Chapter 15 Diencephalon
The diencephalon is part of the prosencephalon (forebrain), which develops from the foremost primary cerebral vesicle and differentiates into a caudal diencephalon and rostral telencephalon. The cerebral hemispheres develop from the sides of the telencephalon, each containing a lateral ventricle. The sites of evagination become the interventricular foramina, through which the two lateral ventricles and midline third ventricle communicate. The diencephalon corresponds largely to the structures that develop lateral to the third ventricle.
The lateral walls of the diencephalon form the epithalamus most superiorly, the thalamus centrally and the subthalamus and hypothalamus most inferiorly. The epithalamus in the mature brain contains the anterior and posterior paraventricular nuclei, the medial and lateral habenular nuclei, the stria medullaris thalami and the pineal gland. The thalamus undergoes proliferation to form numerous nuclear masses that have extensive reciprocal connections with the cerebral cortex. The subthalamic region consists of the subthalamic nucleus, zona incerta and fields of Forel. The subthalamic nucleus is closely related to the basal ganglia and is considered with them in Chapter 14. The hypothalamic rudiment gives rise to most of the subdivisions of the adult hypothalamus.
Thalamus
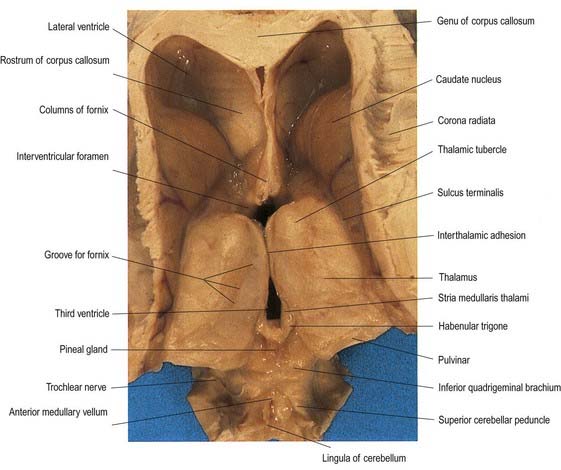
The thalamus is an ovoid nuclear mass, approximately 4 cm long, that borders the dorsal part of the third ventricle (Figs 15.1–15.3; see also Fig. 1.10). The narrow anterior pole lies close to the midline and forms the posterior boundary of the interventricular foramen. Posteriorly, an expansion, the pulvinar, extends beyond the third ventricle to overhang the superior colliculus (Fig. 15.4). The brachium of the superior colliculus (superior quadrigeminal brachium) separates the pulvinar above from the medial geniculate body below. A small oval elevation, the lateral geniculate body, lies lateral to the medial geniculate.
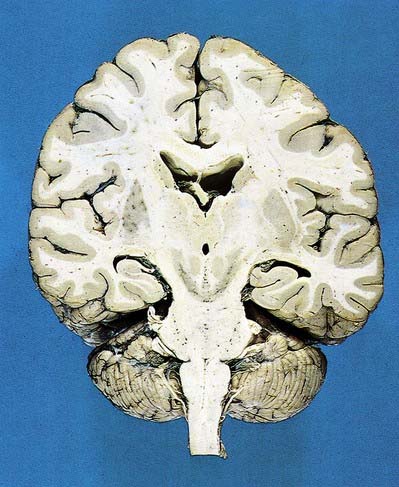
Fig. 15.1 Dorsal half of a brain sectioned in an oblique coronal plane that passes through the cerebral hemispheres, diencephalon, midbrain, pons and medulla oblongata, to show the general disposition of the main structures, some of which are labelled in Figure 15.3. Compare also with Figure 1.10.
(Photograph by Kevin Fitzpatrick on behalf of GKT School of Medicine, London.)
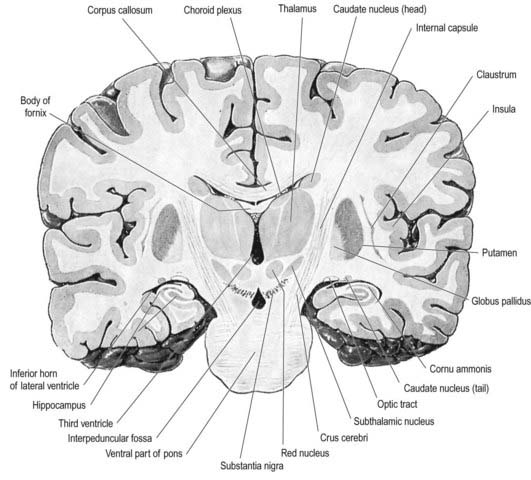
Fig. 15.3 Coronal section of the brain showing the principal parts of the diencephalon and basal ganglia. Compare with Figure 1.10.
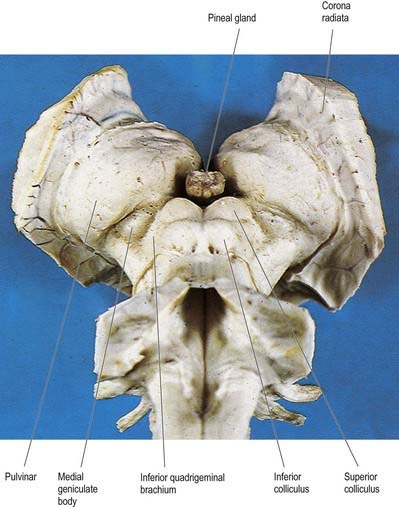
Fig. 15.4 Oblique view of the dorsal aspect of the brain stem and thalamus.
(Photograph by Kevin Fitzpatrick on behalf of GKT School of Medicine, London.)
The superior (dorsal) surface of the thalamus (see Fig. 15.2) is covered by a thin layer of white matter, the stratum zonale. It extends laterally from the line of reflection of the ependyma (taenia thalami) and forms the roof of the third ventricle. This curved surface is separated from the overlying body of the fornix by the choroid fissure, with the tela choroidea within it. More laterally, it forms part of the floor of the lateral ventricle. The lateral border of the superior surface of the thalamus is marked by the stria terminalis and the overlying thalamostriate vein, which separate the thalamus from the body of the caudate nucleus. Laterally, a slender sheet of white matter, the external medullary lamina, separates the main body of the thalamus from the reticular nucleus. Lateral to this, the thick posterior limb of the internal capsule lies between the thalamus and the lentiform complex.
The medial surface of the thalamus is the superior (dorsal) part of the lateral wall of the third ventricle (see Fig. 5.8). It is usually connected to the contralateral thalamus by an interthalamic adhesion behind the interventricular foramina. The boundary with the hypothalamus is marked by an indistinct hypothalamic sulcus, which curves from the upper end of the cerebral aqueduct to the interventricular foramen. The thalamus is continuous with the midbrain tegmentum, the subthalamus and the hypothalamus.
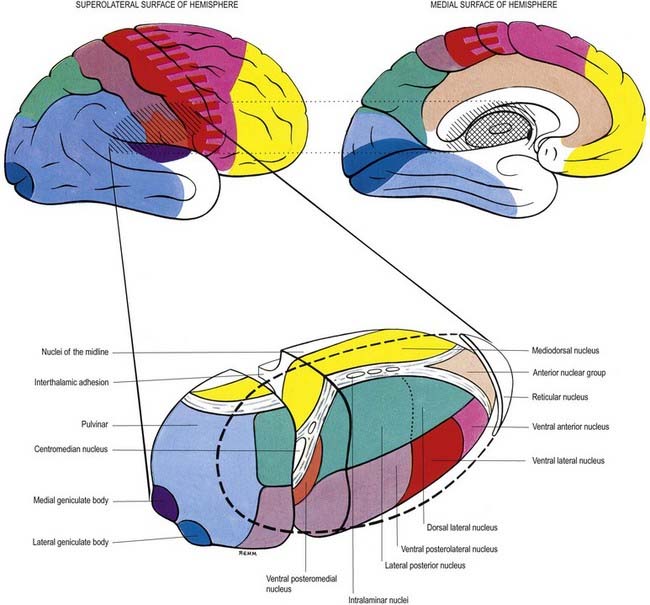
Internally, the thalamus is divided into anterior, medial and lateral nuclear groups by a vertical Y-shaped sheet of white matter, the internal medullary lamina (Figs 15.5, 15.6). In addition, intralaminar nuclei lie embedded within, and surrounded by, the internal medullary lamina. Midline nuclei either abut the ependyma of the lateral walls of the third ventricle medially or lie adjacent to, and to some extent within, the interthalamic adhesion. Reticular nuclei lie lateral to the main nuclear mass, separated from it by the external medullary lamina.
In general, thalamic nuclei both project to and receive fibres from the cerebral cortex (see Fig. 15.6). The whole cerebral cortex, not only the neocortex but also the phylogenetically older palaeocortex of the piriform lobe and archicortex of the hippocampal formation, are reciprocally connected with the thalamus. The thalamus is the major route by which subcortical neuronal activity influences the cerebral cortex, and the greatest input to most thalamic nuclei comes from the cerebral cortex.
Anterior Group of Thalamic Nuclei
The anterior group of nuclei are enclosed between the arms of the Y-shaped internal medullary lamina and underlie the anterior thalamic tubercle (see Fig. 15.2, 15.6). Three subdivisions are recognized. The largest is the anteroventral nucleus; the others are the anteromedial and anterodorsal nuclei.
The cortical targets of efferent fibres from the anterior nuclei of the thalamus lie largely on the medial surface of the hemisphere (see Fig. 15.6). They include the anterior limbic area (in front of and inferior to the corpus callosum), the cingulate gyrus and the parahippocampal gyrus (including the medial entorhinal cortex and the pre- and para-subiculum). These thalamocortical pathways are reciprocal. There also appear to be minor connections between the anterior nuclei and the dorsolateral prefrontal and posterior areas of the neocortex. The anterior thalamic nuclei are believed to be involved in the regulation of alertness and attention and in the acquisition of memory.
Medial Group of Thalamic Nuclei
The single component of this thalamic region is the mediodorsal or dorsomedial nucleus, which is particularly large in humans. Laterally, it is limited by the internal medullary lamina and intralaminar nuclei (see Figs. 15.5, 15.6). Medially, it abuts the midline parataenial and reuniens (medioventral) nuclei. It can be divided into anteromedial magnocellular and posterolateral parvocellular parts.
The larger posterolateral parvocellular division connects reciprocally with the dorsolateral and dorsomedial prefrontal cortex, the anterior cingulate gyrus and the supplementary motor area. In addition, efferent fibres pass to the posterior parietal cortex.
Lateral Group of Thalamic Nuclei
The lateral nuclear complex, lying lateral to the internal medullary lamina, is the largest major division of the thalamus (see Fig. 15.6). It is divided into dorsal and ventral tiers of nuclei. The lateral dorsal nucleus, lateral posterior nucleus and pulvinar all lie dorsally. The lateral and medial geniculate nuclei lie inferior to the pulvinar, near the posterior pole of the thalamus. The ventral tier nuclei are the ventral anterior, ventral lateral and ventral posterior nuclei.
Ventral Anterior Nucleus
The ventral anterior (VA) complex lies at the anterior pole of the ventral nuclear group. It is limited anteriorly by the reticular nucleus and posteriorly by the ventral lateral nucleus, and it lies between the external and internal medullary laminae. It consists of a principal part (VApc) and a magnocellular part (VAmc). The subcortical connections to this region are largely ipsilateral from the internal segment of the globus pallidus and the pars reticulata of the substantia nigra. The terminal fields from these origins do not overlap. Fibres from the globus pallidus end in VApc. The substantia nigra projects to VAmc. Corticothalamic fibres from premotor cortex (area 6) terminate in VApc, and fibres from the frontal eye field (area 8) terminate in VAmc. The VA thalamus does not appear to receive fibres directly from the motor cortex. The efferent projections from VA are incompletely known. Some pass to intralaminar thalamic nuclei, and others project to widespread regions of the frontal lobe and to the anterior parietal cortex. Their functions are unclear. The VA thalamus appears to play a central role in the transmission of the cortical ‘recruiting response,’ a phenomenon in which stimulation of the thalamus can initiate long-lasting, high-voltage repetitive negative electrical waves over much of the cerebral cortex.
Ventral Posterior Nucleus
There is a well-ordered topographical representation of the body in the VP nucleus. The VPl is organized so that sacral segments are represented laterally and cervical segments medially. The latter abut the face area of representation (trigeminal territory) in VPm. Taste fibres synapse anteriorly and ventromedially within the VPl nucleus.
Medial Geniculate Nucleus
The medial geniculate nucleus, which is a part of the auditory pathway (Ch. 12), is located within the medial geniculate body, a rounded elevation situated posteriorly on the ventrolateral surface of the thalamus and separated from the pulvinar by the superior quadrigeminal brachium. It receives fibres travelling in the inferior quadrigeminal brachium. Three major subnuclei—medial, ventral and dorsal—are recognized within it. The inferior brachium separates the medial (magnocellular) nucleus, which consists of sparse, deeply staining neurones, from the lateral nucleus, which is made up of medium-sized, densely packed and darkly staining cells. The dorsal nucleus overlies the ventral nucleus and expands posteriorly; therefore, it is sometimes known as the posterior nucleus of the medial geniculate. It contains small to medium-sized, pale-staining cells, which are less densely packed than those of the lateral nucleus. The ventral nucleus receives fibres from the central nucleus of the ipsilateral inferior colliculus via the inferior quadrigeminal brachium and also from the contralateral inferior colliculus. The nucleus contains a complete tonotopic representation. Low-pitched sounds are represented laterally, and progressively higher-pitched sounds are encountered as the nucleus is traversed from lateral to medial. The dorsal nucleus receives afferents from the pericentral nucleus of the inferior colliculus and from other brain stem nuclei of the auditory pathway. A tonotopic representation has not been described in this subdivision, and cells within the dorsal nucleus respond to a broad range of frequencies. The magnocellular medial nucleus receives fibres from the inferior colliculus and from the deep layers of the superior colliculus. Neurones within the magnocellular subdivision may respond to modalities other than sound. However, many cells respond to auditory stimuli, usually to a wider range of frequencies than do neurones in the ventral nucleus. Many units show evidence of binaural interaction, with the leading effect arising from stimuli in the contralateral cochlea. The ventral nucleus projects primarily to the primary auditory cortex. The dorsal nucleus projects to auditory areas surrounding the primary auditory cortex. The magnocellular division projects diffusely to auditory areas of the cortex and to adjacent insular and opercular fields.
Lateral Geniculate Nucleus
The lateral geniculate body, which is part of the visual pathway (Ch. 12), is a small ovoid ventral projection from the posterior thalamus (Fig. 15.7). The superior quadrigeminal brachium enters the posteromedial part of the lateral geniculate body dorsally, lying between the medial geniculate body and the pulvinar.
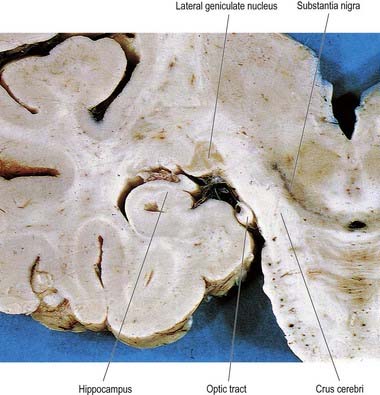
Fig. 15.7 Coronal section through the brain showing the lateral geniculate nucleus.
(Photograph by Kevin Fitzpatrick on behalf of GKT School of Medicine, London.)
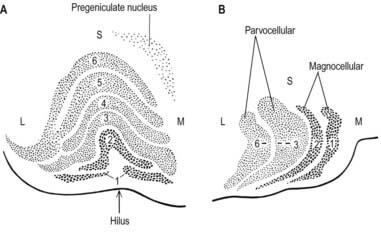
The lateral geniculate nucleus is an inverted, somewhat flattened U-shaped nucleus and is laminated. Its internal organization is usually described on the basis of six laminae, although seven or eight may be present. The laminae are numbered 1 to 6, from the innermost ventral to the outermost dorsal (Fig. 15.8). Laminae 1 and 2 consist of large cells, the magnocellular layers, whereas layers 4 to 6 have smaller neurones, the parvocellular laminae. The apparent gaps between laminae are called the interlaminar zones. Most ventrally, an additional superficial, or S, lamina is recognized.
The lateral geniculate nucleus is organized in a visuotopic manner and contains a precise map of the contralateral visual field. The vertical meridian is represented posteriorly, the peripheral anteriorly, the upper field laterally, and the lower field medially (Ch. 12). Similar precise point-to-point representation is also found in the projection of the lateral geniculate nucleus to the visual cortex. Radially arranged inverted pyramids of neurones in all laminae respond to a single small area of the contralateral visual field and project to a circumscribed area of cortex. The termination of geniculocortical axons in the visual cortex is considered in detail in Chapter 16.
Intralaminar Nuclei
The intralaminar nuclei are collections of neurones within the internal medullary lamina of the thalamus. Two groups of nuclei are recognized. The anterior (rostral) group is subdivided into central medial, paracentral and central lateral nuclei. The posterior (caudal) intralaminar group consists of the centromedian and parafascicular nuclei. The designations central medial and centromedian can lead to confusion, but they are an accepted part of the terminology of thalamic nuclei in common usage. The centromedian nucleus is much larger, is considerably expanded in humans in comparison with other species and is importantly related to the globus pallidus, deep cerebellar nuclei and motor cortex. Anteriorly, the internal medullary lamina separates the mediodorsal nucleus from the ventral lateral complex. It is occupied by the paracentral nucleus laterally and the central medial nucleus ventromedially, as the two laminae converge toward the midline. A little more posteriorly, the central lateral nucleus appears dorsally in the lamina as the latter splits to enclose the lateral dorsal nucleus. More posteriorly, at the level of the ventral posterior nucleus, the lamina splits to enclose the ovoid centromedian nucleus. The smaller parafascicular nucleus lies more medially.
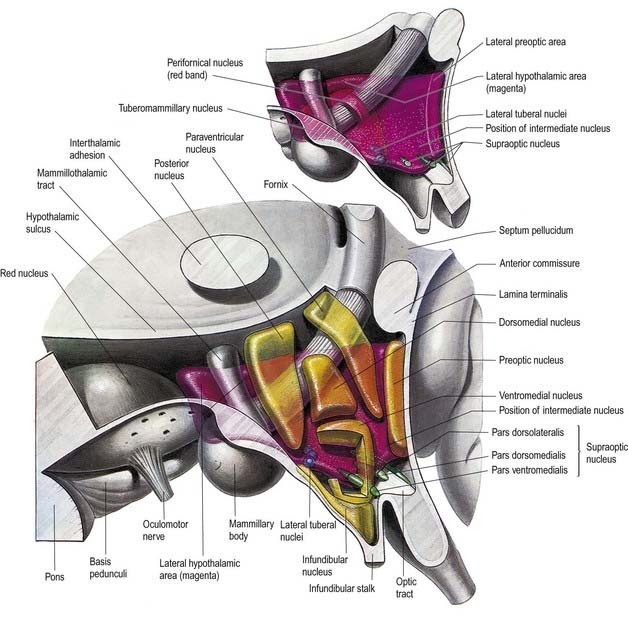
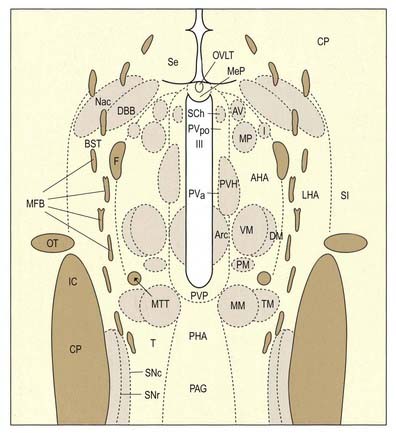
Hypothalamus
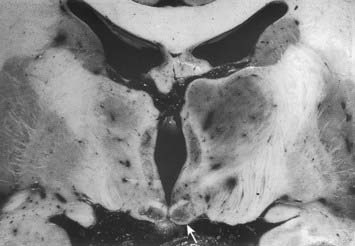
The hypothalamus extends from the lamina terminalis to a vertical plane posterior to the mammillary bodies, and from the hypothalamic sulcus to the base of the brain beneath the third ventricle. It lies beneath the thalamus and anterior to the tegmental part of the subthalamus and the mesencephalic tegmentum (see Figs 15.5, 5.8, 15.10). Laterally, it is bordered by the anterior part of the subthalamus, internal capsule and optic tract. Structures in the floor of the third ventricle reach the pial surface in the interpeduncular fossa (Fig. 15.9). From anterior to posterior, they are the optic chiasma, tuber cinereum, tuberal eminences and infundibular stalk, mammillary bodies and posterior perforated substance. The last lies in the interval between the diverging crura cerebri, pierced by small central branches of posterior cerebral arteries. Within it is the small interpeduncular nucleus, which receives terminals of the fasciculus retroflexus of both sides and has other connections with the mesencephalic reticular formation and mammillary bodies.
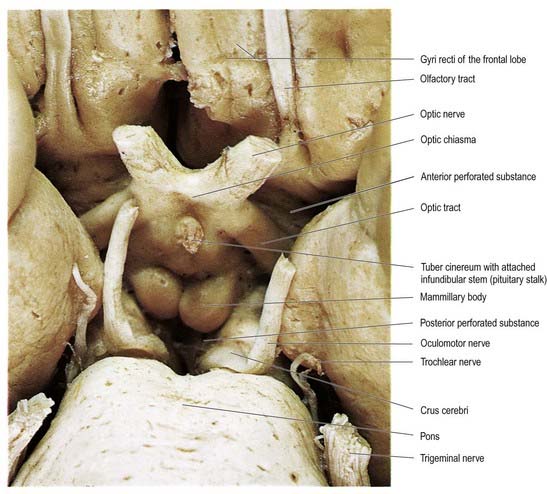
Fig. 15.9 Interpeduncular fossa and surrounding structures.
(Photograph by Kevin Fitzpatrick on behalf of GKT School of Medicine, London.)
Stimulation of the anterior hypothalamus and paraventricular nucleus can cause decreased blood pressure and decreased heart rate. Stimulation in the anterior hypothalamus induces sweating and vasodilatation (and thus heat loss) via projections that pass through the medial forebrain bundle to autonomic centres in the brain stem and cord. Damage to the anterior hypothalamus (e.g. during surgery for suprasellar extensions of pituitary tumours) can result in an uncontrollable rise in body temperature. Projections to the ventromedial hypothalamus conjointly regulate food intake. Stimulation in the posterior part of the hypothalamus induces sympathetic arousal with vasoconstriction, piloerection, shivering and increased metabolic heat production. Circuitry mediating shivering is located in the dorsomedial posterior hypothalamus. This does not imply the existence of discrete parasympathetic and sympathetic ‘centres.’ Stimuli in many different parts of the hypothalamus can cause profound changes in heart rate, cardiac output, vasomotor tone, peripheral resistance, differential bloodflow in organs and limbs, frequency and depth of respiration, motility and secretion in the alimentary tract, erection and ejaculation.
Hypothalamic Nuclei
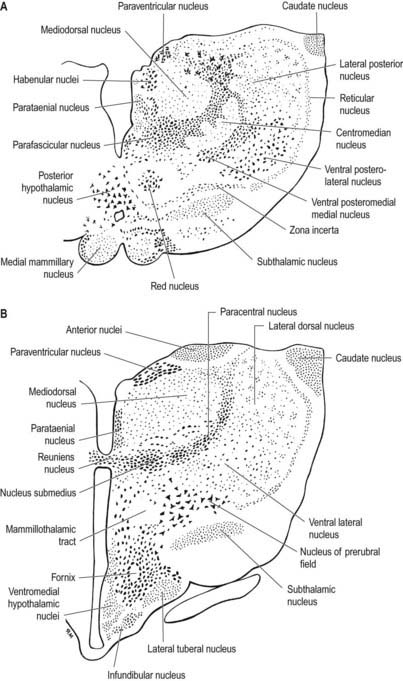
The hypothalamus can be divided anteroposteriorly into chiasmatic (supraoptic), tuberal (infundibulo-tuberal) and posterior (mammillary) regions and mediolaterally into periventricular, intermediate (medial) and lateral zones. Between the intermediate and lateral zones is a paramedian plane that contains the prominent myelinated fibres of the column of the fornix, the mammillothalamic tract and the fasciculus retroflexus. For this reason, some authors group the periventricular and intermediate zones as a single medial zone. These divisions are artificial, and functional systems cross them. The main nuclear groups and myelinated tracts are illustrated in Figures 15.10 and 15.11.
CASE 2 Wernicke’s Encephalopathy
Discussion: This man exhibits a classic Wernicke-Korsakoff syndrome due to thiamine deficiency. Anatomically, the ophthalmoparesis reflects reversible lesions involving the brain stem oculomotor complex bilaterally in the midbrain or pons, and the nystagmus is due to lesions involving the vestibular nuclei. The truncal ataxia is related to observed lesions involving the superior vermis of the cerebellum. The anatomical substrate of the memory defect, the Korsakoff component of the disorder, in all likelihood reflects bilateral subtotal tissue necrosis, which may be haemorrhagic, involving the mammillary bodies or thalamus (Fig. 15.12). Despite vigorous therapy with thiamine, the memory problem may persist indefinitely, posing a major functional disability.
Suprachiasmatic Nucleus
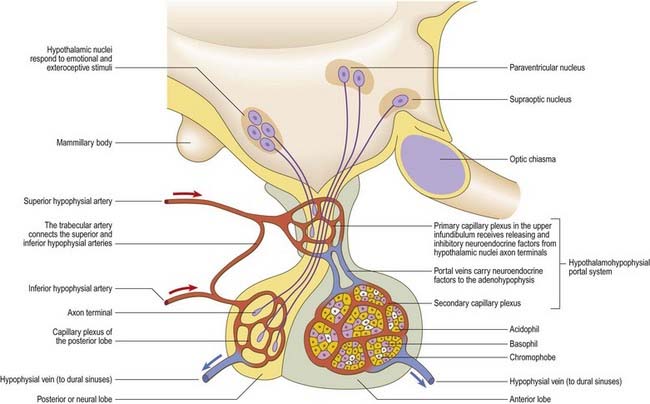
Parvocellular neurosecretory neurones lie within the periventricular zone, in particular the medial parvocellular part of the paraventricular nucleus and the arcuate nucleus. The arcuate nucleus is median in the postinfundibular part of the tuber cinereum. It extends forward into the median eminence and almost encircles the infundibular base but does not meet anteriorly, where the infundibulum adjoins the median part of the optic chiasma. Its numerous neurones are all small and round in coronal section, and oval or fusiform in sagittal section. No glial layer intervenes between the nucleus and the ependymal tanycytes lining the infundibular recess of the third ventricle. Circadian variation in the secretion of all anterior pituitary hormones suggests that projections from the suprachiasmatic nucleus must reach parvocellular neurosecretory neurones. Afferents from the limbic system probably mediate the widespread effects of stress, and serotonin and noradrenaline from the brain stem influence the output of most anterior pituitary hormones. The axons of parvocellular neurones converge on the infundibulum, forming a tubero-infundibular tract, which ends on the capillary loops that form the hypophysial portal vessels.
In addition to these peptide-containing cells, dopamine neurones in the arcuate nucleus (A12 group) have terminals in the median eminence and infundibulum. Dopamine acts as the principal prolactin–release inhibiting hormone and also inhibits the secretion of TSH (likewise, TSH acts as a prolactin-releasing hormone). Noradrenergic terminals are found in the median eminence, where they may act largely in a paracrine manner.
Supraoptic and Paraventricular Nuclei
The paraventricular nucleus extends from the hypothalamic sulcus downward across the medial aspect of the column of the fornix, its ventrolateral angle reaching toward the supraoptic nucleus. Its neurones are more diverse. Magnocellular neurones, which project to the neurohypophysis, tend to lie laterally; parvocellular neurones, which project to the median eminence and infundibulum, lie more medially; and intermediate-sized neurones, which may project caudally, lie posteriorly. The axons of the paraventricular magnocellular neurones pass toward the supraoptic nucleus (paraventriculo-hypophysial tract), where they join axons of supraoptic neurones to form a supraoptico-hypophysial tract. This runs down the infundibulum, superficially, and into the neural lobe, where the axons are distended and branch repeatedly around the capillaries. Vasopressin and oxytocin are produced by separate neurones. Vasopressin neurones tend to cluster in the ventrolateral part of the paraventricular nucleus, and oxytocin cells lie around them.
Connections of the Hypothalamus
Afferent Connections
The hypothalamus receives visceral, gustatory and somatic sensory information from the spinal cord and brain stem. It receives largely polysynaptic projections from the nucleus tractus solitarius, probably directly and indirectly via the parabrachial nucleus and medullary noradrenergic cell groups (ventral noradrenergic bundle); collaterals of lemniscal somatic afferents (to the lateral hypothalamus); and projections from the dorsal longitudinal reticular formation. Many enter via the medial forebrain bundle (see Fig. 15.11) and periventricular fibre system. Others converge in the midbrain tegmentum, forming the mammillary peduncle to the mammillary body.
The medial forebrain bundle is a loose grouping of fibre pathways that mostly run longitudinally through the lateral hypothalamus (see Fig. 15.11). It connects forebrain autonomic and limbic structures with the hypothalamus and brain stem, receiving and giving small fascicles throughout its course. It contains descending hypothalamic afferents from the septal area and orbitofrontal cortex, ascending afferents from the brain stem and efferents from the hypothalamus.
Efferent Connections
Hypothalamic neurones projecting to autonomic neurones are found in the paraventricular nucleus (oxytocin and vasopressin neurones), perifornical and dorsomedial nuclei (atrial natriuretic peptide neurones), lateral hypothalamic area (α-melanocyte-stimulating hormone neurones) and zona incerta (dopamine neurones). These fibres run through the medial forebrain bundle into the tegmentum, ventrolateral medulla and dorsal lateral funiculus of the spinal cord. In the brain stem, fibres innervate the parabrachial nucleus, nucleus ambiguus, nucleus of the solitary tract and dorsal motor nucleus of the vagus. In the spinal cord, they end on sympathetic and parasympathetic preganglionic neurones in the intermediolateral column. Both oxytocin- and vasopressin-containing fibres can be traced to the most caudal spinal autonomic neurones.
The medial mammillary nucleus gives rise to a large ascending fibre bundle that diverges into mammillothalamic and mammillotegmental tracts (see Fig. 15.11). The mammillothalamic tract ascends through the lateral hypothalamus to reach the anterior thalamic nuclei, where massive projections radiate to the cingulate gyrus. The mammillotegmental tract curves inferiorly into the midbrain, ventral to the medial longitudinal fasciculus, and is distributed to the tegmental reticular nuclei.
Pituitary Gland
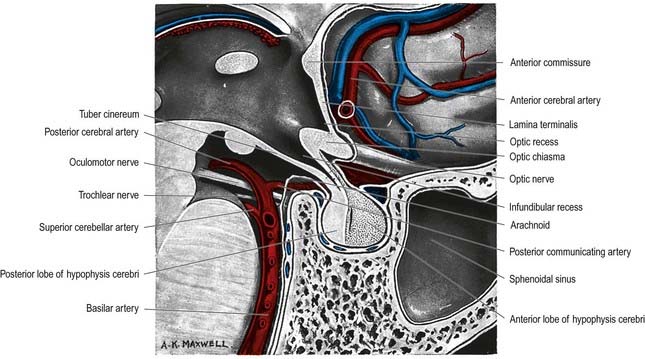
The pituitary gland, or hypophysis cerebri, is a reddish grey ovoid body approximately 12 mm in transverse diameter and 8 mm in anteroposterior diameter, weighing approximately 500 mg (see Fig. 5.8; Fig. 15.13). It is continuous with the infundibulum, a hollow, conical inferior process from the tuber cinereum of the hypothalamus. It lies within the pituitary fossa of the sphenoid bone, where it is covered superiorly by a circular diaphragma sellae of dura mater. The latter is pierced centrally by an aperture for the infundibulum and separates the anterior superior aspect of the pituitary from the optic chiasma. The pituitary is flanked by the cavernous sinuses and their contents. Inferiorly, it is separated from the floor of the pituitary fossa by a venous sinus that communicates with the circular sinus. The meninges blend with the pituitary capsule and are not separate layers.
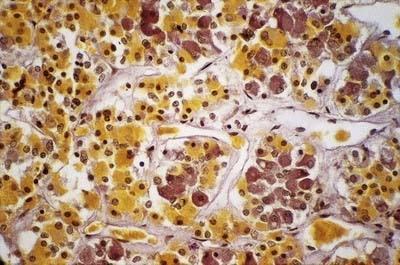
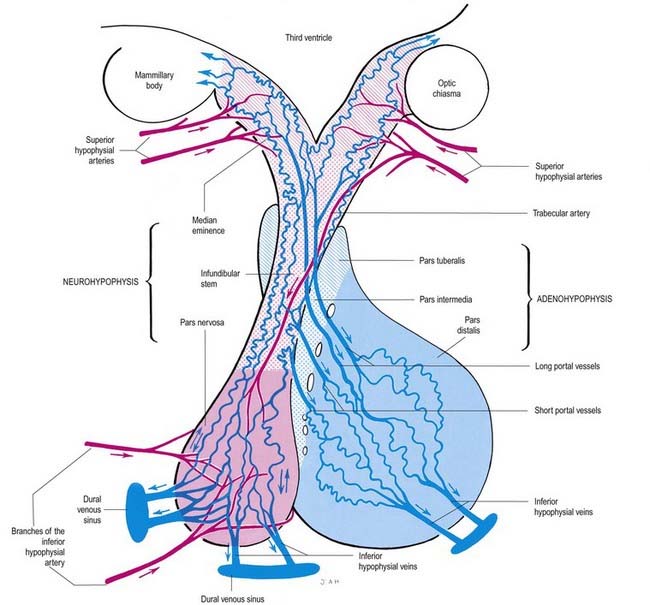
The pituitary has two major parts—neurohypophysis and adenohypophysis—which differ in their origin, structure and function. The neurohypophysis is a diencephalic downgrowth connected with the hypothalamus. The adenohypophysis is an ectodermal derivative of the stomatodeum. Both include parts of the infundibulum (whereas the older terms ‘anterior lobe’ and ‘posterior lobe’ do not). The infundibulum has a central infundibular stem that contains neural hypophysial connections and is continuous with the median eminence of the tuber cinereum. Thus, the neurohypophysis includes the median eminence, infundibular stem and neural lobe or pars posterior. Surrounding the infundibular stem is the pars tuberalis, a component of the adenohypophysis. The main mass of the adenohypophysis can be divided into the pars anterior (pars distalis) and the pars intermedia, which are separated in fetal and early postnatal life by the hypophysial cleft, a vestige of Rathke’s pouch, from which it develops. Although usually obliterated in childhood, remnants may persist in the form of cystic cavities near the adenoneurohypophysial frontier, sometimes invading the neural lobe. The human pars intermedia is rudimentary. It may be partially displaced into the neural lobe, so it has been included in the anterior and posterior parts by different observers. Apart from this equivocation, which is of little significance in view of the exiguous status of the human pars intermedia, the pars anterior and pars posterior can be equated with the anterior and posterior lobes. When the associated infundibular parts continuous with these lobes are included, the names adenohypophysis and neurohypophysis become appropriate and are used here as follows: neurohypophysis includes the pars posterior (pars nervosa, posterior or neural lobe), infundibular stem and median eminence; adenohypophysis includes the pars anterior (pars distalis or glandularis), pars intermedia and pars tuberalis.
Neurohypophysis
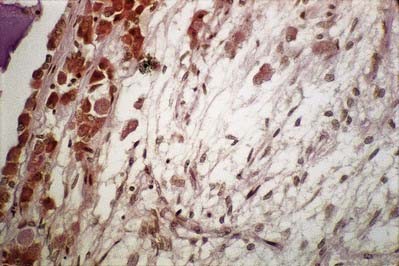
The neurohormones stored in the main part of the neurohypophysis are vasopressin (antidiuretic hormone), which controls reabsorption of water by renal tubules, and oxytocin, which promotes the contraction of uterine smooth muscle in childbirth and the ejection of milk from the breast during lactation. Storage granules containing active hormone polypeptides bound to a transport glycoprotein, neurophysin, pass down axons from their site of synthesis in the neuronal somata. The granules are seen as swellings along the axons and at their terminals, which can reach the size of erythrocytes (Fig. 15.14).
The thin, non-myelinated axons of the neurohypophysis are ensheathed by typical astrocytes in the infundibulum (Figs 15.15, 15.16). Near the posterior lobe, astrocytes are replaced by pituicytes, which constitute most of the non-excitable tissue in the neurohypophysis. Pituicytes are dendritic neuroglial cells of variable appearance, often with long processes running parallel to adjacent axons. Typically, their cytoplasmic processes end on the walls of capillaries and sinusoids between nerve terminals. Axons also end in perivascular spaces. Although they are close to the walls of sinusoids, they remain separated from them by two basal laminae—one around the nerve endings, and the other underlying the fenestrated endothelial cells. Fine collagen fibrils occupy the spaces between basal laminae.
Adenohypophysis
The adenohypophysis is highly vascular. It consists of epithelial cells of varying size and shape arranged in cords or irregular follicles, between which lie thin-walled vascular sinusoids supported by a delicate reticular connective tissue (see Figs 15.14–15.16). Most of the hormones synthesized by the adenohypophysis are trophic. They include the peptides; GH, involved in the control of body growth; and prolactin, which stimulates both the growth of breast tissue and milk secretion. Glycoprotein trophic hormones are the large pro-opiomelanocortin precursor of ACTH, which controls the secretion of certain suprarenal cortical hormones; TSH; FSH, which stimulates the growth and secretion of oestrogens in ovarian follicles and spermatogenesis (acting on testicular Sertoli cells); and LH, which induces progesterone secretion by the corpus luteum and testosterone synthesis by Leydig cells in the testis. Pro-opiomelanocortin is cleaved into a number of different molecules, including ACTH. β-Lipotropin is released from the pituitary, but its lipolytic function in humans is uncertain. β-Endorphin is another cleavage product released from the pituitary.
A small collection of adenohypophysial tissue lies in the mucoperiosteum of the human nasopharyngeal roof. By 28 weeks in utero, it is well vascularized and capable of secretion, receiving blood from the systemic vessels of the nasopharyngeal roof. At this stage, it is covered posteriorly by fibrous tissue. This is replaced in the second half of fetal life by venous sinuses, and a transsphenoidal portal venous system develops, bringing the nasopharyngeal tissue under the same hypothalamic control as the cranial adenohypophysial tissue. The peripheral vascularity of the pharyngeal hypophysis persists until about the fifth year. The organ is then reinvested by fibrous tissue and is presumed to be controlled again by factors present in systemic blood. Although it does not change in size after birth in males, in females it becomes smaller, returning to natal volume during the fifth decade, when it may once again be controlled by a transsphenoidal extension of the hypothalamohypophysial portal venous system. The human pharyngeal hypophysis may be a reserve of potential adenohypophysial tissue, which may be stimulated, particularly in females, to synthesize and secrete adenohypophysial hormones in middle age, when intracranial adenohypophysial tissue begins to fail.
Vessels of the Pituitary
The arteries of the pituitary arise from the internal carotid arteries via a single inferior and several superior hypophysial arteries on each side (Fig. 15.17). The former comes from the cavernous part of the internal carotid artery, and the latter come from its supraclinoid part and from the anterior and posterior cerebral arteries. The inferior hypophysial artery divides into medial and lateral branches, which anastomose across the midline and form an arterial ring around the infundibulum. Fine branches from this circular anastomosis enter the neurohypophysis to supply its capillary bed. The superior hypophysial arteries supply the median eminence, upper infundibulum and, via arteries of the trabeculae, lower infundibulum. A confluent capillary net, extending through the neurohypophysis, is supplied by both sets of hypophysial vessels. Reversal of flow can occur in cerebral capillary beds lying between the two supplies.
The arteries of the median eminence and infundibulum end in characteristic sprays of capillaries, which are most complex in the upper infundibulum. In the median eminence, these form an external or ‘mantle’ plexus and an internal or ‘deep’ plexus. The external plexus, fed by the superior hypophysial arteries, is continuous with the infundibular plexus and is drained by long portal vessels that descend to the pars anterior. The internal plexus lies within and is supplied by the external plexus. It is continuous posteriorly with the infundibular capillary bed and, like the external plexus, is drained by long portal vessels. Short portal vessels run from the lower infundibulum to the pars anterior. Both types of portal vessels open into vascular sinusoids, which lie between the secretory cords in the adenohypophysis and provide most of its blood. There is no direct arterial supply. The portal system carries hormone-releasing factors, probably elaborated in parvocellular groups of hypothalamic neurones, and these control the secretory cycles of cells in the pars anterior. The pars intermedia appears to be avascular.
Subthalamus
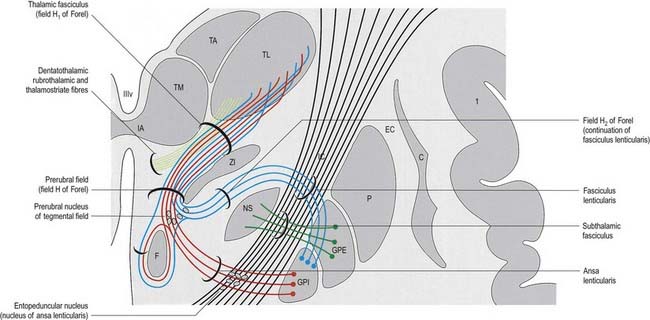
The subthalamus is a complex region of nuclear groups and fibre tracts (Fig. 15.18). The main nuclear groups are the subthalamic nucleus, the reticular nucleus, the zona incerta, the fields of Forel and the pregeniculate nucleus. The rostral poles of the red nucleus and substantia nigra also extend into this area.
Subthalamic Nucleus
The subthalamic nucleus is intimately connected with the basal ganglia and is considered with them in Chapter 14.
Zona Incerta and Fields of Forel
The zona incerta is an aggregation of small cells that lies between the ventral part of the external medullary lamina of the thalamus and the cerebral peduncle. It is linked to the reticular nucleus dorsolaterally. More medially is a scattered group of cells in a matrix of fibres known as the H field of Forel. Field H1 of Forel consists of the thalamic fasciculus, which lies dorsal to the zona incerta. Field H2 of Forel contains the fasciculus lenticularis and lies ventrally, between the zona incerta and the subthalamic nucleus (see Fig. 15.18).
In addition to terminal parts of the lemniscal, dentatothalamic and rubrothalamic tracts, the subthalamus contains massive fibre tracts derived from the globus pallidus. The fasciculus lenticularis is the dorsal component of pallidofugal fibres that traverse the internal capsule. It turns medially near the medial aspect of the capsule, partly intermingled with the dorsal zone of the subthalamic nucleus and the ventral part of the zona incerta, where the fasciculus traverses the H2 field of Forel. Reaching the medial border of the zona incerta, the fasciculus intermingles with fibres of the ansa lenticularis, scattered elements of the prerubral nucleus and dentatothalamic and rubrothalamic fibres. This merging of diverse pathways and associated cell groups is variously called the prerubral, tegmental or H field of Forel.
Epithalamus
Pineal Gland
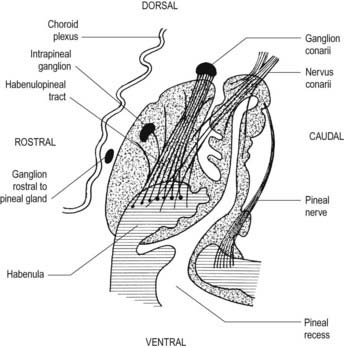
The pineal gland, or epiphysis cerebri (see Fig. 5.8; Fig. 15.19), is a small, reddish grey organ occupying a depression between the superior colliculi. It is inferior to the splenium of the corpus callosum, from which it is separated by the tela choroidea of the third ventricle and the contained cerebral veins. It is enveloped by the lower layer of the tela, which is reflected from the gland to the tectum. The pineal is approximately 8 mm long. Its base, directed anteriorly, is attached by a peduncle, which divides into inferior and superior laminae that are separated by the pineal recess of the third ventricle and contain the posterior and habenular commissures, respectively. Aberrant commissural fibres may invade the gland but do not terminate near parenchymal cells.
The pineal is an endocrine gland of major regulatory importance. It modifies the activity of the adenohypophysis, neurohypophysis, endocrine pancreas, parathyroids, adrenal cortex, adrenal medulla and gonads. Its effects are largely inhibitory. Indolamine and polypeptide hormones secreted by pinealocytes are believed to reduce the synthesis and release of hormones by the pars anterior, either by acting directly on its secretory cells or by indirectly inhibiting the production of hypothalamic releasing factors. Pineal secretions may reach their target cells via the cerebrospinal fluid or the blood stream. Some pineal indolamines, including melatonin and enzymes for their biosynthesis (e.g. serotonin, N-acetyltransferase), show circadian rhythms in concentration. The level rises during darkness and falls during the day, when secretion may be inhibited by sympathetic activity. It is thought that the intrinsic rhythmicity of an endogenous circadian oscillator in the suprachiasmatic nucleus of the hypothalamus governs cyclical pineal behaviour.
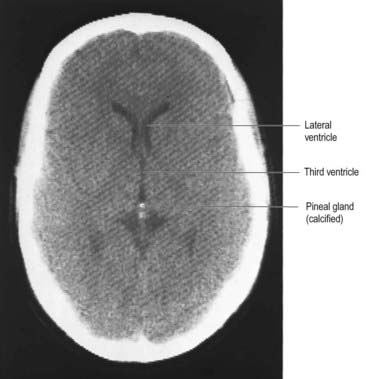
From the second decade, calcareous deposits accumulate in pineal extracellular matrix, where they are deposited concentrically as corpora arenacea or ‘brain sand’ (Fig. 15.20). Calcification is often detectable in skull radiographs, where it can be a useful indicator of a space-occupying lesion if the gland is significantly displaced from the midline.
Benarroch E.E. The midline and intralaminar thalamic nuclei-anatomic and functional specificity and implications in neurologic disease. Neurology. 2008;71:944-949.
Jones, 1985Jones E.G. The Thalamus. 1985. Plenum Press. New York. 403–411.
Describes the nomenclature and connections of thalamic nuclei.
Macchi, Jones, 1997Macchi G. Jones E.G. Toward an agreement on terminology of nuclear and subnuclear divisions of the motor thalamus. J. Neurosurg.. 86:1997;670-685.
Nasreddine Z.S., Saver J.L. Pain after thalamic stroke: right diencephalic predominance and clinical features in 180 patients. Neurology. 1997;48:1196-1199.
Nieuwenhuys, 1985Nieuwenhuys R. Chemoarchitecture of the Brain. 1985. Springer-Verlag. Berlin.
Describes the connections and neurochemistry of the hypothalamus.
Schmahmann J.D. Vascular syndromes of the thalamus. Stroke. 2003:2264-2278.
Victor M., Adams R.D., Collins G.H. The Wernicke-Korsakoff syndrome. Philadelphia: FA Davis Company; 1971.