the Oxford classification described above completely confirmed the results from the original Oxford cohort regarding the predictive value of M, S, and T scores.
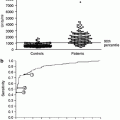
One possible approach to overcoming this apparent limitation of an evidence-based classification would be subclassification within any cohort in order to standardize patient background or treatment as shown in Fig.
5.2. For example, this could be performed by classifying on a number of different parameters including the aforementioned three different disease courses: early phase vs. advanced phase of disease course; immunosuppressant therapy vs. without immunosuppressant therapy; child vs. adults, mild proteinuria vs. severe proteinuria, and long-term follow-up vs. short-term follow-up; and so on. By standardizing the clinical subtypes of cohorts before generating evidence-based histological classification, possible variation limiting the generalizability of any individual classification scheme may be avoided. However, even in this instance, the clinical utility of such approaches may be limited by the necessity of making an initial expert-driven decision as to which cohort a patient belongs to before applying the relevant criteria.
As opposed to evidence-based classification systems, expert’s opinion-based classifications are inherently more subjective and yet have for many years proved of extreme utility in communication between pathologists and clinicians. Expert’s opinion-based classification, including the characterization of histological features decided among experts as applicable to clinical decision-making (e.g., lupus nephritis [
21], antineutrophil cytoplasmic autoantibody-associated glomerulonephritis [
22], focal segmental glomerulosclerosis [
23], and diabetic glomerulosclerosis [
24]) however have not been tested in the development process as to their reproducibility or effectiveness in the same way that evidence-based medicine approaches have been. Even lesions selected in a systematic manner, such as those mentioned previously that are recognized by panels of experts as being important in the analysis of IgAN, are currently grouped into descriptive classes within expert’s opinion classifications rather than semiquantitative grades. Hence, as with the evidenced-based approach, the expert’s opinion-based approach to describing IgAN lesions needs further testing and refinement before being sure that they are the most applicable means of classification. Ideally, this validation would take the form of retrospective studies firstly to test the reliability and reproducibility of expert’s production of classification without quantitative evidence and secondly to test if this classification adequately captures the disease spectrum.
Between these two approaches, that of purely mathematical evidence-based classification and purely descriptive expert opinion-based classification, there is a middle road. Such an approach was taken by the JHGC in the hope that this could combine the strengths while minimizing the disadvantages of the two approaches above. In this manner, the JHGC was developed using evidence-based means but was guided in its development by expert opinion’s understanding of the physiopathology of these lesions. As shown in Table
5.1, each grade consists of a subgroup including A, A/C, and C, which are proposal from expert’s opinion for clinical use. In this approach, the selection of pathological parameters can be weighted on the basis of our understanding of their role in disease development rather than in an entirely blind fashion. While this limits the claim to rigor in method development, it may mean that parameters that are known to be generalizable across a wide disease spectrum can be selected in preference to parameters that may be strongly recognized and unique or specific in the one cohort. This middle road has been used with success in other diseases, and until other approaches are validated with a greater degree of rigor, this approach may be the best means to classify and hence communicate the pathology of IgAN between health-care professionals.
The final goal of any classification system will be an ability to typify an individual case with morphological diversity into a simple category of histological classification, and doing this enables the pathology observed in the lesion to give the appropriate information about the outcome and responsiveness and guide an appropriate and effective therapy for any individual case. At present, none of the classification systems described meets these ideal criteria. However, as a current compromise, the JHGC incorporates some of the lesions diversity captured by the expert opinion approach to grading while also incorporating some of the reproducibility and evidence-based values associated with the Oxford classification.
5.6 Conclusion
The two classifications, Oxford classification and JHGC, were compared concerning classification system (split or lumped) and adopted parameters. Even though JHGC was not produced with the same rigor as the Oxford classification, its utility can be evaluated by the clinical information that it provides. A prospective study from the work of the IgAN Study Group of the Progressive Renal Diseases Study Committee under the auspices of the Ministry of Health, Labor and Welfare is ongoing to show that the evidences that JHGC is reproducible and can predict renal outcome in different cohorts with different follow-up periods, different stages of the disease, and different therapies. In addition to this, the lumped system employed by the JHGC more accurately captures the diversity of IgAN lesions, allowing different courses of the disease to be distinguished from each other. Thus, for the task of effective communication of lesion histology between pathologist and clinician by a reproducible scoring system, the approach taken by the JHGC may prove superior to the four-parameter Oxford classification. In this way, starting with a subjective measure, such as expert option, that is subjected to and refined by empirical validation may prove a more satisfactory approach to the development of a classification system for IgAN. In this way, decades of sound knowledge regarding the physiopathology of IgAN, even if acquired historically by trial and error, can be incorporated into an empirically validated classification. Hence, in addressing the aim to overcome the task of histological classification, lumped system and/or expert’s opinion-based classification that has been developed and validated in a number of studies may not be inferior to purely evidence-based Oxford classification.
Acknowledgment
This study was supported by a Grant-in-Aid for Scientific Research of Japan Society for the Promotion of Science and for Progressive Renal Diseases Research, from the Ministry of Health, Labor and Welfare of Japan. The authors thank Dr. Osamu Hotta, Hotta Osamu Clinic, Sendai, Japan for his precious comments and his contribution to figures.
References
1.
Haas M, Rastaldi MP, Fervenza FC. Histologic classification of glomerular diseases: clinicopathologic correlations, limitations exposed by validation studies, and suggestions for modification. Kidney Int. 2014;85:779–93. doi:
10.1038/ki.2013.375. Epub 2013 Oct 2. Lee SM, Rao VM, Franklin WA, Schiffer MS, Aronson AJ, Spargo BH, Katz AI. IgA nephropathy: morphologic predictors of progressive renal disease. Hum Pathol. 1982;13:314–22.
2.
Lee SM, Rao VM, Franklin WA, Schiffer MS, Aronson AJ, Spargo BH, et al. IgA nephropathy: morphologic predictors of progressive renal disease. Hum Pathol. 1982;13:314–22.
CrossRefPubMed
3.
Lee HS, Lee MS, Lee SM, Lee SY, Lee ES, Lee EY, et al. Histological grading of IgA nephropathy predicting renal outcome: revisiting H.S. Lee’s glomerular grading system. Nephrol Dial Transplant. 2005;20:342–8. Epub 2004 Dec 23.
CrossRefPubMed
4.
Haas M. Histologic subclassification of IgA nephropathy: a clinicopathologic study of 244 cases. Am J Kidney Dis. 1997;29:829–42.
CrossRefPubMed
5.
Cattran DC, Coppo R, Cook HT, Feehally J, Roberts IS, Troyanov S, et al. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int. 2009;76:534–45. doi:
10.1038/ki.2009.243. Epub 2009 Jul 1.
CrossRefPubMed
6.
Roberts IS, Cook HT, Troyanov S, Alpers CE, Amore A, Barratt J, et al. The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int. 2009;76:546–56. doi:
10.1038/ki.2009.168. Epub 2009 Jul 1.
CrossRefPubMed
7.
Kawamura T, Joh K, Okonogi H, Koike K, Utsunomiya Y, Miyazaki Y, et al. A histologic classification of IgA nephropathy for predicting long-term prognosis: emphasis on end-stage renal disease. J Nephrol. 2013;26:350–7. doi:
10.5301/jn.5000151. Epub 2012 Jun 7.
CrossRefPubMed
8.
Coppo R, Troyanov S, Bellur S, Cattran D, Cook HT, Feehally J, et al. Validation of the Oxford classification of IgA nephropathy in cohorts with different presentations and treatments. Kidney Int. 2014;86:828–36. doi:
10.1038/ki.2014.63. Epub 2014 Apr 2.
CrossRefPubMedPubMedCentral
9.
Coppo R, Troyanov S, Camilla R, Hogg RJ, Cattran DC, Cook HT, et al. The Oxford IgA nephropathy clinicopathological classification is valid for children as well as adults. Kidney Int. 2010;77:921–7. doi:
10.1038/ki.2010.43. Epub 2010 Mar 3.
CrossRefPubMed
11.
Katafuchi R, Ninomiya T, Nagata M, Mitsuiki K, Hirakata H. Validation study of oxford classification of IgA nephropathy: the significance of extracapillary proliferation. Clin J Am Soc Nephrol. 2011;6:2806–13. doi:
10.2215/CJN.02890311.
CrossRefPubMedPubMedCentral
12.
Shi SF, Wang SX, Jiang L, Lv JC, Liu LJ, Chen YQ, et al. Pathologic predictors of renal outcome and therapeutic efficacy in IgA nephropathy: validation of the oxford classification. Clin J Am Soc Nephrol. 2011;6:2175–84. doi:
10.2215/CJN.11521210. Epub 2011 Aug 18.
CrossRefPubMedPubMedCentral
13.
Zeng CH, Le W, Ni Z, Zhang M, Miao L, Luo P, et al. A multicenter application and evaluation of the oxford classification of IgA nephropathy in adult Chinese patients. Am J Kidney Dis. 2012;60:812–20. doi:
10.1053/j.ajkd.2012.06.011. Epub 2012 Jul 20.
CrossRefPubMed
14.
Shima Y, Nakanishi K, Hama T, Mukaiyama H, Togawa H, Hashimura Y, et al. Validity of the Oxford classification of IgA nephropathy in children. Pediatr Nephrol. 2012;27:783–92. doi:
10.1007/s00467-011-2061-0. Epub 2011 Dec 2.
CrossRefPubMed
15.
Lee H, Yi SH, Seo MS, Hyun JN, Jeon JS, Noh H, et al. Validation of the Oxford classification of IgA nephropathy: a single-center study in Korean adults. Korean J Intern Med. 2012;27:293–300. Epub 2012 Sep 1.
CrossRefPubMedPubMedCentral
16.
El Karoui K, Hill GS, Karras A, Jacquot C, Moulonguet L, Kourilsky O, et al. A clinicopathologic study of thrombotic microangiopathy in IgA nephropathy. J Am Soc Nephrol. 2012;23:137–48. doi:
10.1681/ASN.2010111130. Epub 2011 Nov 3.
CrossRefPubMedPubMedCentral
18.
Kang SH, Choi SR, Park HS, Lee JY, Sun IO, Hwang HS, et al. The Oxford classification as a predictor of prognosis in patients with IgA nephropathy. Nephrol Dial Transplant. 2012;27:252–8. doi:
10.1093/ndt/gfr295. Epub 2011May 23.
CrossRefPubMed
19.
Alamartine E, Sauron C, Laurent B, Sury A, Seffert A, Mariat C. The use of the Oxford classification of IgA nephropathy to predict renalsurvival. Clin J Am Soc Nephrol. 2011;6:2384–8. doi:
10.2215/CJN.01170211. Epub 2011 Sep 1.
CrossRefPubMedPubMedCentral
20.
Sato R, Joh K, Komatsuda A, Ohtani H, Okuyama S, Togashi M, et al. Validation of the Japanese histologic classification 2013 of immunoglobulin A nephropathy for prediction of long-term prognosis in a Japanese single-center cohort. Clin Exp Nephrol. 2014 Jul 8. [Epub ahead of print].
21.
Weening JJ, D’Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int. 2004;65:521–30.
CrossRefPubMed
22.
Berden AE, Ferrario F, Hagen EC, Jayne DR, Jennette JC, Joh K, et al. Histopathologic classification of ANCA-associated glomerulonephritis. J Am Soc Nephrol. 2010;21:1628–36. doi:
10.1681/ASN.2010050477. Epub 2010 Jul 8.
CrossRefPubMed
23.
D’Agati VD, Fogo AB, Bruijn JA, Jennette JC. Pathologic classification of focal segmental glomerulosclerosis: a working proposal. Am J Kidney Dis. 2004;43:368–82.
CrossRefPubMed
24.
Tervaert TW, Mooyaart AL, Amann K, Cohen AH, Cook HT, Drachenberg CB, et al. Pathologic classification of diabetic nephropathy. J Am Soc Nephrol. 2010;21:556–63. doi:
10.1681/ASN.2010010010. Epub 2010 Feb 18.
CrossRefPubMed
Conflict of Interest
The authors declare that they have no conflict of interest.