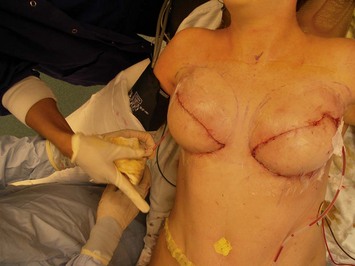
CHAPTER 9 DIEP Flap Breast Reconstruction
Summary
The lower abdominal region has evolved as the workhorse donor site for use in breast reconstruction. In particular, the deep inferior epigastric artery perforator (DIEP) flap has become popular in the hands of microsurgeons because of the balance between protection of the donor site and reliability for autologous breast reconstruction. The transverse rectus abdominis myocutaneous (TRAM) flap for breast reconstruction was first described by Holstrom1 and Robbins.2 It was Koshima and Soeda3 who brilliantly transformed this into a flap with complete muscle preservation, now known as the DIEP flap. When compared with the traditional pedicled TRAM flap, the DIEP flap has a lower incidence of fat necrosis or partial flap loss and has a lower incidence of hernia and abdominal bulge rates. When compared with the free TRAM flap, the DIEP flap, when properly raised, preserves all muscle function and abdominal fascia so that hernia and bulge rates are again lower4 and flap success rates are comparable. It should be noted that as the free TRAM flap has evolved to become more muscle-sparing, these structural abdominal donor site differences may be less.5 While the superficial inferior epigastric artery (SIEA) flap maintains the privilege of absolutely no violation of the abdominal wall, it cannot be carried out in all patients because of anatomical absence of the SIEA. Furthermore, the SIEA may not be able to reliably supply as much of the lower abdominal tissue for transfer as the DIEP flap, and failure rates in reported series are higher than for DIEP flap reconstruction. Although the DIEP flap appears to provide an ideal balance between reliability and low donor site morbidity, successful breast reconstruction using the DIEP is multifactorial, and proper patient selection and intraoperative decision making is essential.
Patient Selection
It has been shown repeatedly that obesity (body mass index, BMI > 30) and smoking result in increased rates of fat necrosis in the breast reconstruction and increased donor site complications.6–11 Patients should stop smoking a minimum of 3 weeks prior to elective flap surgery. There should be sufficient lower abdominal tissue to provide adequate volume for the breast reconstruction.
Scars in the lower abdomen require careful consideration. With appropriate perforator selection, there should be no increase in rate of total or partial flap loss or fat necrosis rate. However, the extent of intramuscular scarring that may interfere with perforator dissection may extend for a greater distance than can be appreciated by the external linear skin scar and it cannot be detected on preoperative imaging that may be used to map out dominant perforators. Furthermore, pre-existing abdominal scars may increase the incidence of donor site morbidity, with greater chance of wound breakdown, seroma, and abdominal bulge or laxity.12 Although abdominal liposuction is regarded by some as a contraindication to elevation of a DIEP flap,13 a series of DIEP flaps in abdominal liposuction patients has been successfully carried out after preoperative Duplex Doppler imaging.14 However, the newer modality of laser-assisted liposuction which is geared towards coagulating vessels and tightening the dermis has not been included in this study group and based on the mechanism of action, this form of liposuction would cause more damage to vessels than would traditional liposuction and should be considered a contraindication. Cosmetic abdominoplasty should also be considered a contraindication to elevation of a DIEP flap. A further discussion on avoiding complications when abdominal scars are present can be found in the Pitfalls section of this chapter.
Operative Technique
Surgical anatomy
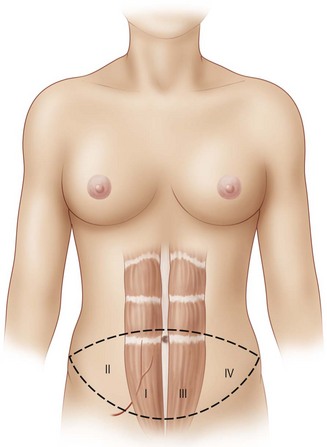
For free flaps, the best perfusion of the lower abdominal skin and fat is on the hemi-abdomen ipsilateral to the pedicle of the flap, as opposed to the central portion of the abdomen across the midline.15 This has been recognized to be the case both clinically and in anatomical dissections of DIEP flaps that have demonstrated variability in the presence of crossing branches of the venous plexus across the midline.16 It has also been supported by the work of Taylor investigating angiosomes.17,18 Therefore, Zone I constitutes the area directly over the ipsilateral rectus abdominis muscle from which the pedicle will arise, and Zone II lies lateral to the ipsilateral rectus abdominis muscle. Zones I and II constitute the ipsilateral hemiabdomen with the best zones of perfusion. Conversely, the contralateral hemiabdomen is composed of Zone III (the area adjacent to Zone I which lies across the midline, overlying the contralateral rectus abdominis muscle) and Zone IV is the remaining portion lateral to the contralateral rectus abdominis muscle which is furthest from the blood supply of the flap (see Fig. 9.1).
Elevation of a DIEP flap necessitates dissection through the rectus sheath as well as through a variable amount of rectus abdominis muscle because its source vessel, the deep inferior epigastric artery, arises from the external iliac artery deep to the inguinal ligament. The deep inferior epigastric artery is accompanied by paired venae comitantes. It pierces the deep surface of the rectus abdominis muscle as it ascends towards the umbilicus most commonly in the middle third (78%) and less commonly in its lower third (17%) or upper third (5%).19 It also has a variable branching pattern within the muscle. The deep inferior epigastric artery most commonly divides into a dominant lateral branch with an additional medial branch (54%). However, it may have a central course with multiple small branches (28%) or a dominant medial branch (18%).20 Both the level at which the deep inferior epigastric artery pierces the deep surface of the muscle and its branching pattern have implications for the difficulty of dissection of the DIEP pedicle, and this is where some of the variability and the learning curve in perforator selection arises. Although there are between two and eight perforators greater than 0.5 mm in diameter that pierce each side of the anterior rectus fascia in the paraumbilical region between 2 cm cranial and 6 cm caudal to the umbilicus and between 1 and 6 cm lateral to the umbilicus,21 perforators that are lateral tend to have a more direct perpendicular course through the muscle. Medial perforators, however, may have a long, complicated and oblique intramuscular course with numerous subsidiary muscular branches along the way. This is why preoperative imaging may be helpful in predetermining which perforator, if they appear to have comparable caliber where they are piercing the rectus abdominis fascia and entering the flap, would result in a technically easier pedicle dissection with potentially less disruption of the muscle during the dissection. However, it is also important to note that a more medial perforator may be more likely to supply a greater portion of the flap on the contralateral side of the abdomen.22
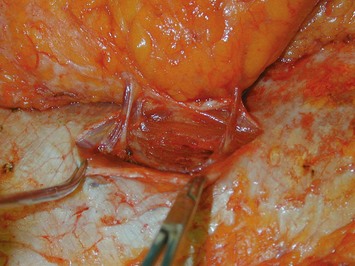
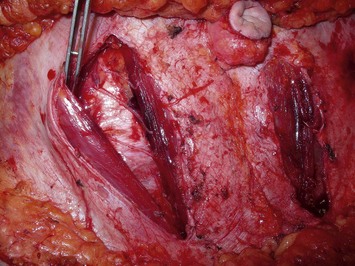
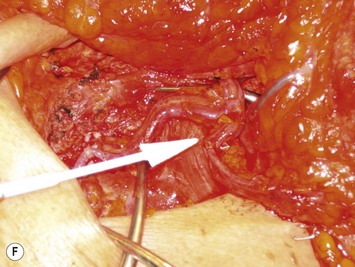
The relationship of nerves relative to the intramuscular course of the deep inferior epigastric artery is also an important consideration. Lateral perforators tend to be accompanied by larger sensory segmental nerves (see Fig. 9.2), which can potentially be used to neurotize the DIEP flap if an appropriate donor nerve in the mastectomy site can be located for neurorraphy. Motor nerves from intercostal contributions tend to cross superficial to the main branches of the deep inferior epigastric vessels (see Fig. 9.3) so that they can potentially be carefully dissected off of the main pedicle. However, because the motor nerves are segmental and travel in an oblique direction, they are often found traveling between sets of perforators arising from the same medial, lateral or central intramuscular row of the deep inferior epigastric. The implication here is that if two or more perforators in a row are used as the pedicle for the DIEP flap, then intervening segmental motor nerves need to be divided for removal of the flap.
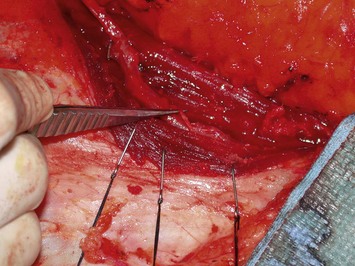
Finally, venous drainage may not be reliable. This is of particular concern for drainage across the midline. In some patients, the superficial venous drainage system may be dominant so that knowledge of the vascular anatomy of the superficial system is also important for DIEP flap elevation. Although a full description of the anatomy of the SIEA is found in Chapter 10, the essentials of the accompanying venous drainage system anatomy is highlighted as it relates to DIEP flaps. The landmark for the location of the SIEA – when it is present – at the level of the inguinal ligament is the midpoint between the anterior superior iliac spine and the pubic bone, or slightly lateral to this point,23 superficial to Scarpa’s fascia. It is accompanied by venae comitantes that travel inferiorly, piercing the cribiform fascia to descend to drain into the femoral vein. However, there is an additional superficial draining vein (medial epigastric vein) that is often present several centimeters medial to the artery which is often larger than the venae comitantes of the SIEA. Both the SIEA and accompanying venae comitantes and medial epigastric vein are shown in Figure 9.4A. This vein can be used as an alternate or adjunctive venous drainage for a DIEP making it essential that it be identified, dissected, and preserved if present. If a large vein from the superficial inferior epigastric system is encountered during flap elevation, this can be taken as a warning that drainage through the small perforating veins may be inadequate (see Fig. 9.4B and C).
Preoperative planning
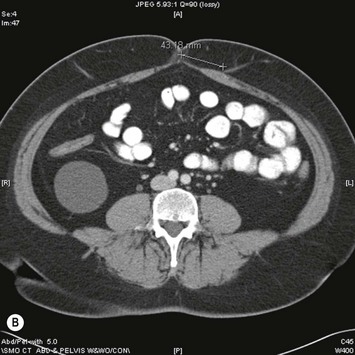
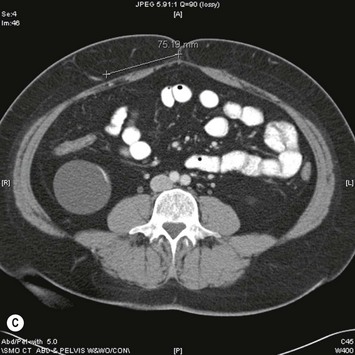
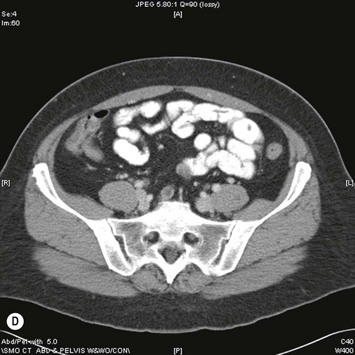
Assessment of the perforator size and course can be carried out in advance with Duplex Doppler, computed tomography angiography (CTA), or even magnetic resonance angiography (MRA). If these modalities are not available, then surface handheld Doppler assessment can be used as a guide but it does not correlate as well to the exact location of where the perforator is piercing the fascia and it cannot delineate the vessel’s course. In contrast, Duplex Doppler scan of the lower abdominal wall can provide information on the position, flow, and diameter of the intramuscular perforators and can also be used to determine if the SIEA is present. A grid centered on the umbilicus and marked out in x– and y-axes at 1 cm intervals can be used to map the perforators and the grid can be laid out again on the day of surgery to localize the pertinent perforators. However, Duplex Doppler is operator-dependent and does not leave a permanent imaging record. In contrast, CTA can provide a permanent record of the preoperative roadmap for flap elevation.24,25 Dominant perforator locations can be measured on the images relative to the umbilicus, with these measurements then repeated on the patient and marked out on the skin surface. The intramuscular course can also be identified, so that the surgeon will know in advance which direction the perforator turns when it lies beneath the fascia, and whether it is lying just beneath the fascia or whether it plunges into the muscle. The length of the intramuscular course can be calculated and the branching pattern of the deep inferior epigastric vessels with which the perforator is associated can be determined. An example of CTA perforator mapping and clinical correlation is shown in Figure 9.5. Disadvantages of CTA include radiation dose and CT contrast that is administered during the study. To circumvent these disadvantages, MRA has been described to be used in a similar manner, but an MR image takes longer to perform than does a CT image. Preoperative imaging may also lead a surgeon to alter the limits of the markings of their donor site to a slightly higher location above the umbilicus in an attempt to have a greater amount of the flap centered on the selected dominant perforator. In addition, preoperative imaging may play a particular role in the case of extensive or unusual prior abdominal scarring where there is a question as to whether DIEA vessels are present or whether perforators exist to allow for DIEP flap elevation. Although each of the described imaging tools are adjuncts that have been shown in some hands to significantly decrease operative time and may be particularly useful for microsurgeons who are starting in practice, a DIEP flap can still successfully be elevated without their use based on intraoperative assessment, vascular anatomy knowledge, and meticulous technique. These fundamental principles apply even when the operating surgeon has preoperative imaging. There are very strong advocates of preoperative imaging, and similarly there are well-known experienced surgeons with equally successful outcomes who do not routinely obtain preoperative imaging. Thus, practice patterns vary and the surgeon should do what in his or her hands gives a successful outcome.
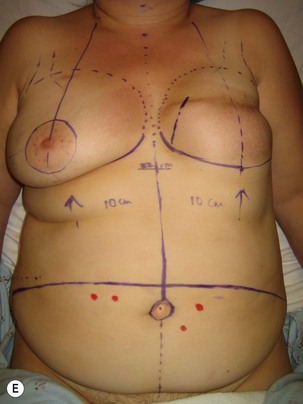
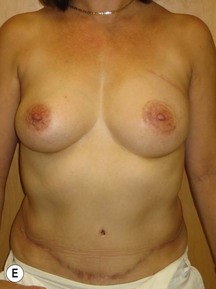
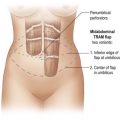
Preoperative markings are usually carried out in the standing position and confirmed once the patient is supine under anesthesia (see Fig. 9.5E). A fusiform ellipse is outlined on the lower abdomen that extends from the suprapubic crease inferiorly to just above the umbilicus superiorly and laterally to each anterior superior iliac spine (ASIS), although the territory may be extended to the midaxillary line.26,27 The amount of tissue that can be taken and still allow for closure of the donor site should be estimated by pinching the tissues. The amount that can be incorporated depends on the patient’s tissue laxity. It should also be noted that scars above the umbilical level (midline, paramedian, or subcostal Kocher scars) allow for less excursion of the remaining abdominal skin for closure. When one is attempting to maximize the amount of tissue that can be taken and it is suspected the closure may be unduly tight, the lowest portion of the marked ellipse can be adjusted in the operating room after elevation of the upper abdominal skin from above the umbilicus to the xiphisternum, followed by flexing the OR table at the patient’s waist, testing the excursion of the upper abdominal flap, and then remarking the inferior portion of the ellipse. The thickest fat of the flap is in the superior portion of the flap where the perforators are also the largest, so that the overall volume of the flap that is lost by having to superiorly adjust the inferior flap cut is usually not substantial and this maneuver can prevent abdominal wound problems even though it may add to the operative time.
Procedure
Mastectomy site and vessel preparation
In the case of an immediate reconstruction, mastectomy flaps should be assessed for hemostasis, viability, and extent of dissection. Any clearly non-viable areas should be excised to avoid the complication of mastectomy flap necrosis. If there is uncertainty and large areas are in question, modalities such as fluorescein imaging can be used or alternatively a delayed definitive flap inset can be carried out with the patient being returned to the OR approximately 48 hours later for excision of demarcated necrotic mastectomy skin flaps and de-epithelialization of the parts of the flap to be buried and final inset.28 Any overdissection of the mastectomy site, which most commonly occurs inferiorly or laterally, should be addressed with internal tacking sutures to recreate the inframammary fold and the lateral breast contour. The latter is an often underemphasized and very important aesthetic line of the breast, and it avoids having a large volume of the flap fall out laterally while helping to achieve medial fullness for cleavage and for masking of an internal mammary vessel recipient site.
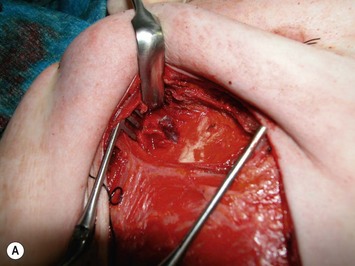
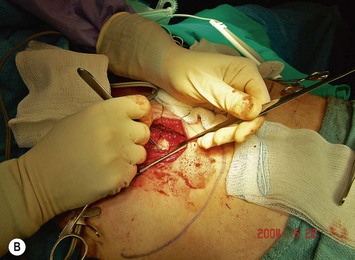
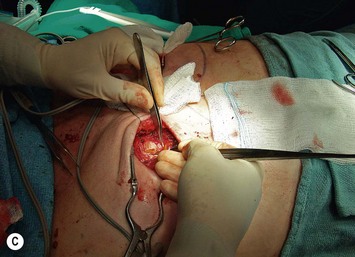
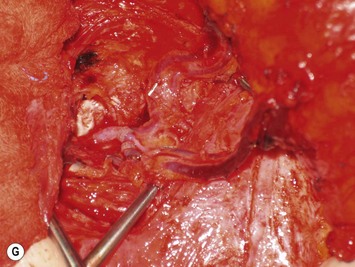
Recipient vessels are prepared. The author’s preference is to use the internal mammary system as opposed to the thoracodorsal vessels. Use of the thoracodorsal vessels often results in more difficulty in medialization of the breast reconstruction flap and often more lateral breast fullness results (see Fig. 9.6A), although this can be addressed by maintaining long length of the DIEP flap pedicle and placing internal tacking sutures to hold the flap in the appropriate position. Furthermore, in the case of a delayed reconstruction the axilla is often scarred and the thoracodorsal vessels are more prone to spasm as compared to the internal mammary vessels. Finally, if a sentinel node biopsy is carried out at the time of an immediate reconstruction, there is the possibility that a completion axillary dissection will need to be carried out in the postoperative time period if the sentinel node is positive for metastatic disease.29 The implications are that the oncologic breast surgeon would have to carry out the axillary node dissection with repositioned thoracodorsal vessels and the relatively fresh pedicle to the DIEP flap within the field. The internal mammary vessels offer the advantages of more medial positioning of the flap (see Fig. 9.6B), negative intrathoracic pressure which aids in venous drainage, more protection from scarring and radiotherapy in the case of a delayed reconstruction, and protection from manipulation should a completion axillary dissection be required at a later date in the case of an immediate reconstruction with sentinel node biopsy. Although the internal mammary perforators (shown in Fig. 9.7A) can be used if they are of adequate caliber, the main vessels are often a superior size match to the DIEA and their venae comitantes. There is evidence to suggest that the left sided internal mammary vessels are smaller and in particular the veins are more often smaller, paired vessels. Despite this, in most cases they are usually still usable as even a small vein has the added advantage of the negative intrathoracic pressure to aid in venous drainage. The thoracodorsal vessels or rarely even the cephalic vein or external jugular vein can be used as an alternate drainage system. The author’s preferred technique for exposure of the recipient vessels is to first select a rib cartilage that will be covered by the DIEP flap reconstruction when the patient would be in a standing position in order to avoid a visible chest contour abnormality as shown in Figure 9.8 and even visible indrawing of the site with respiration. This has lead to selecting a lower rib than originally described, usually the fourth costal cartilage. The other advantages of a lower position are that vessel dissection and microvascular anastomosis does not require as much retraction of the mastectomy skin flap resulting in fewer traction-related ischemic mastectomy flap issues and easier exposure; the internal mammary vessels at this rib are also on a level plane, whereas at the location of the second rib they start to dive deeper into the chest with the curvature of the chest as they head toward the mediastinum. Finally, if the recipient vessels are inadvertently injured in the course of their dissection or in the case of a takeback for revision of an anastomosis there is sufficient extra length available by dissecting out a higher rib interval. The removal of the fourth rib cartilage, with exposure in the third and fourth intercostal spaces can give more than 3 cm of vessel length. Exposure is further optimized once the internal mammary artery and vein are divided at the inferior portion of the fourth intercostal space and allowed to curve more laterally. Excising too inferior a rib cartilage can lead to more technical challenges. For instance, the ribs start to travel more obliquely with small intercostal space intervals so that less vessel exposure is obtained. Furthermore, the internal mammary vessels can become smaller, with a shorter distance before the intercostal branches are given off, requiring more hemoclips in the field of dissection close to the area of the vessels that will be transected for the site of the anastomoses.
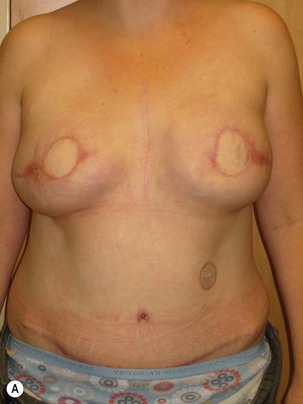
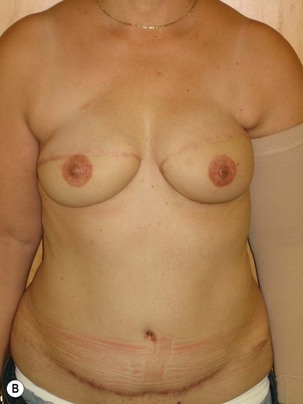
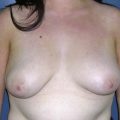
Fig. 9.6 Differences in flap inset and final appearance dependent on choice of recipient vessels.
(With permission, J.E. Lipa.)
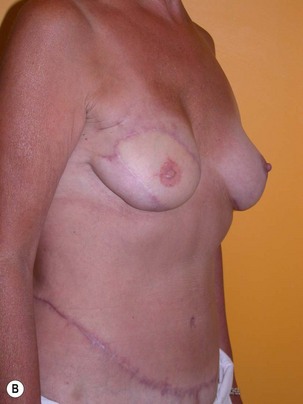
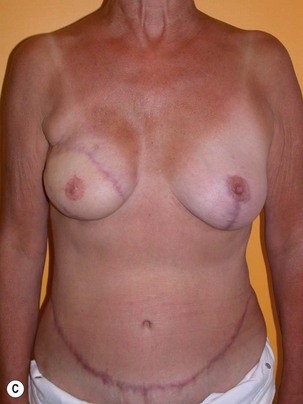
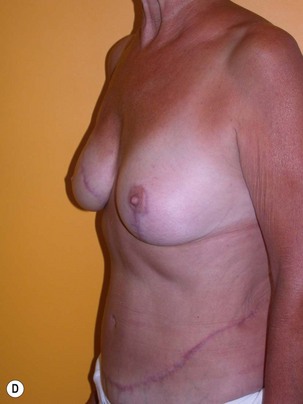
Fig. 9.8 Internal mammary recipient vessel contour deformity can occur when a more superior site is chosen.
(With permission, J.E. Lipa.)
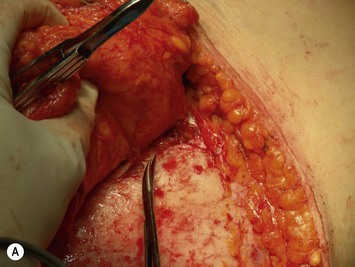
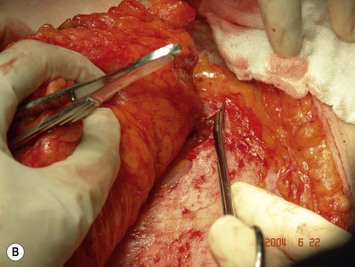
The inferior aspect of the selected rib cartilage is palpated, and the pectoralis major muscle is divided using cautery, parallel to the muscle fiber direction, from the sternocostal junction to the bone/cartilage junction of the rib. These junctions can be palpated, with the latter being visualized as an angle in the rib once the muscle is split. The sternal portion of the pectoralis major muscle is then divided vertically upwards along the sternocostal junction. As shown in Figure 9.7A, this creates good exposure of the rib cartilage, visualization of internal mammary perforators if present, and it allows for the pectoralis major muscle to form a flap, which naturally migrates superiorly serving to increase exposure, potentially minimize contour deformity, and prevent a dynamic compression of the pedicle with muscle contraction that could result if just a split in the fibers was made. Next, the anterior perichondrium overlying the costal cartilage is scored in an ‘H’ fashion so that the perichondrium can be elevated superiorly and inferiorly from the transverse limb of the ‘H’ using a Cobb elevator or alternatively Freer or Howarth elevators. The advantage of using a broader elevator such as the Cobb is that the surgeon has a lower likelihood of perforating the posterior perichondrium as it is being elevated off of either the superior or inferior aspects of the rib. The posterior perichondrium is then elevated from the cartilage. Where possible, when it peels off easily, a Doyan rib elevator can be positioned around the lateral costal cartilage, advanced medially, and then used to excise the cartilage. If it is not possible to slide this in safely, two periosteal elevators can be positioned between a lateral portion of the cartilage and the posterior perichondrium, one from above and one from below, and then a 15-blade scalpel can cut down onto the hard surface of the two elevators that should be protecting the posterior perichondrium and underlying pleura and vessels (see Fig. 9.7B). The posterior perichondrium can be further elevated in this fashion more medially (Fig. 9.7C), and another cartilage cut carried out in order to remove a block of cartilage. If the cartilage is calcified or impossible to separate from the posterior perichondrium, it can be carefully resected piecemeal using a rongeur. Even when using a Doyan or the scalpel, it is often necessary to remove more cartilage medially to the sternocostal junction with a rongeur. Once the costal cartilage is removed, self-retaining retractors and dural hooks, or alternatively, two Gilpie retractors, can be inserted which are sometimes less cumbersome than self-retainers to remove once the flap is anastomosed. When Gilpie retractors are used, one is positioned into the cut lateral remaining rib with the other blade placed at the medial reflection of the mastectomy skin flap from the chest wall. The second retractor is used to spread the superior and inferior portions of the pectoralis major muscle interval. This creates wide exposure and the tension of the retractor between the rib and skin flap aids in the next step: the posterior perichondrium (shown in Fig. 9.7D) is carefully incised at its lateral exposure. This can be done with cautery at a lower setting or if the surgeon is more comfortable using scissors or a bipolar over a dissecting instrument this can also be done. The reflection of the posterior perichondrium adjoining the intercostal muscles must also be divided superiorly and inferiorly. As a result of the tension of the retractor, this perichondrial incision spreads open several millimeters, exposing a thin layer of fat that tends to protect underlying pleura and vessels. This incision should end up lateral to the location of the vessels. The posterior perichondrial flap can now be gently elevated from this underlying fatty layer using a combination of blunt dissection to free it (such as with the broad cautery tip when it is not in use, just utilizing it as a pusher/elevator). Any tiny vessels can be controlled with bipolar cautery. This should now reveal the internal mammary vessels. Usually, if there is only one vein, it is positioned medial to the artery (see Fig. 9.7E). If there is a vein seen lateral to the artery, it is likely that this is the smaller of two venae comitantes, and the dissection should be carried medial to the internal mammary artery to identify a larger paired medial vein. The vessels can then be exposed on their superficial aspect by division of the intercostal muscles superior and inferior to the rib cartilage location, using cautery or bipolar as often these muscles are quite vascular. Usually branches from the internal mammary artery and vein travel out perpendicular to the main vessels from both the medial and lateral aspects. Any branch needs to be meticulously controlled with either bipolar cautery or micro-hemoclips, with control of the branch a couple of millimeters away from the main vessel to avoid damage to the recipient vessels and also to ensure that if a clip comes off or if it bleeds that there will be enough length on the side branch to re-secure it. These principles should also be followed when controlling branches from the DIEP vessels during flap elevation. Next, the internal mammary vessels can be gently teased off of the underlying fatty layer that separates them from the parietal pleura. There may be a lymphatic channel and/or lymph nodes that are identified. The author’s preference is to preserve the lymphatic channel if possible. Often the lymph node requires resection with control of a feeding arterial branch and venous branch in order to expose the internal mammary vessels. Occasionally in the case of prior chemotherapy and radiotherapy the lymph nodes are very scarred and difficult to separate from the vessels and then often this marks the endpoint for vessel mobilization and a decision must be made as to whether there is enough length for anastomosis. If not, or if the vessels are too small for anastomosis, then consideration of removal of another adjacent costal cartilage should be given prior to abandoning this site for recipient vessels. The vessels can be irrigated with a vasodilator substance such as papaverine, packed with a moistened saline sponge, and the retractors removed until the flap is ready for anastomosis.
Flap elevation
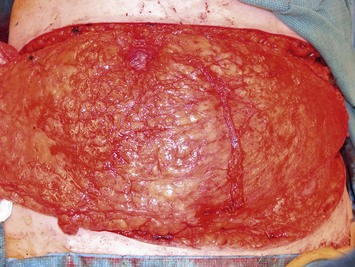
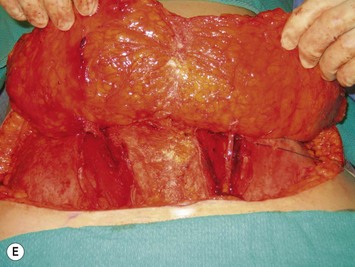
Elevation of the DIEP flap can be carried out concurrently with the mastectomy and/or mastectomy site and recipient vessel preparation if two surgical teams are available. The outline of the lower abdominal ellipse is incised and the dissection taken down the abdominal wall fascia. Along the inferior incision, the superficial system is always carefully explored as shown in Figure 9.4A. This system may be necessary for conversion to an SIEA flap if it is felt that the vessels are sufficient for microvascular anastomosis and that a large enough territory of the flap can be perfused for the breast reconstruction, or else to preserve the medial superficial epigastric vein to have it as a potential ‘lifeboat’ for flap venous drainage. This is particularly important if the vein is of larger caliber, at least 1.5 mm, as it may be the dominant venous drainage system for the flap. An incision can be fashioned around the umbilicus and the umbilical stalk dissected down to the fascial wall to facilitate access for exploration of perforators. If this is a bilateral reconstruction, the incision down the center of the abdominal pannus is also carried out.
If preoperative imaging is available, dissection should be directed toward the dominant perforator. However, if no imaging is available, then dissection must proceed, using cautery to lift the fat of the flap off of the abdominal wall fascia until all perforators are visualized and each can be assessed for caliber and position within the flap. Both sides can be assessed, and dissection should proceed from all directions to include visualization of the medial paraumbilical perforators. An adequate perforator should have a vein >1 mm in diameter where it is entering into the flap and a palpable or visibly pulsating artery.30 If several perforators are in the same longitudinal row, then they can all be preserved within the flap. Any dissection which approaches or involves the perforators should be done only when the patient is paralyzed, with paralysis being monitored and maintained by the anesthesiologist. This may mean that perforator dissection cannot be completed until any axillary dissection work by the oncologic breast surgeon is completed if their routine is to not have the patient paralyzed during this portion of the surgery. Paralysis should be maintained until the flap is elevated and detached, anastomosed, and the abdomen closed. Because dissection of the perforators involves staying immediately on the vessels after splitting the fascia and following them through their intramuscular course, and because the rectus abdominis muscle is kept innervated with minimal fiber disruption, any movement of the muscle, caused either by direct stimulation by the cautery or if the patient becomes light and coughs, can cause damage to the perforator or avulsion of the perforator from its entry point into the flap.
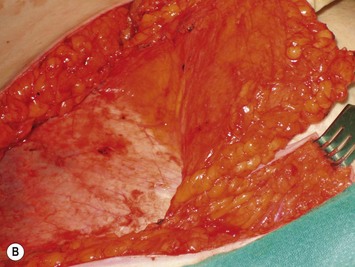
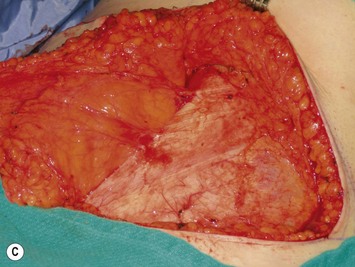
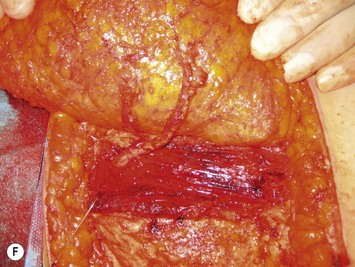
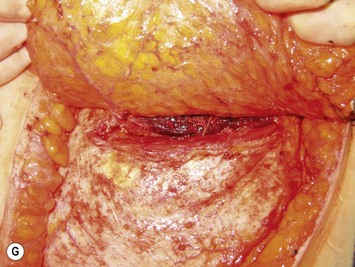
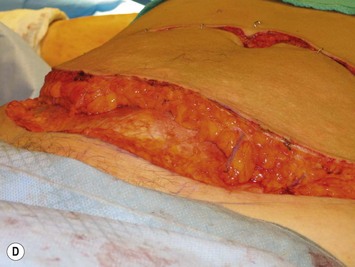
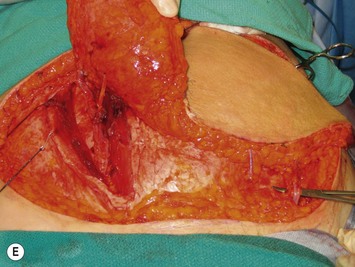
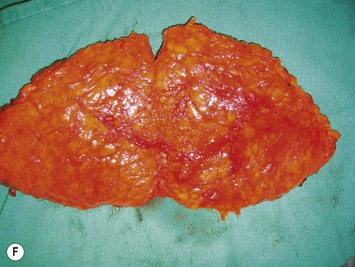
Once the perforator(s) to supply the flap is chosen based on location and caliber, the fascia surrounding the perforator is mobilized away from it as shown in Figure 9.9A (usually there is small rent in the fascia and fatty tissue that surrounds the perforator, but this is not always the case) or if it is strongly adherent or if the surgeon does not feel comfortable with this maneuver a small fascial cuff can be cut around the vessel. The fascia is then split longitudinally for a distance of 1–2 cm superior to the perforator and several centimeters inferior to the perforator or until the next perforator in the same medial or lateral row is encountered (see Fig. 9.9B). Exposure should always be maintained along an advancing front, as opposed to making a small keyhole in the fascia. Good exposure will demonstrate the course of the perforator and will allow for visualization of small muscular branches that must be controlled along the way in order to maintain meticulous hemostasis to improve perforator visualization and in order to avoid avulsion of small branches which can then either damage the main perforator or make it go into spasm. As well, the fascial incision must be made without diving into deeper tissues because sometimes the perforator tracks immediately deep to the fascia and superficial to the underlying rectus abdominis muscle. Until it can be seen diving between the muscle fibers, great care must be taken in incising the rectus fascia. The perforator is then followed through the muscle once it enters it, and this is carried out by laying open the interval in the muscle through dissection between the muscle fibers above and below the perforator (see Fig. 9.9C). Retraction of the muscle at this interval is facilitated by use of dural hooks. Small muscle branches need to be controlled as the perforator follows through the muscle, but if there is any doubt as to which is the perforator and the main DIEA/V branch, vessels should not be clipped off until the junction of the perforator entering into the main vessels is visualized. Once visualized, branches can be clipped or controlled with bipolar cautery, and the superior continuation of the main DIEA/V row or vessels can be divided between hemoclips. If one is considering carrying out an intraflap anastomosis (see Pitfalls section) then extra length on the superior continuation of these vessels can be maintained. Dissection through the muscle is continued, accompanied by incision of the overlying fascia as needed, until adequate length is obtained to reach the recipient vessels. This is impacted by the planned flap orientation and by the caliber of the vessels required for a favorable size match to the recipient vessels. This may be at the take-off from the DIEA/V of the main medial or lateral branch, or it may require dissection further to the main DIEA/V. However, it is usually not necessary to dissect the pedicle to its origin from the external iliac vessels. The group from Gent advocates making a separate fascial incision corresponding to the lateral inferior border of the rectus abdominis muscle to carry out pedicle dissection if it is necessary to get to the main DIEA/V because it is felt that two parallel cuts in the fascia in this lower part of the abdomen may be protective to development of hernia as opposed to one continual fascial incision.25,27 However, an alternative is to limit the inferior extent of this fascial cut but this requires adequate retraction to get under the muscle for pedicle dissection. The flap should perfuse well in situ (see Fig. 9.9D).
During intramuscular dissection, any time that the pedicle appears to be tethered to muscle fibers there is usually a muscular branch that needs to be controlled either by bipolar cautery or by hemoclips. There may also be tethering when a lateral perforator is being dissected. Often there is a communication with a segmental intercostal artery and venae comitantes and intercostal nerve. It is sometimes possible that the lateral perforator connects through a short segment of intercostal vessel before entering into the lateral row branch of the DIEA/V so that these vessels must be carefully exposed and the vascular anatomy confirmed before any branches are ligated. With respect to nerves, those that are following the perforators up into the subcutaneous fat are sensory nerves and will require transection. However, motor nerves typically travel superficial to the pedicle and can usually be dissected off of the underlying pedicle and the pedicle, once it is transected at its origin, can be passed under the nerves as with the donor site shown in Figure 9.10. In the event that a segmental motor nerve passes between two perforators that will be used to supply the flap, it is necessary to divide the motor nerve. The author prefers to carry out reparative epineural neurorraphy using 9-0 nylon. However, there is no clear data on incidence of hernia, bulge, or abdominal function with DIEP flaps depending on whether motor nerves are sacrificed, preserved, or repaired. The concept of the DIEP flap is to minimize muscle disruption so it follows that nerves should be preserved where possible.
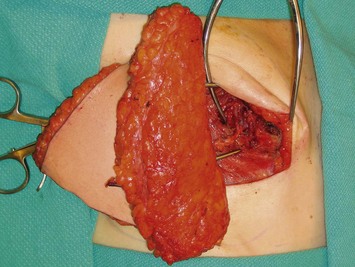
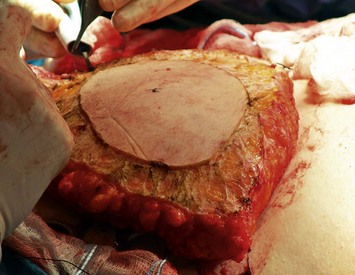
During DIEP flap elevation and prior to division of all of the muscular branches, it is prudent to isolate a few other perforators within the flap and temporarily clamp them. The flap should then be observed for the amount of tissue that has good capillary refill and bleeding. If the flap does not appear to be well-perfused on just the selected perforator(s), then an additional blood supply must be included. This may involve additional perforators along the same row if, when their clamps are removed, perfusion improves. Alternatively the flap can be converted to a MS-TRAM flap if many more perforators are required to be unclamped to allow for good flap perfusion. Finally, it may be that perforators on both sides of the abdomen are required to perfuse adequate volume for unilateral breast reconstruction, as demonstrated in Figure 9.11.
The flap pedicle is divided. The author’s preference is to use hemoclips to separately ligate each vessel of the pedicle, placing double hemoclips on the iliac side, and then marking the flap vessels with the following sequence – a small hemoclip on the smaller vena comitans, a larger hemoclip on the artery, and the second, larger vena comitans is left open to drain the flap and to indicate when the flap is sitting in the breast site that this is the vein to be used, if possible, for anastomosis. When the flap is lifted out of the donor site, the pedicle can be laid along the fat of the flap to maintain its orientation (see Fig. 9.12) as opposed to leaving it to dangle with the potential to rotate and result in a kink once it is anastomosed.
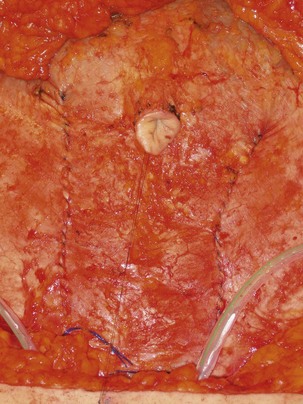
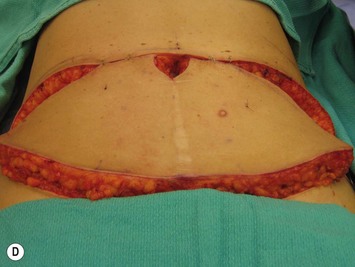
The flap is then handed up to the breast site and while it is being set up for the anastomosis (see below), the fascial incision can be closed. Non-absorbable sutures such as 0 Prolene interrupted buried sutures followed by a running 0 Prolene suture are used (see Fig. 9.13). The remainder of the abdominoplasty flap can be elevated if this was not already done, plication of any diastasis recti carried out, and then the bed can be placed into a reflex position followed by Trendelenburg. This allows for tension to be taken off of the abdomen for skin closure while still having the chest region lie flat for the microvascular anastomoses. The abdomen is closed over two drains brought out through separate suprapubic stab incisions, with Scarpa’s fascia closed using absorbable sutures such as 2-0 Vicryl and the skin closed using layered absorbable sutures such as 3-0 Monocryl interrupted deep dermal sutures and 4-0 Monocryl running intracuticular suture. Prior to this closure, the midline markings that were made in the suprapubic location and just above the umbilicus should be realigned. These markings often disappear by the time of the abdominal closure, so that it is useful to mark them at the start of the case with skin staples. As well, the position of the umbilicus is palpated following abdominal incision reapproximation. Even though the ideal anatomic location is at the level of the ASIS, a short stalk may limit its excursion and final location of inset. It can be brought out through an inverted ‘U’ incision in the abdominoplasty flap with some defatting and secured with interrupted buried dermal absorbable sutures. This is just one of the many ways to fashion the umbilicus.
Microvascular anastomosis and flap inset
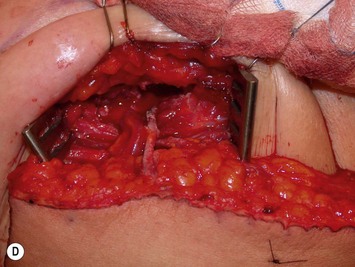
The internal mammary vessel exposure is recreated with the retractors as described in the vessel preparation, the flap temporarily secured with staples or sutures to the surrounding chest skin with the pedicle draped appropriately to meet the costal cartilage resection site, and the operating microscope brought in. The internal mammary artery and vein(s) are ligated distally with hemoclips and temporary microvascular clamps are placed proximally. The vessels are transected, the lumens irrigated with heparinized saline, arterial flow is tested, and the vessel ends are trimmed back to be ready for anastomosis. The venous anastomosis can be carried out with either suture technique or it is amenable to anastomosis using a coupling device. The arterial anastomosis is usually hand-sewn. The set-up is shown in Figure 9.14. When clamps are removed perfusion is readily appreciated and venous outflow confirmed. Papaverine irrigation may be used if there is any evidence of spasm. Flow in the flap is confirmed and the portion of the DIEP flap that will be inset under mastectomy skin flaps can be de-epithelialized at its periphery, as shown in Figure 9.15. The temporary stabilizing staples/sutures are removed, retractors removed, and the flap can be placed under the mastectomy flaps. With the abdomen already closed, the shaping of the flap and location of the inframammary fold can be confirmed, so that the definitive de-epithelialization can be carried out. A drain should be placed in the mastectomy pocket away from the pedicle in order to avoid having it inadvertently put mechanical compression on the pedicle. At the location of the flap-mastectomy skin inset, the flap dermis can be scored to the deeper dermis in order to allow for the skin of the flap to come up to meet the mastectomy skin and avoid having a retracted skin paddle which can be difficult to correct secondarily.
Pitfalls and Their Correction
Insufficient abdominal tissue volume
If 70% or less of the abdominal pannus is required for the breast reconstruction, a DIEP flap can be used because Zones I, II and III are generally reliable (i.e., Zone IV is discarded).31 However, if more than 70% of the abdomen is needed, then a free TRAM can reliably supply this entire territory of Zones I–IV. Alternatively, flaps elevated from both sides of the abdomen can be transferred and anastomosed in the breast site for unilateral breast reconstruction32,33 in a stacked fashion,34 or a ‘Siamese’ flap can be fashioned connecting vascular supplies from each side of the abdomen together in order to create one flap that perfuses the entire lower abdominal territory.35 This may either be bilateral DIEP flaps for unilateral breast reconstruction or a DIEP flap combined with an SIEA flap for unilateral breast reconstruction (see Fig. 9.16). This is also a strategy to deal with a midline lower abdominal scar, as shown in Figure 9.17. The DIEP-to-DIEP anastomosis can be carried out while the flap is still perfusing in the abdomen, but it is more expeditious to detach the flap and either carry out these anastomoses on a side table or after one DIEP circulation is attached to the recipient vessels in the chest.
Venous congestion
If the flap appears venous congested while still in situ or with multiple temporarily clamped perforators, it may mean the perforator which has been selected to drain the flap is not adequate. It may be necessary to unclamp other identified perforators and if the flap now has normal appearing color and capillary refill it should be converted to a MS-TRAM flap. The other possibility is, particularly if a large medial epigastric vein is present, that the superficial venous system is the dominant drainage or that it should be used as an alternate or second venous drainage system for the DIEP flap. Because the DIEA is deep to the flap, it needs to be anastomosed first. The medial (superficial) epigastric vein comes off the cut side of the fat of the flap, so that it will need to travel down along the edge of the flap to the internal mammary vein. This can make inset slightly more challenging, as one must be aware to avoid kinking the vein within the mastectomy pocket during shaping. This set up is shown in Figure 9.18. As previously alluded to, a large SIEV may be a sign that venous drainage of a DIEP flap or even a TRAM flap may have insufficient venous drainage through the deep venous system. The superficial vein, if larger than 1.5 mm, should therefore be dissected out to preserve length as it may be used either as the primary venous drainage system, as a second venous anastomosis,16,36 or as a venous supercharge to augment drainage in Zone III of a DIEP flap which shows signs of venous congestion across the midline.37
Fat necrosis
Reports in the literature of fat necrosis range from 6 to 18% in DIEP flaps6,38 (compared with rates of 2.3–16% in TRAM flaps11,39 and 5.1–13.5% in pedicled TRAM flaps11,40). Fat necrosis can be minimized by any strategy that ensures the best vascularized portion of the lower abdominal flap is used for the breast reconstruction. Therefore, the portion of the flap that is used for the breast reconstruction should be centered around the dominant perforator to provide the best flap perfusion and avoid fat necrosis.27 As described in the vascular anatomy, a lateral perforator perfuses Zones I and II best with variable extension past the midline, whereas a medial perforator has better perfusion across the midline. Only the portion of the flap that appears to have good perfusion in the abdomen in situ should be transferred for the breast reconstruction, because there is no guarantee that perfusion will be any better once it is anastomosed. The part of the flap that has the poorest perfusion is the sub-Scarpal fat, so that when defatting for shaping and tapering the flap around the edges of the reconstruction is carried out, the deeper fat should be removed rather than the dermis and more superficial fat. If the entire flap is too thick to match the contralateral breast it is best to leave sculpting to second stage revisionary surgery with liposuction and some direct excision as opposed to aggressive immediate defatting. In addition, flaps which appear to have marginal venous drainage are likely to have some fat necrosis so that anything that needs to be done to improve drainage such as a second vein or secondary drainage system anastomosis should be considered. Finally, longer flap harvest time has been correlated with greater incidence of fat necrosis.41 This could be indicative of a more difficult dissection, or it could result from increased desiccation of, or traction injury to, the perforator.
Flap failure
Total flap loss rates are 0–4% in DIEP flaps,6,38,42 0–4.3% in TRAM flaps,11,39 0–3% in pedicled TRAM flaps,39,43 and 7% in SIEA flaps.44,45 Apart from technical proficiency in microvascular anastomoses, there are other technical considerations specific to DIEP flaps that can be used to avoid flap loss. These include avoidance of overstretching or desiccation of the delicate perforators, ligation of side branches 1–2 mm away from the main pedicle in order to avoid damage to the vessels, liberal use of bipolar cautery in the dissection of the muscle from the pedicle to avoid avulsion or transection of tiny muscular branches that again can lead to damage or spasm of the vessel, and to pay particular attention to vessel orientation to make sure there is no kinking. Most often vascular thrombosis is a result of a geometry problem as opposed to an anastomotic failure. In particular, the point of entry of the perforator into the subcutaneous tissue can twist because it doesn’t have muscle helping to hold it in proper position. Any twist can be amplified by the weight of the flap upon inset. When more than one perforator is used in a DIEP flap, this can happen more frequently because often one perforator will be longer than the other, so that the shorter perforator causes the longer perforator to fold back or kink on itself if care is not taken to look at the lie of the vessels and ensure that there is just a gentle curve to the vessels as they are draped under the flap. Finally, if paralysis is not maintained during the dissection, this can cause avulsion of the perforator where it is entering into the flap if there is sudden muscle movement or if the patient bucks if they become light under the anesthetic.
Abdominal complications in the presence of prior surgical scars
Subcostal Kocher incisions confer an increased risk of abdominal skin necrosis46,47 so that although these incisions do not interfere with the flap blood supply, they have an impact on the vascularity of the remaining abdominal wall bridge that remains between that scar and the superior edge of the flap donor site. Strategies that can be employed in an attempt to decrease abdominal necrosis rely on leaving an adequate bridge of skin below the scar with minimal undermining laterally and immediately inferior to it. To maximize the width of the skin bridge, the donor site can be positioned asymmetrically on the lower abdomen, with the horizontal axis of the flap tilted obliquely downward on the right (Kocher) side of the abdomen and raised superiorly on the contralateral left abdomen. The resulting oblique abdominal donor site scar can then be revised at a second surgery.28
Postoperative Care
The author’s preference is to use a semiocclusive transparent dressing over the flap and its incisions as a simple postoperative dressing that alleviates the need for frequent dressing changes and still allows for assessment of clinical monitoring and Doppler through the dressing. This is shown in Figure 9.19.
Summary
While there is good documentation that hernia rates for DIEP flaps (0–4.1%)38,42,48 are lower than for either free TRAM (3–10%)39,49 or pedicled TRAM flaps (1–15.6%),50,51 the long-term functional consequences of taking tissue from the lower abdomen have been more difficult to determine because of the lack of well-controlled validated assessments comparing various forms of flap donor sites. One recent study comparing patient-perceived abdominal function in those undergoing DIEP flaps compared specifically with muscle-sparing TRAM flaps failed to show any difference.52 In addition, DIEP flaps whose perforators have a transverse intramuscular component may require division of some muscle fibers53 and this may result in postoperative functionality similar to that of a muscle-sparing free TRAM flap. However this is still theoretical and speculative, and data are still required to make definitive conclusions.
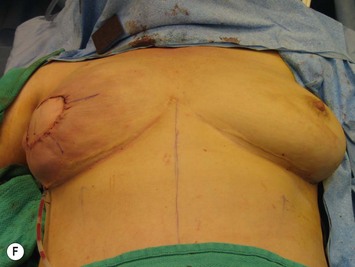
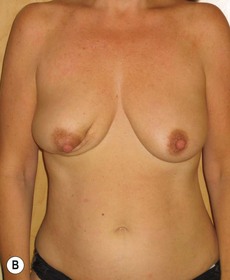
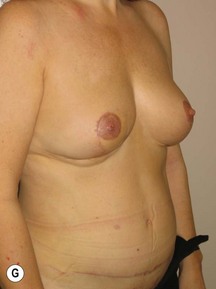
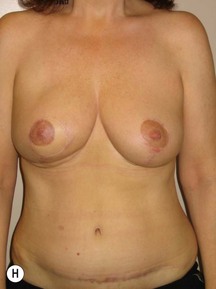
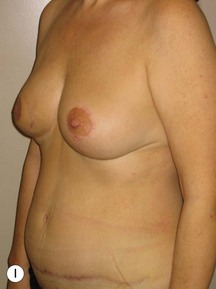
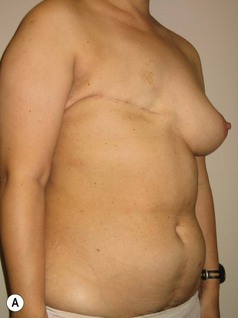
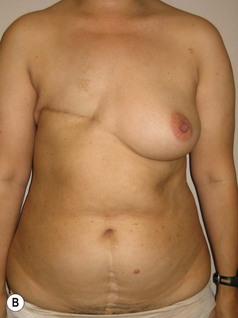
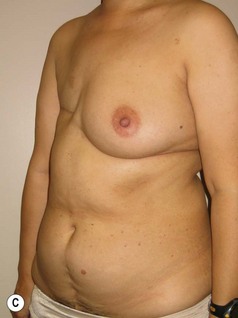
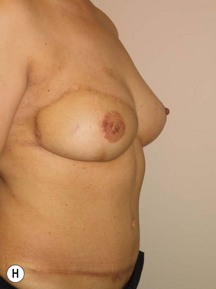
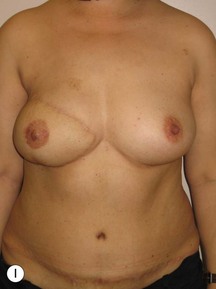
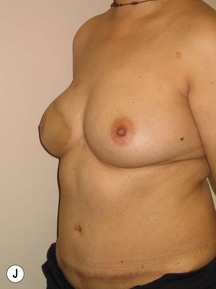
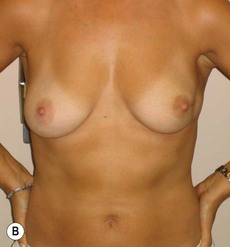
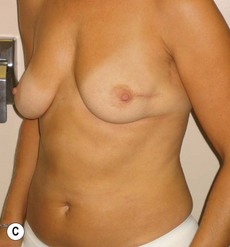
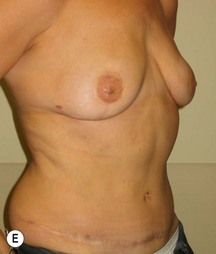
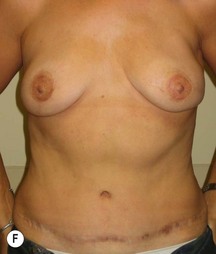
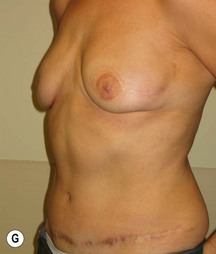
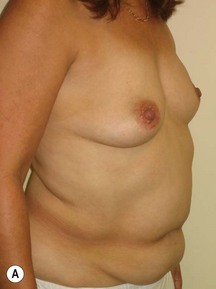
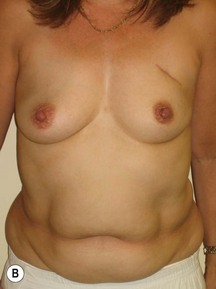
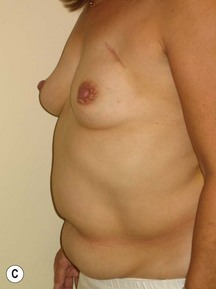
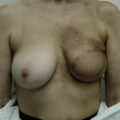
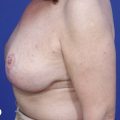
In any event, the DIEP flap – although it does have a learning curve for its intraoperative decision making – can be carried out with great success to create an aesthetic breast reconstruction with minimal donor site morbidity. Additional cases are shown in Figures 9.20 and 9.21. Patient factors such as tissue volume requirements, smoking status, BMI, and prior surgery must be taken into account in order to optimize patient outcomes.
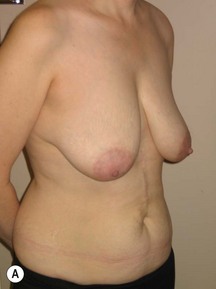
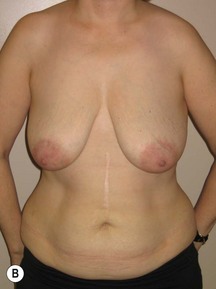
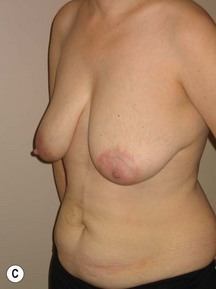
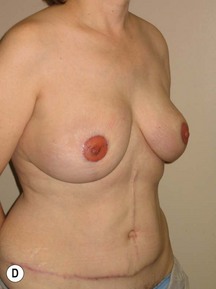
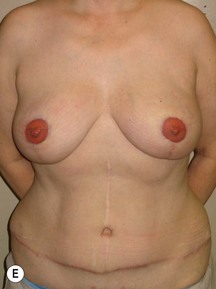
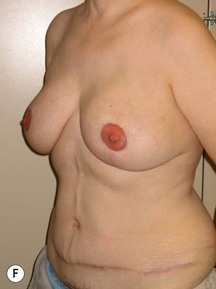
Fig. 9.20 Aesthetic reconstruction for bilateral prophylactic mastectomy in a BRCA2 gene mutation carrier.
(With permission, J.E. Lipa.)
1 Holmstrom H. The free abdominoplasty flap and its use in breast reconstruction. An experimental study and clinical case report. Scand J Plast Reconstr Surg. 1979;13(3):423-427.
2 Robbins TH. Rectus abdominis myocutaneous flap for breast reconstruction. Aust N Z J Surg. Oct 1979;49(5):527-530.
3 Koshima I, Soeda S. Inferior epigastric artery skin flaps without rectus abdominis muscle. Br J Plast Surg. 1989;42(6):645-648.
4 Vyas RM, Dickinson BP, Fastekjian JH, Watson JP, Dalio AL, Crisera CA. Risk factors for abdominal donor-site morbidity in free flap breast reconstruction. Plast Reconstr Surg. 2008;121(5):1519-1526.
5 Nahabedian MY, Tsangaris T, Momen B. Breast reconstruction with the DIEP flap or the muscle-sparing (MS-2) free TRAM flap: is there a difference? Plast Reconstr Surg. 2005;115(2):436-444.
6 Kroll SS, Gherardini G, Martin JE, et al. Fat necrosis in free and pedicled TRAM flaps. Plast Reconstr Surg. 1998;102(5):1502-1507.
7 Chang DW, Reece GP, Wang B, et al. Effect of smoking on complications in patients undergoing free TRAM flap breast reconstruction. Plast Reconstr Surg. 2000;105(7):2374-2380.
8 Chang DW, Wang B, Robb GL, et al. Effect of obesity on flap and donor-site complications in free transverse rectus abdominis myocutaneous flap breast reconstruction. Plast Reconstr Surg. 2000;105(5):1640-1648.
9 Scheer AS, Novak CB, Neligan PC, Lipa JE. Complications associated with breast reconstruction using a perforator flap compared with a free TRAM flap. Ann Plast Surg. 2006;56(4):355-358.
10 Mehrara BJ, Santoro TD, Arcilla E, Watson JP, Shaw WW, Da Lio AL. Complications after microvascular breast reconstruction: experience with 1195 flaps. Plast Reconstr Surg. 2006;118(5):1100-1109.
11 Padubidri AN, Yetman R, Browne E, et al. Complications of postmastectomy breast reconstructions in smokers, ex-smokers, and nonsmokers. Plast Reconstr Surg. 2001;107(2):342-349.
12 Parrett BM, Caterson SA, Tobias AM, Lee BT. DIEP flaps in women with abdominal scars: are complication rates affected? Plast Reconstr Surg. 2008;121(5):1527-1531.
13 Granzow JW, Levine JL, Chiu ES, Allen RJ. Breast reconstruction using perforator flaps. J Surg Oncol. 2006;94(6):441-454.
14 De Frene B, Van Landuyt K, Hamdi M, et al. Free DIEAP and SGAP flap breast reconstruction after abdominal/gluteal liposuction. J Plast Reconstr Aesthet Surg. 2006;59(10):1031-1036.
15 Dinner MI, Dowden RV, Scheflan M. Refinements in the use of the transverse abdominal island flap for postmastectomy reconstruction. Ann Plast Surg. 1983;11(5):362-372.
16 Blondeel PN, Arnstein M, Verstraete K, et al. Venous congestion and blood flow in free transverse rectus abdominis myocutaneous and deep inferior epigastric perforator flaps. Plast Reconstr Surg. 2000;106(6):1295-1299.
17 Taylor GI, Palmer JH. The vascular territories (angiosomes) of the body: experimental study and clinical applications. Br J Plast Surg. 1987;40(2):113-141.
18 Moon HK, Taylor GI. The vascular anatomy of rectus abdominis musculocutaneous flaps based on the deep superior epigastric system. Plast Reconstr Surg. 1988;82(5):815-832.
19 Milloy FJ, Anson BJ, McAfee DK. The rectus abdominis muscle and the epigastric arteries. Surg Gynecol Obstet. 1960;110:293-302.
20 Kikuchi N, Murakami G, Kashiwa H, Homma K, Sato TJ, Ogino T. Morphometrical study of the arterial perforators of the deep inferior epigastric perforator flap. Surg Radiol Anat. 2001;23(6):375-381.
21 Blondeel PN, Beyens G, Verhaeghe R, et al. Doppler flowmetry in the planning of perforator flaps. Br J Plast Surg. 1998;51(3):202-209.
22 Schaverien M, Saint-Cyr M, Arbique G, Brown SA. Arterial and venous anatomies of the deep inferior epigastric perforator and superficial inferior epigastric artery flaps. Plast Reconstr Surg. 2008;121(6):1909-1919.
23 Ninkovic M. Superficial inferior epigastric artery perforator flap. In: Blondeel PN, Morris SF, Hallock GG, Neligan PC, editors. Perforator flaps. Anatomy, technique, & clinical applications, Vol I. St. Louis: Quality Medical Publishing, Inc.; 2006:405-419.
24 Masia J, Clavero JA, Larranaga JR, Alomar X, Pons G, Serret P. Multidetector-row computed tomography in the planning of abdominal perforator flaps. J Plast Reconstr Aesthet Surg. 2006;59(6):594-599.
25 Hamdi M, Rebecca A. The deep inferior epigastric artery perforator flap (DIEAP) in breast reconstruction. Semin Plast Surg. 2006;20(2):95-102.
26 Kroll SS. Bilateral breast reconstruction in very thin patients with extended free TRAM flaps. Br J Plast Surg. 1998;51(7):535-537.
27 Blondeel PN. Deep inferior epigastric artery perforator flap. In: Blondeel PN, Morris SF, Hallock GG, Neligan PC, editors. Perforator flaps. anatomy, techniques & clinical applications, Vol I. St. Louis: Quality Medical Publishers, Inc.; 2006:385-403.
28 Beahm EK, Walton RL. Revision in autologous breast reconstruction: principles and approach. Clin Plast Surg. 2007;34(1):139-162.
29 Kronowitz SJ, Chang DW, Robb GL, et al. Implications of axillary sentinel lymph node biopsy in immediate autologous breast reconstruction. Plast Reconstr Surg. 2002;109(6):1888-1896.
30 Kroll SS. Fat necrosis in free transverse rectus abdominis myocutaneous and deep inferior epigastric perforator flaps. Plast Reconstr Surg. 2000;106(3):576-583.
31 Blondeel PN. One hundred free DIEP flap breast reconstructions: a personal experience. Br J Plast Surg. 1999;52(2):104-111.
32 Ng RL, Youssef A, Kronowitz SJ, Lipa JE, Potochny J, Reece GP. Technical variations of the bipedicled TRAM flap in unilateral breast reconstruction: effects of conventional versus microsurgical techniques of pedicle transfer on complication rates. Plast Reconstr Surg. 2004;114(2):374-384.
33 Beahm EK, Walton RL. The efficacy of bilateral lower abdominal free flaps for unilateral breast reconstruction. Plast Reconstr Surg. 2007;120(1):41-54.
34 Figus A, Fioramonti P, Ramakrishnan V. Stacked free SIEA/DIEP flap for unilateral breast reconstruction in a thin patient with an abdominal vertical midline scar. J Reconstr Microsurg. 2007;23(8):523-525.
35 Agarwal JP, Gottlieb LJ. Double pedicle deep inferior epigastric perforator/muscle-sparing TRAM flaps for unilateral breast reconstruction. Ann Plast Surg. 2007;58(4):359-363.
36 Villafane O, Gahankari D, Webster M. Superficial inferior epigastric vein (SIEV): ‘lifeboat’ for DIEP/TRAM flaps. Br J Plast Surg. 1999;52(7):599.
37 Wechselberger G, Schoeller T, Bauer T, Ninkovic M, Otto A, Ninkovic M. Venous superdrainage in deep inferior epigastric perforator flap breast reconstruction. Plast Reconstr Surg. 2001;108(1):162-166.
38 Hamdi M, Weiler-Mithoff EM, Webster MH. Deep inferior epigastric perforator flap in breast reconstruction: experience with the first 50 flaps. Plast Reconstr Surg. 1999;103(1):86-95.
39 Moran SL, Serletti JM. Outcome comparison between free and pedicled TRAM flap breast reconstruction in the obese patient. Plast Reconstr Surg. 2001;108(7):1954-1960.
40 Clough KB, O’Donoghue JM, Fitoussi AD, Vlastos G, Falcou MC. Prospective evaluation of late cosmetic results following breast reconstruction: II. TRAM flap reconstruction. Plast Reconstr Surg. 2001;107(7):1710-1716.
41 Figus A, Mosahebi A, Ramakrishnan V. Microcirculation in DIEP flaps: a study of the haemodynamics using laser Doppler flowmetry and lightguide reflectance spectrophotometry. J Plast Reconstr Aesthet Surg. 2006;59(6):604-612.
42 Nahabedian MY, Momen B, Galdino G, Manson PN. Breast reconstruction with the free TRAM or DIEP flap: patient selection, choice of flap, and outcome. Plast Reconstr Surg. 2002;110(2):466-475.
43 Watterson PA, Bostwick J3rd, Hester TR, Bried JT, Taylor GI. TRAM flap anatomy correlated with a 10-year clinical experience with 556 patients. Plast Reconstr Surg. 1995;95(7):1185-1194.
44 Ulusal BG, Cheng MH, Wei FC, Ho-Asjoe M, Song D. Breast reconstruction using the entire transverse abdominal adipocutaneous flap based on unilateral superficial or deep inferior epigastric vessels. Plast Reconstr Surg. 2006;117(5):1395-1403.
45 Chevray PM. Breast reconstruction with superficial inferior epigastric artery flaps: a prospective comparison with TRAM and DIEP flaps. Plast Reconstr Surg. 2004;114(5):1077-1083.
46 Losken A, Carlson GW, Jones GE, Culbertson JH, Schoemann M, Bostwick J3rd. Importance of right subcostal incisions in patients undergoing TRAM flap breast reconstruction. Ann Plast Surg. 2002;49(2):115-119.
47 Takeishi M, Shaw WW, Ahn CY, Borud LJ. TRAM flaps in patients with abdominal scars. Plast Reconstr Surg. Mar 1997;99(3):713-722.
48 Keller A. The deep inferior epigastric perforator free flap for breast reconstruction. Ann Plast Surg. 2001;46(5):474-479.
49 Kroll SS, Baldwin B. A comparison of outcomes using three different methods of breast reconstruction. Plast Reconstr Surg. 1992;90(3):455-462.
50 Hartrampf CRJr, Black PW, Beegle PHJr. Breast reconstruction following mastectomy. J Med Assoc Ga. 1987;76(5):328-334.
51 Petit JY, Rietjens M, Garusi C, et al. Abdominal complications and sequelae after breast reconstruction with pedicled TRAM flap: is there still an indication for pedicled TRAM in the year 2003? Plast Reconstr Surg. 2003;112(4):1063-1065.
52 Bajaj AK, Chevray PM, Chang DW. Comparison of donor-site complications and functional outcomes in free muscle-sparing TRAM flap and free DIEP flap breast reconstruction. Plast Reconstr Surg. 2006;117(3):737-746.
53 Rozen WM, Ashton MW, Pan WR, Taylor GI. Raising perforator flaps for breast reconstruction: the intramuscular anatomy of the deep inferior epigastric artery. Plast Reconstr Surg. 2007;120(6):1443-1449.